Determining the Mass Percent Composition in an Aqueous Solution
Genel Bakış
Source: Laboratory of Dr. Neal Abrams — SUNY College of Environmental Science and Forestry
Determining the composition of a solution is an important analytical and forensic technique. When solutions are made with water, they are referred to as being aqueous, or containing water. The primary component of a solution is referred to as the solvent, and the dissolved minor component is called the solute. The solute is dissolved in the solvent to make a solution. Water is the most common solvent in everyday life, as well as nearly all biological systems. In chemistry labs, the solvent may be another liquid, like acetone, ether, or an alcohol. The solute can be a liquid or a solid, but this experiment only addressesthe determination of solids.
İlkeler
When a solute is dissolved in a solvent, the mass of the resultant solution is a sum of the mass of the solute and the solvent. This follows the Law of Conservation of Mass:
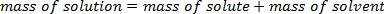
This differs from the addition of volumes, which is notadditive, since molecules of the solute fit into open spaces between the molecules of the solvent and keeps the total volume lower than expected. Determining percent by mass is a simple and important technique for analyzing a solution's makeup. By definition, a percent is a fractional,unitless value of one component compared to the whole. In this case, the percent by mass can be represented mathematically as:

Although percent by mass is unit-less, this value is sometimes represented with the units (w/w) to convey that the comparison is by mass (weight) only and not by volume. Percent by volumes are indicated as weight per volume (w/v) or volume per volume (v/v). In w/w calculations, it is important to note that the denominator is the mass of the entire solution, not the solvent. While percent by mass is used to determine solution concentration, percent by mole is typically used to calculate the percent of anelement or group in a molecule. It is difficult to calculate percent by mole in a solution, since the total moles of solute and solvent would need to be known. If those values were known, the overall calculation would be irrelevant.
In some cases, obtaining the mass of the solvent or solute individually can be difficult or impractical. In these cases, the density of a solution can be used to determine mass percent by first producing a calibration curve of solutions with known compositions. This first requires the following to be known: the identity of the solute anda reasonable range of the solution's concentration.More complex analytical methods are required when more than one solute is present in solution. For example, selective precipitation or ion exchange could be used to analyze for a solute of interest. For single solute solutions, enough material must be available to create a series of solutions of known concentration.Two percent by mass methods are demonstrated here.
Prosedür
1. Percent by Mass - Direct
- Place a small volume of a solution into a clean and oven-dried beaker or crystallization dish.
- After accurately determining the precise total mass of the solution, heat the beaker or dish on a hotplate or in an oven to drive off the water. Slow evaporation is the best method, as boiling can result in splattering of the solution.
- Once the solvent has evaporated, cool the remaining solid (solute) and determine the mass.
- Calculate the mass percent as:

2. Percent by Mass - Using a Calibration Curve
- Make a series of standard solutions by dissolving known quantities of solute into a solvent. Five standards are recommended and should range from the minimum to the maximum expected percent compositions.
- If the approximate value is unknown, produce a series of solutions ranging from 0% through the solubility maximum of the solute in water. Reference tables are available to indicate the maximum solubility for many solids in water over a variety of temperature ranges.
- For example, to produce a 35% (w/w) sodium chloride (NaCl) solution, add 35 g to a flask, then add 65 g (or 65 mL, as volume can also be used when measuring pure water since the density is known to be 1.0 g/mL at ambient temperatures) of water, and mix the solution until the solid is dissolved. Because mass is additive, this would be the same as adding enough water so the total solution has a mass of 100 g.
- After measuring the mass of a precise volume of each of the standard solutions, calculate the density as:
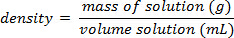
3. Data Analysis
- Plot the density values against the percentage by mass of the standard solutions and determine the slope of the line. This slope corresponds to the density vs. mass percent of the solution, fitting a linear equation, y = mx + b (Figure 1).
- Calculate the density of the unknown sample solution by obtaining the mass of the specific volume of solutions. Now that the slope of the line is known, determine the percent by mass of solute by solving for "x" using the measured density and slope from the calibration curve.
Sonuçlar
Using the example shown in Figure 1, a set of sodium chloride standards was prepared with mass percent compositions of 5.000%, 10.00%, 15.00%, 20.00%, and 25.00% of solute in solution. The measured densities were 1.025, 1.042, 1.060, 1.070, and 1.090 g/mL, respectively. After plotting these data, a linear trendline is applied, fitting the equation y = 3.446 x 10-3x + 1.0048, where y is the density and x is the mass percent composition.
Next, the volume of 10.00 g of solution with unknown percent composition is measured and determined to be 9.497 mL. By dividing the mass by the volume, the density is then calculated as 1.053 g/mL. Inserting the density value into the linear equation, the mass percent is determined as x:
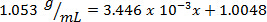

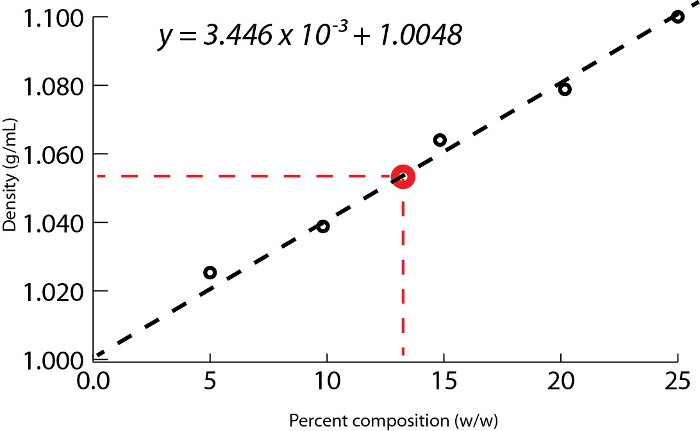
Figure 1. Aqueous sodium chloride solution density as a function of percent composition by mass.
Başvuru ve Özet
The percentage of sugar in soda, could easily be determinedusing the principle of mass percent composition. The procedure for doing this experiment would be to measure the mass and volume of degassed soda (no bubbles) and to calculate the solution's density. A calibration curve of density vs. percent by mass for several standard sucrose (sugar) solutionswould need to be created, and then that calibration could be used to solve for the percent of sucrose in the soda. One assumption is that sucrose is the major contributor to a change in density for soda, which is true for non-sugar-free soda.
Seawater is composed of several different salts dissolved in water; for example, NaCl, MgCl2, and NaBr2. While it would be difficult to determine mass percent using a calibration curve, evaporation provides a simple solution to the problem. By taking a known mass of seawater and evaporating it, the mass can be determined by the amount of solute remaining after the solvent is evaporated. Then, the mass of solute needs to be divided by the total mass of the original solution to calculate the mass percent of salts (dissolved solids) in seawater.
There are a variety of ionic compounds that have water molecules and are referred to as hydrates. The number of water molecules on a compound can be determined by percent composition. For example, cobalt (II) chloride is normally blue, but turns pink when it is hydrated. If 5.0 grams of pink cobalt (II) chloride hydrate is heated to the point it turns blue, then the final mass of the blue solid is recorded as 2.73. The difference, 2.27 g, corresponds to the mass of water that was on the 5 g of hydrated solid. The original sample was 45.4 % water by mass. By converting mass values to moles, it can be calculated that 0.02101 moles of cobalt chloride (129.93 g/mol) and 0.126 moles of water (18 g/mol) are present. Dividing by the smallest value, it can be determined that 6 moles of water are present for every one mole of cobalt chloride.
Atla...
Bu koleksiyondaki videolar:

Now Playing
Determining the Mass Percent Composition in an Aqueous Solution
General Chemistry
383.3K Görüntüleme Sayısı

Common Lab Glassware and Uses
General Chemistry
655.3K Görüntüleme Sayısı

Solutions and Concentrations
General Chemistry
273.7K Görüntüleme Sayısı

Determining the Density of a Solid and Liquid
General Chemistry
555.6K Görüntüleme Sayısı

Determining the Empirical Formula
General Chemistry
180.7K Görüntüleme Sayısı

Determining the Solubility Rules of Ionic Compounds
General Chemistry
141.3K Görüntüleme Sayısı

Using a pH Meter
General Chemistry
345.1K Görüntüleme Sayısı

Introduction to Titration
General Chemistry
423.9K Görüntüleme Sayısı

Ideal Gas Law
General Chemistry
78.3K Görüntüleme Sayısı

Spectrophotometric Determination of an Equilibrium Constant
General Chemistry
158.3K Görüntüleme Sayısı

Le Châtelier's Principle
General Chemistry
264.9K Görüntüleme Sayısı

Freezing-Point Depression to Determine an Unknown Compound
General Chemistry
160.6K Görüntüleme Sayısı

Determining Rate Laws and the Order of Reaction
General Chemistry
195.9K Görüntüleme Sayısı

Using Differential Scanning Calorimetry to Measure Changes in Enthalpy
General Chemistry
44.4K Görüntüleme Sayısı

Coordination Chemistry Complexes
General Chemistry
91.3K Görüntüleme Sayısı
JoVE Hakkında
Telif Hakkı © 2020 MyJove Corporation. Tüm hakları saklıdır