Method Article
Implantation of Tissue-Engineered Vascular Graft in Mouse Carotid Artery via Cuff Technique
In This Article
Summary
Here, a protocol is presented for the implantation of a tissue-engineered vascular graft into the mouse carotid artery using the cuff technique, providing a suitable animal model for investigating vascular tissue regeneration mechanisms.
Abstract
The development of small-diameter vascular grafts has been a global endeavor, with numerous research groups contributing to this field. Animal experimentation plays a pivotal role in assessing the efficacy and safety of vascular grafts, particularly in the absence of clinical applications. Compared to alternative animal models, the mouse implantation model offers several advantages, including a well-defined genetic background, a mature method for disease model construction, and a straightforward surgical procedure. Based on these advantages, the present study devised a simple cuff technique for the implantation of tissue-engineered vascular grafts in the mouse carotid artery. This technique began with the fabrication of polycaprolactone (PCL) small-diameter vascular grafts via electrostatic spinning, followed by the seeding of macrophages onto the grafts through perfusion adsorption. Subsequently, the cellularized tissue-engineered vascular grafts were transplanted into the mouse carotid artery using the cuff technique to evaluate patency and regenerative capability. After 30 days of in vivo implantation, vascular patency was found to be satisfactory, with evidence of neo-tissue regeneration and the formation of an endothelial layer within the lumen of the grafts. All data were analyzed using statistical and graphing software. This study successfully established a mouse carotid artery implantation model that can be used to explore the cellular sources of vascular regeneration and the mechanisms of action of active substances. Furthermore, it provides theoretical support for the development of novel small-diameter vascular grafts.
Introduction
The prevalence and mortality of cardiovascular diseases are increasing globally, representing a significant public health concern1. Vascular bypass grafting is an effective intervention for severe coronary heart disease and peripheral vascular disease2. The use of artificial vascular grafts with diameters exceeding 6 mm has been well-documented in clinical settings. Conversely, those with a diameter below 6 mm are prone to thrombosis and intimal hyperplasia, which can lead to a considerable risk of restenosis3. Despite significant advancements in the research and development of small-diameter vascular grafts in recent years, with several products approaching clinical application, multiple challenges remain4,5. These include a relatively low long-term patency rate, limited vascular regeneration, and an insufficient understanding of the regeneration mechanism.
Preclinical evaluation of novel small-diameter vascular grafts relies on in vivo implantation in various animal models. The most commonly used models include the sheep carotid artery, dog femoral artery, rabbit carotid artery, and rat abdominal artery implantation models6,7,8,9. The patency of vascular grafts can be assessed in medium- to large-sized animals, such as sheep, pigs, and dogs. However, these studies involve substantial costs due to the expertise and equipment required. Additionally, their technical complexity poses a challenge to implementation. In contrast, small animal models such as rabbits and rats lack well-established transgenic species with clearly defined genetic backgrounds, presenting a significant obstacle in studying vascular regeneration mechanisms.
Compared to the aforementioned animal models, the mouse model offers a relatively straightforward surgical procedure, a well-established methodology for generating genetically engineered mice, and a clearly defined genetic background. However, the small diameter of mouse blood vessels makes end-to-end anastomosis in vascular grafting technically complex, requiring significant expertise and yielding a relatively low success rate. To reduce the complexity of the procedure and improve the success rate of vascular graft implantation, the present study employed the cuff technique in a mouse carotid artery implantation model.
Following in vivo implantation, vascular grafts can recruit endogenous cells that contribute to vascular tissue regeneration. The presence of these cells facilitates the endothelialization and regeneration of the smooth muscle layer of grafts.10. However, the source and type of cells involved in vascular tissue regeneration remain unclear, and multiple competing theories are under investigation11. Among these, research has focused on the roles of inflammatory and stem cells. Breuer et al. seeded human bone marrow-derived monocytes (hBMCs) onto vascular grafts and found that the seeded cells recruited host cells into the graft wall through the release of monocyte chemoattractant protein-1 (MCP-1), thereby promoting vascular tissue regeneration12. In this study, an efficient perfusion adsorption cell-seeding method was proposed and successfully used to seed macrophages onto polycaprolactone (PCL) small-diameter vascular grafts. Following implantation, these cells exhibited sustained viability.
This article details the methodology for preparing tissue-engineered vascular grafts and the carotid artery implantation procedure in mice using the cuff technique. The process begins with the fabrication of PCL small-diameter vascular grafts with defined parameters via electrostatic spinning. Subsequently, grafts deemed suitable for implantation undergo mechanical testing. Macrophages are then seeded onto the vascular grafts using the perfusion adsorption method. Finally, macrophage-seeded vascular grafts are implanted into the mouse carotid artery using the cuff technique, and the patency and regenerative properties are analyzed after one month of in vivo implantation.
This technique has the potential to enhance the efficacy and success rates of vascular grafting in mouse models. Furthermore, the model can be used to investigate the mechanisms underlying cell sources, pivotal genes, and active factors in vascular regeneration, providing theoretical and methodological support for the functional modification and development of novel small-diameter vascular grafts.
Protocol
All animal procedures were approved by the Animal Experiments Ethical Committee of the Institute of Radiation Medicine, Chinese Academy of Medical Sciences, and complied with the Guidelines for the Care and Use of Laboratory Animals. Male C57BL/6 mice, 6-8 weeks old, with a body weight of 25-30 g, were used in this study. Details of the reagents and equipments used in this study are listed in the Table of Materials.
1. Fabrication of small-diameter vascular grafts
NOTE: Fabricate small-diameter PCL vascular grafts using the electrospinning technique13.
- Prepare PCL solutions at 10%, 15%, and 20% (w/v) in hexafluoroisopropanol (HFIP) at room temperature (RT) for 12 h.
- Load the PCL solutions into a 10 mL syringe and position the syringe with a 21 G stainless-steel needle.
- Place a tungsten-steel mandrel (0.7 mm in diameter, 20 cm in length) on the collection instrument.
- Fabricate nine groups of PCL vascular grafts using the electrospinning technique. Connect a high-voltage power supply to the needle. Position a tungsten steel rod with an inner diameter of 0.7 mm at a fixed distance in front of the needle as a receiving device.
NOTE: The description of vascular graft groups is provided in Table 1, and the parameters of the vascular grafts are detailed in Table 2. - Subject the fabricated vascular grafts to an evacuation process. Place the vascular grafts in a vacuum-drying oven for 72 h to remove residual solvents.
- Sterilize the grafts by immersing them in medical alcohol for 30 min and exposing them to UV light overnight.
- Observe the microscopic morphology of the vascular grafts using a scanning electron microscope (SEM). Attach the vascular grafts to the SEM specimen stage with conductive adhesive and place them in the gold-sputtering device for coating.
- Observe the structure and fiber morphology of the vascular grafts using SEM at an accelerating voltage of 15 kV. Measure fiber diameter and pore size from SEM images (n = 5) using ImageJ software.
- Evaluate the mechanical properties (tensile and elastic properties) of the vascular grafts using a tensile testing machine. Clamp the upper and lower ends of the vascular graft with fixed clamps spaced 1 cm apart.
- Stretch the vascular graft at a rate of 10 mm/min until rupture. Collect the stress-strain curve from the tensile test machine.
- Calculate mechanical parameters, including maximum load (2-15 N), stress at break (5-30 MPa), strain at break (200%-1500%), and elastic modulus (1-20 MPa)14.
- Perform statistical analysis using statistical and graphing software. Express data as mean ± standard deviation. Analyze and compare univariate differences between multiple groups using Tukey's post hoc test in one-way ANOVA. *P < 0.05, **P < 0.01, ***P < 0.001.
2. Seeding of macrophages onto vascular grafts
NOTE: Ensure that all solutions and materials are sterile. Conduct all operations within the cell culture room.
- Culture RAW264.7 (mouse monocyte macrophage) in flasks under adherent conditions (Figure 1A). Prepare the cell culture medium using complete Dulbecco's modified Eagle's medium (DMEM) supplemented with 1% penicillin-streptomycin and 10% fetal bovine serum. Place the flasks in a 37 °C incubator containing 5% carbon dioxide.
- Collect macrophages using a cell scraper. Discard the medium using a 1 mL pipette and wash the cells with PBS.
- Add 2 mL of fresh medium to the culture flask and gently scrape the surface with a cell scraper. Transfer the collected cells to a tube and centrifuge at 1,000 x g for 5 min at room temperature. Resuspend 5 × 106 cells in 100 µL of complete medium.
- Place a PCL vascular graft (1 cm in length) in a 15 mL tube filled with DMEM and centrifuge at 4,000 x g for 5 min. Repeat this process several times until the graft sinks to the bottom of the tube, ensuring complete infiltration of the material with DMEM.
- Prepare a 10 cm Petri dish lined with a layer of filter paper. Place the moistened PCL vascular graft on the filter paper and roll it to remove excess medium.
- Take 10 µL of the cell suspension using a 10 µL pipette and inject it into one end of the vascular graft. Rotate the vascular graft over the filter paper to facilitate uniform distribution of the suspension. Inject each end five times for a total of ten injections (Figure 1B,C).
- Place the macrophage-loaded vascular graft in a 24-well plate containing 1 mL of complete medium and incubate for 2 h in a cell culture incubator before implantation.
- Determine the distribution of cells within the graft wall. Embed the macrophage-loaded vascular graft in optical coherence tomography (OCT) compound for cryosectioning. Stain cell nuclei with 4',6-diamidino-2-phenylindole (DAPI) and observe under a fluorescence microscope.
3. Mouse carotid artery implantation model
NOTE: Maintain a sterile surgical area for animal procedures. Sterilize all surgical instruments and disposables prior to surgery.
- Select three healthy male C57BL/6 mice, each weighing 25-30 g. Fast the mice the day before surgery. Implant one vascular graft in each animal, for a total of three grafts.
- Construct cuffs from a nylon tube (Figure 2A) and present the schematic diagram of the vascular cuff technique in Figure 2B.
- Anesthetize the mice by intraperitoneal injection of sodium pentobarbital at a concentration of 50 mg/kg (following institutionally approved protocols). Confirm effective anesthesia by ensuring muscle relaxation and even breathing. Apply petrolatum ophthalmic ointment to the eyes to prevent dryness during anesthesia.
- Position the mouse in a supine position on the operating table and remove the neck hair. Sterilize the surgical area with iodophor. Cover the non-surgical area with sterile gauze to maintain a sterile environment (Figure 3A).
- Use ophthalmic scissors to make a midline incision (1.5-2 cm in length) from the mandible to the sternum. Elevate the left salivary glands and excise the left cleidomastoid muscle to increase the surgical field of view. Expose the left common carotid artery using micro tweezers (Figure 3B).
- Isolate the left carotid artery using microforceps (Figure 3C).
- Ligate the carotid artery in two locations in the mid-portion using a 9-0 suture. Transect the artery between the two ligatures using microscissors. Pass the cuff through the arteries at each end and secure the artery and cuff together using arterial clips (Figure 3D).
- Turn the artery outward to cover the cuff body and secure it to the cuff using a 9-0 suture with microforceps (Figure 3E).
- Implant a vascular graft between the two ends of the carotid artery by sliding the graft ends over the artery cuff and securing them with 9-0 sutures (Figure 3F).
NOTE: The dimensional data of the cuff, carotid artery, and vascular graft are presented in Table 3. Although the diameters of the graft and cuff closely match those of the mouse carotid arteries, a mismatch exists between the anastomotic lumen diameter and the graft diameter. The anastomotic lumen diameter is calculated by subtracting twice the thickness of the carotid artery wall (0.1 mm) from the inner diameter of the cuff (0.5 mm), resulting in a lumen diameter of 0.3 mm. The vascular graft has an inner diameter of 0.7 mm, leading to a mismatch of approximately 2.3 times (0.7 mm / 0.3 mm = 2.3). - Remove the arterial clips at both ends and irrigate the implantation site with saline. Assess the patency of the vascular graft by observing distal arterial pulsation (Figure 3G).
- Reposition the left salivary gland and close the surgical site using 6-0 sutures (Figure 3H).
4. Post-procedural care and analysis
- Transfer the mouse to a 37 °C incubator and continuously monitor its vital signs until it regains consciousness.
- Inject 5 mg/kg carbinol for postoperative pain relief.
- Monitor the mouse during the recovery period and do not return it to group housing until it has fully recovered.
- Anesthetize the mice one month after implantation and harvest the vascular graft samples for histological analysis9.
- Perform euthanasia via CO2 asphyxiation followed by cervical dislocation following ethical guidelines.
Results
Small-diameter vascular grafts with different parameters were successfully prepared via electrospinning. SEM images revealed that the fibers were uniformly distributed and exhibited an irregular arrangement within the graft wall, with the presence of pore structures (Figure 4). As the concentration of PCL increased, both the fiber diameter and pore size increased. Specific values for each vascular graft group are presented in Table 2. The results of mechanical testing demonstrated that all vascular grafts met the required mechanical standards. The maximum load and strain at break increased with higher PCL concentrations and greater vascular wall thickness (Figure 5A-C), while the modulus of elasticity decreased (Figure 5D). Based on these findings, small-diameter vascular grafts with a PCL concentration of 15% and a wall thickness of 150 µm were selected as the optimal grafts for subsequent experiments.
In this study, macrophages were seeded onto vascular grafts using the perfusion adsorption method. SEM analysis confirmed that macrophages were successfully seeded onto the vascular grafts and exhibited a uniform distribution (Figure 6A). Cross-sectional grafts subjected to DAPI staining revealed that the seeded macrophages had infiltrated from the graft lumen into the graft wall (Figure 6B).
Thirty days after in vivo implantation, the survival rate of the animals was 100%, and two of the three implanted vascular grafts remained patent, which was a satisfactory outcome in mouse models. No aneurysms or discernible fibrous encapsulation of the grafts were observed under a stereomicroscope (Figure 7A). The extent of cellular infiltration and tissue regeneration was further evaluated through histological analysis. Hematoxylin and eosin (H&E) staining revealed substantial cellular infiltration within the graft wall and neotissue formation within the graft's inner lumen (Figure 7B). CD31 immunofluorescence staining demonstrated the regeneration of a single layer of endothelial tissue within the inner lumen of the grafts, with relatively intact endothelial cell coverage (Figure 7C).

Figure 1: Cell seeding procedure. (A) Culture of RAW264.7 cells. Scale bar: 100 µm. (B) Schematic of the cell seeding process. (C) RAW264.7 cells seeded onto the graft wall using the perfusion-adsorption method. Please click here to view a larger version of this figure.

Figure 2: Cuff technique. (A) Preparation of vascular cuffs. (B) Schematic representation of the vascular cuff technique. Vascular grafts were implanted into the carotid artery using this method. Please click here to view a larger version of this figure.

Figure 3: Surgical procedure for vascular graft implantation in a mouse model. (A) Injection of anesthetics, positioning of the mouse in the supine position, and immobilization of the paws. (B) Exposure of the surgical area. (C) Isolation of the carotid artery. (D) Placement of the cuff around the carotid artery. (E) Eversion of the artery over the cuff body and fixation using a 9-0 suture. (F) Sleeving of the vascular graft over the arterial cuff and suturing. (G) Removal of vascular clamps. (H) Skin closure. Please click here to view a larger version of this figure.
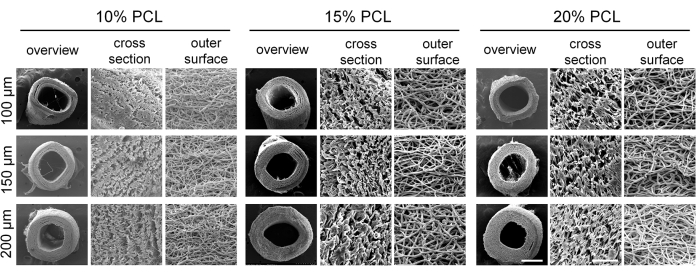
Figure 4: Morphology of electrospun polycaprolactone (PCL) vascular grafts. Representative scanning electron microscopy (SEM) images demonstrating the well-defined fibrous structure of the vascular grafts. Scale bars: 500 µm and 50 µm (magnified images). Please click here to view a larger version of this figure.
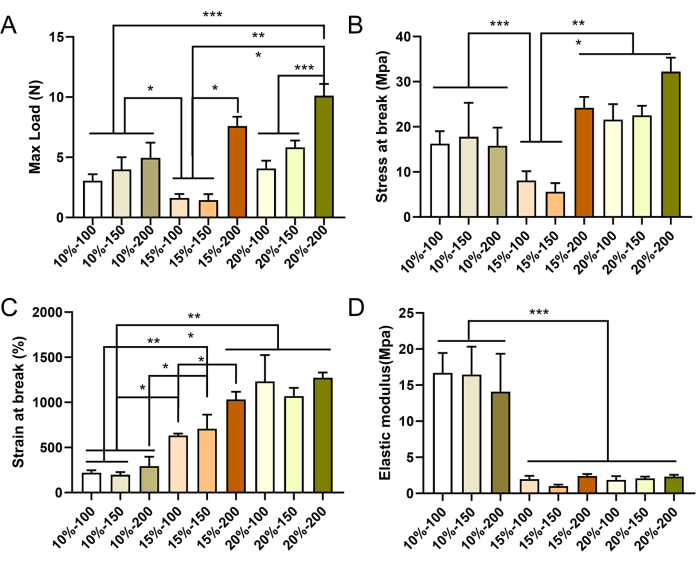
Figure 5: Mechanical properties of electrospun PCL vascular grafts. Mechanical characterization of the vascular grafts in the longitudinal direction, showing maximum load (A), maximum stress (B), strain at break (C), and elastic modulus (D). Data are expressed as mean ± standard deviation (SD) (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001, one-way ANOVA followed by Tukey's post hoc test. Please click here to view a larger version of this figure.
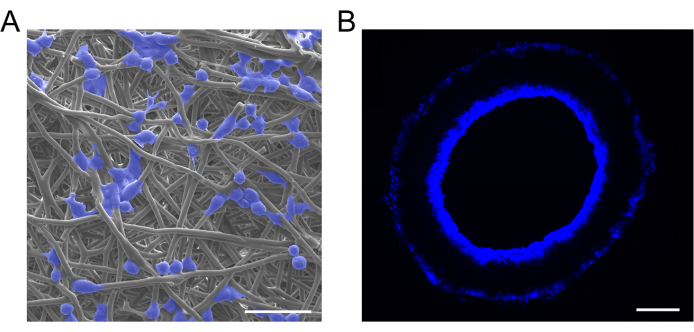
Figure 6: Characterization of cell-loaded grafts. (A) Representative SEM images and (B) 4',6-diamidino-2-phenylindole (DAPI) staining of cell-loaded vascular grafts, showing successful macrophage implantation and uniform distribution. Scale bars: 30 µm and 100 µm. Please click here to view a larger version of this figure.
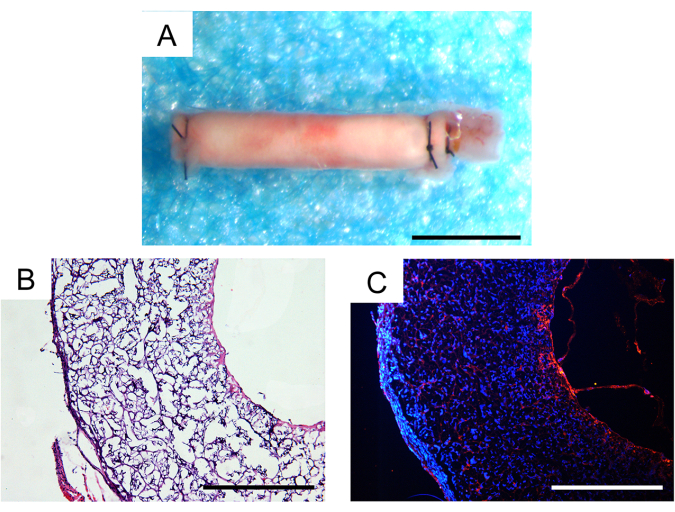
Figure 7: Histological analysis of explanted vascular grafts one month after implantation. (A) Stereoscopic image showing no aneurysm formation or fibrous encapsulation. Scale bar: 500 µm. (B) Hematoxylin and eosin (H&E) staining demonstrating good cellularization and tissue regeneration. Scale bar: 200 µm. (C) CD31 immunofluorescence staining revealing a monolayer of endothelial cells on the luminal surface. Scale bar: 200 µm. Please click here to view a larger version of this figure.
| Group | Concentration of PCL solution (%) | thickness of vascular graft (μm) | needle-collector distance (cm) | flow rate (mL/h) | voltage (kV) |
| 10%-100 | 10 | 100 | 15 | 2 | 18 |
| 10%-150 | 10 | 150 | 15 | 2 | 18 |
| 10%-200 | 10 | 200 | 15 | 2 | 18 |
| 15%-100 | 15 | 100 | 15 | 2 | 18 |
| 15%-150 | 15 | 150 | 15 | 2 | 18 |
| 15%-200 | 15 | 200 | 15 | 2 | 18 |
| 20%-100 | 20 | 100 | 13 | 8 | 12 |
| 20%-150 | 20 | 150 | 13 | 8 | 12 |
| 20%-200 | 20 | 200 | 13 | 8 | 12 |
Table 1: Description of vascular graft groups. PCL, polycaprolactone.
| Group | Fiber diameter (μm) | Pore size (μm) |
| 10%-100 | 0.51±0.12 | 2.48±1.38 |
| 10%-150 | 0.91±0.4 | 1.83±0.84 |
| 10%-200 | 0.73±0.35 | 2.24±0.83 |
| 15%-100 | 1.85±0.3 | 8.91±2.87 |
| 15%-150 | 1.82±0.34 | 8.41±2.72 |
| 15%-200 | 2.18±0.47 | 9.59±3.01 |
| 20%-100 | 3.61±1.02 | 13.95±4.7 |
| 20%-150 | 3.06±0.56 | 13.12±3.36 |
| 20%-200 | 3.46±0.66 | 13.92±4.19 |
Table 2: Structural parameters of different vascular grafts.
| Group | internal diameter(mm) | external diameter(mm) | wall thickness(μm) | length(cm) |
| carotid artery | 0.5-0.6 | 0.6-0.7 | 100 | / |
| vascular graft | 0.7 | 1 | 150 | 0.8 |
| cuff | 0.5 | 0.63 | 65 | / |
Table 3: Dimensional data of the cuff, carotid artery, and vascular graft.
Discussion
The use of the cuff technique for implanting tissue-engineered vascular grafts in the mouse carotid artery represents a significant advancement in cardiovascular research15. The critical steps of this technique include cell seeding and graft implantation. This study employed a perfusion adsorption approach to enhance macrophage seeding density to address issues related to non-uniform cell seeding and low cell viability. This method allowed macrophages to infiltrate the vascular graft wall and distribute it evenly.
With regard to vascular implantation, the cuff technique, as opposed to end-to-end anastomosis16, may be associated with a higher incidence of stenosis, particularly when applied to vessels with smaller diameters. To minimize this effect, cuffs with internal and external diameters closely matching those of the mouse carotid arteries were selected. The relevant dimensions are provided in Table 3.
This technique facilitates a more straightforward surgical procedure for vascular graft implantation in the mouse carotid artery. The animal models most frequently used in previous studies of small-diameter vascular grafts include the sheep jugular arteriovenous fistula and the rat abdominal artery implantation models17,18. These models are effective for assessing the mechanical strength and tissue remodeling capacity of vascular grafts; however, they are limited in their ability to elucidate underlying mechanisms. Genetically modified mice have emerged as an important model for investigating vascular regeneration mechanisms, addressing the limitations of other animal models.
For example, Apolipoprotein E (ApoE) knockout mice spontaneously develop hypercholesterolemia and atherosclerotic lesions19, making them a valuable model for simulating clinical conditions that require vascular transplantation. Evaluating vascular patency and regeneration in ApoE-knockout mice provides important reference data for the clinical translation of vascular grafts. Additionally, gene-deficient mice can be utilized to investigate the roles of key genes in vascular regeneration. Endothelial nitric oxide synthase (eNOS), a critical regulator of vascular function, is a primary source of nitric oxide20. eNOS gene-deficient mice serve as a research model for elucidating the mechanisms by which this gene influences vascular remodeling. Furthermore, the origin of cells involved in vascular tissue regeneration can be explored through in vivo cell labeling and lineage tracing using reporter gene tool mice21,22.
This technique has certain limitations concerning the number of animals used and the duration of transplantation. The present study primarily focused on evaluating the feasibility of this grafting method using a short-term in vivo approach. Future studies should increase the sample size and conduct long-term evaluations to comprehensively assess the impact of this method on aneurysm formation, patency, and intimal hyperplasia.
Additionally, the graft patency rate was 66.6%, which may have been influenced by a diameter mismatch (2.3-fold) at the anastomosis site. This mismatch can lead to localized blood flow disturbances, increasing the risk of thrombosis. Further studies are required to optimize cuff design to reduce diameter discrepancies and investigate their effects on patency rates.
In conclusion, the mouse carotid artery implantation model based on the cuff technique provides a simple and effective animal model for the biological evaluation of small-diameter vascular grafts. Moreover, it enables the investigation of the roles of various cell types in vascular tissue remodeling and the underlying regenerative mechanisms.
Disclosures
The authors have no conflicting financial interests.
Acknowledgements
Funding for this study was provided by the National Natural Science Foundation of China projects (no. 32101098, 32071356, and 82272158) and the CAMS Innovation Fund for Medical Sciences (no. 2022-I2M-1-023).
Materials
| Name | Company | Catalog Number | Comments |
| 1% penicillin-streptomycin | Solarbio | P1400 | |
| 10% fetal bovine serum | Gibco | A5256701 | |
| 4% paraformaldehyde | Solarbio | P1110 | |
| 4',6-Diamidino-2-Phenylindole (DAPI) | SouthernBiotech | 0100-20 | |
| Alcohol | Tianjin Chemical Reaggent Company | 1083 | |
| Anti-Mouse CD31 primary antibody | BD Bioscience | 553370 | |
| Arterial clips | RWD Life Science | R31005-06 | |
| C57BL/6 mice | Beijing Vital River Laboratory Animal Technology Company | ||
| Dulbecco's modified eagle medium (DMEM) | Gibco | 11966025 | |
| Electrostatic spinning machine | Yunfan Technology | DP30 | |
| Goat anti-rat IgG (Alexa Fluor 555) | Invitrogen | A-21434 | |
| Hematoxylin and eosin (H&E) | Solarbio | G1120 | |
| Hexafluoroisopropanol (HFIP) | McClean | H811026 | |
| Iodophor | LIRCON | V273068 | |
| Microscissors | World Precision Instruments | 14124 | |
| Microtweezers | World Precision Instruments | 500338 | |
| Normal goat serum | Boster | AR0009 | |
| Normal saline | Cisen Pharmaceutical company | H20113369 | |
| Nylon tube for cuff | Portex | ||
| Optimal cutting temperature compound (OCT) | Sakara | 4583 | |
| Pentobarbital sodium | Sigma | P3761 | |
| Phosphate Buffered Saline (PBS) | Solarbio | P1003 | |
| Poly(ε-caprolactone) (PCL) pellets (Mn = 80,000) | Sigma | 704067 | |
| RAW264.7 macrophages | Biyuntian Biotechnology | ||
| Scanning electron microscope (SEM) | Zeiss | PHENOM-XL-G2 | |
| Surgical sutures 6-0 | Ningbo Chenghe microapparatus factory | 220919 | |
| Surgical sutures 9-0 | Ningbo Chenghe microapparatus factory | 221006 | |
| Syringe | Changqiang Medical Devices | 0197 | |
| Tensile testing machine | Instron | WDW-5D |
References
- Adhikary, D., Barman, S., Ranjan, R., Stone, H. A systematic review of major cardiovascular risk factors: A growing global health concern. Cureus. 14 (10), e30119 (2022).
- Alexander, J. H., Smith, P. K. Coronary-artery bypass grafting. N Engl J Med. 374 (20), 1954-1964 (2016).
- Jeong, Y., Yao, Y., Yim, E. K. F. Current understanding of intimal hyperplasia and effect of compliance in synthetic small diameter vascular grafts. Biomater Sci. 8 (16), 4383-4395 (2020).
- Lawson, J. H., et al. Bioengineered human acellular vessels for dialysis access in patients with end-stage renal disease: Two phase 2 single-arm trials. Lancet. 387 (10032), 2026-2034 (2016).
- Kirkton, R. D., et al. Bioengineered human acellular vessels recellularize and evolve into living blood vessels after human implantation. Sci Transl Med. 11 (485), eaau6934 (2019).
- Wang, C., Li, Z., Zhang, L., Sun, W., Zhou, J. Long-term results of triple-layered small diameter vascular grafts in sheep carotid arteries. Med Eng Phys. 85, 1-6 (2020).
- Tanaka, T., et al. Evaluation of small-diameter silk vascular grafts implanted in dogs. JTCVS Open. 6, 148-156 (2021).
- Jin, X., et al. Preparation of small-diameter tissue-engineered vascular grafts electrospun from heparin end-capped PCL and evaluation in a rabbit carotid artery replacement model. Macromol Biosci. 19 (8), e1900114 (2019).
- Xiao, Y., et al. Fabrication of small-diameter in situ tissue engineered vascular grafts with core/shell fibrous structure and a one-year evaluation via rat abdominal vessel replacement model. Biomater Adv. 165, 214018 (2024).
- Wei, Y., Wang, F., Guo, Z., Zhao, Q. Tissue-engineered vascular grafts and regeneration mechanisms. J Mol Cell Cardiol. 165, 40-53 (2022).
- Cleary, M. A., et al. Vascular tissue engineering: The next generation. Trends Mol Med. 18 (7), 394-404 (2012).
- Roh, J. D., et al. Tissue-engineered vascular grafts transform into mature blood vessels via an inflammation-mediated process of vascular remodeling. Proc Natl Acad Sci U S A. 107 (10), 4669-4674 (2010).
- Wang, Z., et al. The effect of thick fibers and large pores of electrospun poly(ε-caprolactone) vascular grafts on macrophage polarization and arterial regeneration. Biomaterials. 35 (22), 5700-5710 (2014).
- Wu, Y., et al. Peptide-tethered vascular grafts enable blood vessel regeneration via endogenous cell recruitment and neovascularization. Compos B Eng. 252, 110504 (2023).
- Hu, Y., Xu, Q. Vessel graft atherosclerosis in murine models. Curr Drug Targets. 9 (3), 239-250 (2008).
- Qin, K., et al. Implantation of electrospun vascular grafts with optimized structure in a rat model. J Vis Exp. (136), e57340 (2018).
- Geelhoed, W. J., et al. A novel method for engineering autologous non-thrombogenic in situ tissue-engineered blood vessels for arteriovenous grafting. Biomaterials. 229, 119577 (2020).
- Wu, P., et al. Construction of vascular graft with circumferentially oriented microchannels for improving artery regeneration. Biomaterials. 242, 119922 (2020).
- Vasquez, E. C., Peotta, V. A., Gava, A. L., Pereira, T. M., Meyrelles, S. S. Cardiac and vascular phenotypes in the apolipoprotein e-deficient mouse. J Biomed Sci. 19 (1), 22 (2012).
- Garcia, V., Sessa, W. C. Endothelial nos: Perspective and recent developments. Br J Pharmacol. 176 (2), 189-196 (2019).
- He, L., et al. Enhancing the precision of genetic lineage tracing using dual recombinases. Nat Med. 23 (12), 1488-1498 (2017).
- Wang, F., et al. Nitric oxide improves regeneration and prevents calcification in bio-hybrid vascular grafts via regulation of vascular stem/progenitor cells. Cell Rep. 39 (12), 110981 (2022).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved