Proton Exchange Membrane Fuel Cells
Overview
Source: Laboratories of Margaret Workman and Kimberly Frye - Depaul University
The United States consumes a large amount of energy – the current rate is around 97.5 quadrillion BTUs annually. The vast majority (90%) of this energy comes from non-renewable fuel sources. This energy is used for electricity (39%), transportation (28%), industry (22%), and residential/commercial use (11%). As the world has a limited supply of these non-renewable sources, the United States (among others) is expanding the use of renewable energy sources to meet future energy needs. One of these sources is hydrogen.
Hydrogen is considered a potential renewable fuel source, because it meets many important criteria: it’s available domestically, it has few harmful pollutants, it’s energy efficient, and it’s easy to harness. While hydrogen is the most abundant element in the universe, it is only found in compound form on Earth. For example, it is combined with oxygen in water as H2O. To be useful as a fuel, it needs to be in the form of H2 gas. Therefore, if hydrogen is to be used as a fuel for cars or other electronics, H2 needs to be made first. Thusly, hydrogen is often called an “energy carrier” rather than a “fuel.”
Currently, the most popular way to make H2 gas is from fossil fuels, through steam reforming of hydrocarbons or coal gasification. This does not reduce dependence on fossil fuels and is energy intensive. A less-used method is by electrolysis of water. This also requires an energy source, but it can be a renewable source, like wind or solar power. In electrolysis, water (H2O) is split into its component parts, hydrogen gas (H2) and oxygen gas (O2), through an electrochemical reaction. The hydrogen gas made through the process of electrolysis can then be used in a Proton Exchange Membrane (PEM) fuel cell, generating an electric current. This electric current can be used to power motors, lights, and other electrical devices.
Principles
Part I of this experiment involves the generation of hydrogen gas through electrolysis. In electrolysis, water is split into its component parts, hydrogen and oxygen, through the following electrochemical reaction:
2 H2O(l) → 2 H2(g) + O2(g)
There are twice as many hydrogen molecules produced as oxygen molecules. This reaction does not happen spontaneously and needs a source of electrical energy, e.g., a solar panel. This is an oxidation-reduction reaction. These types of chemical reactions can be split into two parts: the oxidation reaction and the reduction reaction. These are called half-reactions. In the oxidation half-reaction, electrons are released. In the reduction half-reaction, electrons are accepted.
Oxidation: 2 H2O(l) → O2(g) + 4 H+(aq) + 4 e-
Reduction: 4 H+(aq) + 4 e- → 2 H2(g)
The hydrogen gas can be collected and stored for use at a later time in a (PEM) fuel cell (Figure 1).
Part II of this experiment involves using the stored hydrogen gas as a fuel to produce electricity to power a fan. The fuel cell used in this experiment is a PEM fuel cell. The PEM fuel cell is like a battery, in that it creates electricity through a chemical reaction that involves the transfer of electrons. In the PEM fuel cell, the half reactions are as follows:
Oxidation: 2 H2(g) → 4 H+(aq) + 4 e-
Reduction: 4 H+(aq) + O2(g) + 4 e- → 2 H2O(l)
The overall reaction is: 2 H2(g) + O2(g) → 2 H2O(l) + energy
These half-reactions occur at the electrodes (conductors through which electricity passes). In the PEM fuel cell, there are two electrodes: an anode and a cathode. Oxidation occurs at the anode. Reduction occurs at the cathode. So, in the PEM fuel cell at the anode, hydrogen gas is oxidized, and electrons are released into the circuit. At the cathode, oxygen gas is reduced and water is formed. In the PEM fuel cell, a proton exchange membrane separates the two electrodes. This membrane allows protons (H+) to flow through, but prevents electrons from entering the membrane. Thus the electrons are forced to flow through the electrical circuit (Figure 2).

Figure 1: Diagram of an electrolyzer.
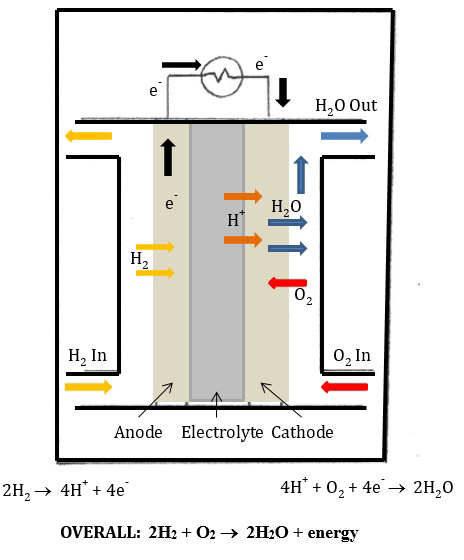
Figure 2: PEM Fuel Cell.
Procedure
1. Using the Electrolyzer to Produce Hydrogen Gas
- Setup the electrolyzer (Figure 3).
- Set up the gas collection cylinders, making sure the distilled water level in the outer cylinder is at the 0 mark (Figure 4).
- Connect the electrolyzer to the gas collection cylinders (Figure 5).
- Connect a solar panel to the electrolyzer using jumper wires and expose to direct sunlight (Figure 6). Note, if the weather is not cooperating that day, use a lamp with a light bulb to simulate the sun.
- H2 and O2 gas begins entering the inner cylinders (Figure 7). Monitor the volume of each gas produced in 30-s intervals, using the scale marked on the outer cylinder. It takes approximately 10 min to fill up the inner cylinder with H2 gas.
- When the inner cylinder is completely full of H2 gas, some bubbles should emerge from the inner cylinder, eventually reaching the surface. At this point, disconnect the solar panel from the electrolyzer and close the cincher on the H2 gas tube, so none of the H2 gas escapes. Notice there is twice as much hydrogen gas produced as oxygen gas, as predicted in the balanced chemical equation.
2. Fuel Cell
- Setup a fuel cell (Figure 8).
- Disconnect the H2 gas tubing from the electrolyzer and connect it to the fuel cell.
- Connect the fuel cell to a fan (or an LED light, if a fan is not available (Figure 9)) and release the cinch on the H2 gas tube (Figure 10). The fan should begin spinning. If not, press the purge valve on the fuel cell to get the gas flowing.
- The fan continues spinning until all of the H2 gas is consumed. This should last approximately 5 min.
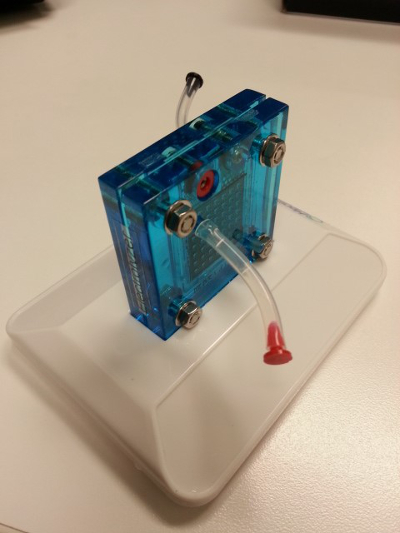
Figure 3: A picture of the electrolyzer.

Figure 4: Gas collection cylinders with distilled water levels equal to 0.
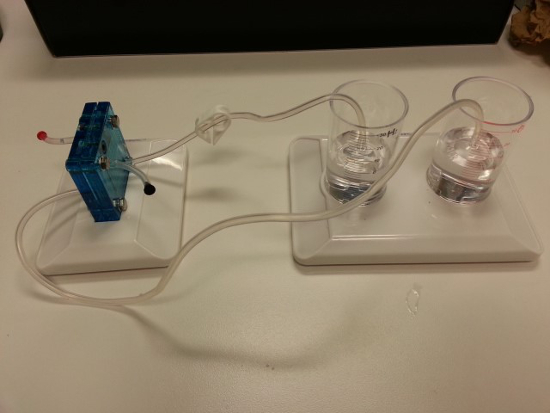
Figure 5: A picture of the electrolyzer connected to the gas collection cylinders.
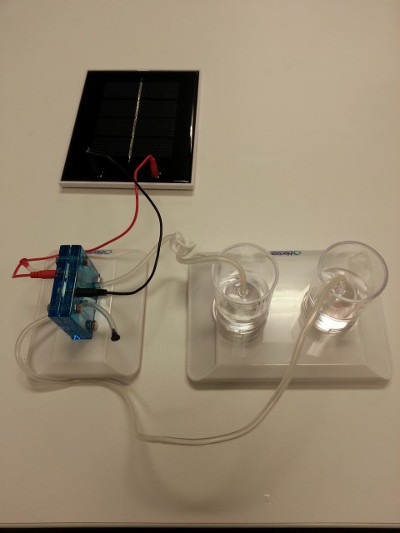
Figure 6: The solar panel connected to the electrolyzer with jumper wires.

Figure 7: An example of the gas entering the cylinders.

Figure 8: A picture of a fuel cell.
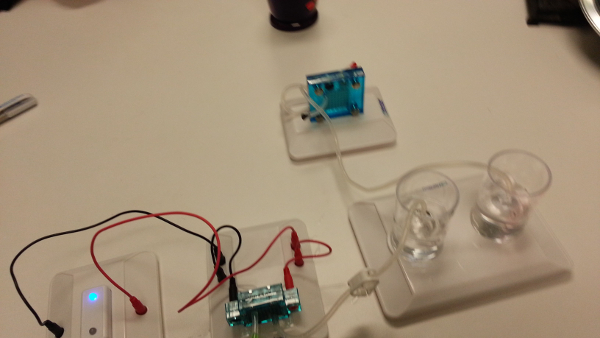
Figure 9: The fuel cell connected to an LED light instead of a fan.

Figure 10: The electrolyzer connected with the fuel cell, which is connected with the fan.
Results
During the electrolysis procedure, hydrogen and oxygen gas are generated once the solar panel is connected and exposed to sunlight. It takes approximately 10 min to generate enough H2 gas to fill the inner cylinder (Table 1). Note that there is twice as much H2 generated as O2, as seen in the balanced equation:
2 H2O(l) → 2 H2(g) + O2(g)
Once the H2 gas is generated and the tubing is connected to the fuel cell, the fuel cell generates electricity and causes the fan to spin. This lasts approximately 10 min on a full cylinder of H2 gas.
| Time (s) | Hydrogen Generated (mL) | Oxygen Generated (mL) |
| 0 | 0 | 0 |
| 30 | 4 | 2 |
| 60 | 8 | 4 |
| 90 | 10 | 6 |
| 120 | 12 | 6 |
| 150 | 14 | 6 |
| 180 | 14 | 8 |
| 210 | 16 | 8 |
| 240 | 18 | 8 |
| 270 | 20 | 10 |
| 300 | 22 | 10 |
| 330 | 22 | 10 |
| 360 | 24 | 12 |
| 390 | 24 | 12 |
| 420 | 26 | 12 |
| 450 | 26 | 14 |
| 480 | 28 | 14 |
| 510 | 28 | 14 |
| 540 | 28 | 14 |
| 570 | 30 | 16 |
| 600 | 30 | 16 |
Table 1: Time Required for Generating Different Hydrogen and Oxygen Quantities
Application and Summary
Hydrogen is a flexible fuel. It can be produced on-site in small quantities for local use or in large quantities at a centralized facility. The hydrogen can then be used to produce electricity with only water as a byproduct (provided a renewable source of energy, like a wind turbine, was used to generate the hydrogen gas). For example, in Boulder, Colorado, the Wind2H2 project has wind turbines and solar panels connected to electrolyzers that produce hydrogen gas from water and then stores it to be used in their hydrogen fueling station.
This process can also be used to make cars run on hydrogen gas (H2) instead of fossil fuels. If a PEM fuel cell is installed in a car, electricity can be used to make the motor run. The only exhaust would be water (H2O). From an air pollution perspective, this is advantageous. There are many prototype fuel cell cars being developed by major car manufacturers. Due to the amount of space currently required to store the compressed hydrogen tanks on a vehicle, hydrogen fuel cells are mainly seen on buses. Fuel cell buses can be found in several countries around the world. There are some technological issues that need to be addressed before fuel cell cars are a viable alternative to internal combustion engine cars including providing more infrastructure, reducing costs, and an increased use of renewable energy sources when making H2 gas.
In addition, hydrogen fuel cells can be used in place of batteries for things like video cameras and radios. An example is the UPP device, which is a portable power pack based on hydrogen fuel cell technology that can be used to charge USB compatible devices.
Skip to...
Videos from this collection:
Copyright © 2025 MyJoVE Corporation. All rights reserved
