Method Article
Exploring the Arginine Methylome by Nuclear Magnetic Resonance Spectroscopy
* These authors contributed equally
In This Article
Summary
The present protocol describes the preparation and quantitative measurement of free and protein-bound arginine and methyl-arginines by 1H-NMR spectroscopy.
Abstract
Protein-bound arginine is commonly methylated in many proteins and regulates their function by altering the physicochemical properties, their interaction with other molecules, including other proteins or nucleic acids. This work presents an easily implementable protocol for quantifying arginine and its derivatives, including asymmetric and symmetric dimethylarginine (ADMA and SDMA, respectively) and monomethyl arginine (MMA). Following protein isolation from biological body fluids, tissues, or cell lysates, a simple method for homogenization, precipitation of proteins, and protein hydrolysis is described. Since the hydrolysates contain many other components, such as other amino acids, lipids, and nucleic acids, a purification step using solid-phase extraction (SPE) is essential. SPE can either be performed manually using centrifuges or a pipetting robot. The sensitivity for ADMA using the current protocol is about 100 nmol/L. The upper limit of detection for arginine is 3 mmol/L due to SPE saturation. In summary, this protocol describes a robust method, which spans from biological sample preparation to NMR-based detection, providing valuable hints and pitfalls for successful work when studying the arginine methylome.
Introduction
During the last two decades, methylation of arginine residues has been recognized as an essential posttranslational modification of proteins. It affects fundamental biological processes like regulation of transcription, signal transduction, and many more1. The main proteins involved in the regulation of arginine methylation are protein arginine methyltransferases (PRMTs)2. The main derivatives of arginine are ω-(NG,NG)-asymmetric dimethylarginine (ADMA), ω-(NG,N'G)-symmetric dimethylarginine (SDMA), and ω-NG-monomethylarginine (MMA)2.
PRMTs use S-adenosyl-l-methionine to transfer methyl groups to the terminal guanidino group (with two equivalent amino groups) of protein-bound arginine1. Two main enzymes can be distinguished: Both type I and type II enzymes catalyze the first methylation step to form MMA (which thereby loses its symmetry). Following this step, type I enzymes (e.g., PRMT1, 2, 3, 4, 6, 8) use MMA as the substrate to form ADMA, whereas type II enzymes (primarily PRMT5 and PRMT9) produce SDMA. PRMT1 was the first protein arginine methyltransferase to be isolated from mammalian cells3. Still, PRMTs have been evolutionarily conserved4 in other animals like non-mammalian vertebrates, invertebrate chordates, echinoderms, arthropods, and nematodes cnidarians5, plants6, and protozoa, including fungi like yeast7. In many cases, knockout of one of the PRMTs leads to loss of viability, revealing the essential role of methylated arginine species involved in fundamental cellular processes like transcription, translation, signal transduction, apoptosis, and liquid-liquid phase separation (meaning the formation of membrane-less organelles, e.g., nucleoli), which regularly involves arginine-rich domains8,9,10. In turn, this influences physiology and disease states, including cancer11,12,13, multiple myeloma14, cardiovascular diseases15, viral pathogenesis, spinal muscular atrophy16, diabetes mellitus17, and aging1. Increased ADMA levels in the bloodstream, e.g., derived from lung18 due to protein breakdown, are thought to be connected with endothelial dysfunction, chronic pulmonary disease19, and other syndromes of cardiovascular disease20. Overexpression of PRMTs has been found to accelerate tumorigenesis and is associated with poor prognosis21,22. Besides, ablation of PRMT6 and PRMT7 triggers a cellular senescence phenotype23. Significant decreased ADMA and PRMT1 have been found during the aging of WI-38 fibroblasts24.
The challenge is understanding how methylation acts in (patho)physiological processes is identifying and quantifying protein arginine methylation. Most of the current approaches use antibodies to detect methylated arginines. However, these antibodies are still context-specific and might fail to recognize different motifs of arginine methylated proteins25,26. In the described protocol, all of the arginine derivatives mentioned afore can be quantified reliably by nuclear magnetic resonance (NMR) spectroscopy, i.e., alone, in combination, or, as in most cases, within complex biological matrices like eukaryotic cells (e.g., from yeast, mouse, or human origin) and tissues27, as well as serum28. For proteins and those complex matrices, protein hydrolysis29 is a prerequisite to generate free (modified) amino acids, such as arginine, MMA, SDMA, and ADMA. Solid-phase extraction (SPE)30 enables the enrichment of the compounds of interest. Finally, 1H-NMR spectroscopy allows the parallel detection of arginine and all the major methyl derivatives of arginine. NMR spectroscopy comes with the advantage that it is genuinely quantitative, highly reproducible, and a robust technique31,32. The final NMR measurements can be done afterward when many samples have been collected and prepared. Finally, this protocol mainly focuses on sample preparation as this does not require an own NMR spectrometer. It can be performed in most biochemical laboratories. Still, some hints on which NMR spectroscopy measurements should be done are provided in this work.
Protocol
Yeast protein hydrolysates were used as samples for the Representative results of this work. The entire protocol is summarized in Figure 1.
1. Preparation of materials and reagents
- Methanol/water: prepare a mixture of two parts of 99% methanol (MeOH) and one part of H2O, designated as MeOH/H2O. Store pure MeOH and MeOH/H2O at -20 °C to minimize alcohol evaporation and increase sample stability.
- Hydrochloric acid (HCl): dilute 9 mol/L from concentrated HCl (12.0 mol/L or 37%) as well as 0.1 mol/L of HCl. The higher concentrated (9 mol/L) solution is used for protein hydrolysis, while the lower concentrated (0.1 mol/L) one is used for solid-phase extraction.
CAUTION: Work inside the fume hood. - Prepare a Replacement solution for SPE containing 10% of a saturated ammonia solution (stock: 30% w/w ammonia), 50% of methanol, and 40% of water (v/v).
- NMR Buffer: Dissolve 5.56 g of disodium hydrogen phosphate (Na2HPO4, 0.08 mol/L), 0.4 g of 3-(trimethylsilyl) propionic acid-2,2,3,3-d4 sodium salt (TSP, 5 mmol/L), 0.04 % (w/v) of sodium azide (NaN3) in 500 mL of deuterium dioxide (D2O) and adjust to pH 7.4 (using HCl or NaOH, respectively) (see Table of Materials). Check the purity of each new buffer batch by NMR spectroscopy.
NOTE: The amount of any contaminants (e.g., ethanol) should be as low as possible. - Fill pulping tubes with zirconium oxide beads (2 mL tubes and 1.4 mm beads are suitable for most applications, but different variants are available) (see Table of Materials). Fill roughly 10-20 beads by hand or buy pre-filled tubes, which are also available.
- Keep pipettes and the respective tips ready in the range of 10-1,000 µL.
NOTE: Highly pure water, designated as H2O, defined by a high resistance of ≥18.2 MΩ·cm-1, was used throughout the work. It corresponds to double distilled water.
2. Sample collection and storage
- Flash freeze the liquid samples (serum/plasma, cell culture supernatant, etc.) with liquid nitrogen and store them at -80 °C until usage.
- Prepare the solid materials as mentioned below.
- Collect the cells from cell cultures (3-5 million cells) either by collecting adherent cells, after washing with cold phosphate-buffered saline, scraping and centrifugation, or direct centrifugation of cells grown in suspension.
- Remove the cell supernatants (which might be collected for further analysis, either by consecutive direct measurement, see step 6, or after precipitation of soluble proteins, see step 3.1), and then flash freeze the pellets with liquid nitrogen and store at -80 °C. For further processing, see step 3.2.
- Store and collect the tissues as per the aim of the study. For very homogenous tissues, such as the liver or muscle, analyze any part of it. For example, in the case of a mouse brain, determine global arginine methylation of the entire brain or brain sections.
NOTE: The ideal weight of tissues to be processed is 30-60 mg. It is advisable to freeze and pulverize the entire brain and use aliquots thereof. Alternatively, specific brain parts (e.g., frontal, cerebellum, occipital regions, or one hemisphere, weighing ~250 mg) may be used.
3. Preliminary sample preparation
NOTE: Perform the work on ice, keeping MeOH cold. The samples must also be kept on ice to avoid protein degradation.
- For liquid samples, add 400 µL of ice-cold methanol to 200 µL of the sample. Continue with step 3.3.
NOTE: If less sample is available, adjust the volumes accordingly; NMR sensitivity is usually high enough for smaller sample amounts, but has to be tested individually. - In the case of solid materials, prepare enough 1.5 mL tubes for the lysates (use for centrifugation after homogenization).
- Carefully, but thoroughly, resuspend the cell pellets in 600 µL of MeOH/H2O and transfer the entire liquid phase into the pulping tubes.
- Place tissues into pulping tubes (you may directly weigh them into the tubes) and add 600 µL of MeOH/H2O (for 30-60 mg; adjust if weight differs substantially).
- Put the tubes into the tissue homogenizer (see Table of Materials) and either homogenize one time for 20 s with heavy shaking (for cell pellets, soft tissues) or two times for 20 s each (or longer if necessary) with an interval of 5 min in between (for stiff tissues).
- After homogenization, immediately put samples on the ice again and transfer the whole lysates into new 1.5 mL tubes.
- Store samples for at least 30 min (up to several days) at -20 °C before further processing.
- Centrifuge at 10,000 x g for 30 min at 4 °C. In the meantime, prepare enough 1.5 mL tubes to collect the supernatants.
NOTE: This supernatant (designated "(1)" in Figure 1) might be discarded, be lyophilized, and directly processed for NMR (step 6) or analyzed otherwise. - In this protocol, the MeOH precipitate (= pellet obtained after step 3.1 or 3.2.2, respectively) is used for further analysis containing, among other things, components such as nucleic acids and lipids, arginine-methylated proteins. Continue with protein hydrolysis or store the pellets at -20 °C for a few days.
4. Protein hydrolysis
- Add 500 µL of 9 mol/L of HCl to each sample. Then, truncate the caps of each tube with scissors and place them into the glass culture tubes (Figure 2A). Carefully, but tightly, close the red caps (thereby checking the sealing).
NOTE: Before use, always check each sealing (gray PTFE foil inside the red screw caps) for proper tightening and regularly clean them with water. - When finished, place the tubes into a beaker partly filled with sand (for better heat transfer) and hydrolyze samples for about 16 h at 110 °C in a drying chamber.
- Following hydrolysis, cool down the samples (~1 h).
- After that, lyophilize overnight using a centrifuge with a high vacuum (below 100 Pa).
- Dissolve the pellets - if completely dry (if not, continue lyophilization) - in 1 mL of 0.1 mol/L HCl and add 50 µL of chloroform (CHCl3) to each tube; thoroughly dissolve the pellet with a 1,000 µL pipette, thereby mixing the liquids, and then transfer the complete volume into a new tube.
CAUTION: Always work with small amounts to minimize extensive evaporation - Then, centrifuge at 8,000 x g for 10 min at room temperature.
- Carefully collect the upper phase of the biphasic liquid containing the water-soluble fraction (Figure 2B, the fraction with the lower density) with a pipette and fill it into new tubes. Use 1.5 mL tubes for manual SPE (section 5.2) or glass vials for robotic SPE (section 5.3 and Figure 2D). Avoid spilling over CHCl3 and its contents (Figure 2C).
5. Solid-phase extraction (SPE)
- Before the first usage, clean the SPE cartridges two times with 1 mL of the replacement solution (step 1.3) followed by centrifugation at 800 x g for 1 min at room temperature (placed into 15 mL tubes). They can be used 10-20 times each.
- Manual SPE
- Pre-condition the cartridges (see Table of Materials) in every run with 1 mL of pure methanol and 2 x 1 mL of PBS, each time followed by centrifugation at 800 x g for 1 min at room temperature (placed into 15 mL tubes).
- After that, load the samples (from step 4.7) onto the SPE cartridges and centrifuge them at 600 x g for 2 min at room temperature.
- Then, apply a washing cycle as mentioned below.
- Add three times 1 mL of H2O (each followed by centrifugation for 1 min at 800 x g at room temperature).
- Add five times 1 mL of 0.1 mol/L HCl (each followed by centrifugation for 1 min at 800 x g at room temperature).
- Add two times 1 mL of MeOH (each followed by centrifugation for 1 min at 800 x g at room temperature).
- Then, eluate arginine and its derivatives with 3 x 1 mL of replacement solution (followed by centrifugation 1 min at 800 x g at room temperature) into a single 15 mL tube.
NOTE: The replacement solution serves as eluent for arginine and derivatives and is also used for cleaning the cartridges (all substances are either washed out before elution or at the basic pH value of the replacement solution). Still, to avoid contamination over time, the re-usage should be limited (see step 5.1).
- Robotic SPE
NOTE: Here, a pipetting robot (the main part consisting of a pipetting needle, a washing station for the needle, a reagent supply, and positions for samples and eluates, see Table of Materials) is supplied with a cartridge holder for the SPE columns. For other instruments, make sure to be fully equipped with all things listed.- Fill the supernatant (from step 4.7) directly into glass vials with PTFE sealing (Figure 2D), prepare enough reagents (100 mL of each, i.e., replacement solution, 99% of MeOH, PBS, H2O, and 0.1 of mol/L HCl), 5 mL tubes for elution and washing solution for the needle of the robot (usually H2O).
- Use an application or method in the software of the robot based precisely on the steps described for manual SPE (step 5.2).
NOTE: The details may vary a lot among instrument suppliers, so refer to the software manual of the respective robot for programming. A pipetting robot usually works by applying air pressure onto the cartridges instead of centrifugation, which is the main difference. If the protocol is established and run for the first time, include controls (e.g., L-arginine of known concentration) to verify an optimal workflow.
6. Final preparation for NMR
- Lyophilize samples overnight to complete dryness to get rid of ammonia and H2O.
- Dissolve each sample in 500 µL of NMR buffer (step 1.4) and transfer to NMR tubes. Importantly, the volume must be the same in all tubes, and samples must be homogenous (free of residues and lipids, which tend to aggregate).
NOTE: The details of NMR spectroscopy go beyond this method review and are described in more detail elsewhere27. Here, only the main requirements and steps are explained. The quantification of arginine and its metabolites are performed on a 600 MHz NMR spectrometer (see Table of Materials). In principle, any other NMR spectrometer/NMR field strengths can be used, provided that the NMR signals of arginine and methylated arginines can be detected with sufficient sensitivity and without signal overlap. Any probe head with z-axis gradients able to record 1H-spectra can be used, provided that the pulse sequences are set up accordingly. - Record a 1D spectrum using the CPMG (Carr-Purcell-Meiboom-Gill) pulse sequence33,34 (cpmgpr1d, 512 scans, size of fid 73728, 11904.76 Hz spectral width on a 600 MHz NMR, recycle delay 4 s) with water signal suppression using presaturation.
- In these experiments, remove the 1H scalar couplings by virtual decoupling, reducing signal overlap. Record additional for specific questions and/or complex samples or matrices, e.g., 1H-13C-heteronuclear single quantum coherence (HSQC) spectra27.
NOTE: For more complex matrices in which 1H NMR signals of methylated arginines could be partially overlapped with 1H NMR signals of other metabolites (e.g., lysine), 1H homo-nuclear J-resolved spectra (JRES)35 are required. - Perform absolute quantification by integrating the respective peak intensities of standards of a known concentration, e.g., 100 µmol/arginine.
NOTE: Routinely, ADMA, SDMA, and MMA (see Table of Materials) are reported as a ratio relative to arginine. This has a significant advantage that no separate normalization (e.g., cell number, tissue mass, protein concentrations) needs to be performed. In that case, arginine serves as an internal standard, and relative quantification is sufficient to gain biologically relevant information. - For differentiation of SDMA and MMA, lyophilize the samples again be to get rid of D2O (if they have been resuspended in NMR buffer before), which is then replaced by deuterated dimethyl sulfoxide (d6-DMSO), enabling the resolution of methyl resonances27. Therefore, aspirate the samples from the NMR tubes with Pasteur pipettes, lyophilize, and dissolve them in 500 µL of d6-DMSO.
Results
Routinely, 1H 1D projections of 2D J-resolved (JRES), virtually decoupled NMR spectra are used for peak assignments and quantifications in our laboratory36. Figure 3 shows representative JRES spectra of yeast protein hydrolysates purified using the present SPE protocol. Though at very different concentrations, both substances can be separated and quantified in a cellular matrix. Based on the number of protons of the specific methyl (-CH3) or methylene (-CH2-) group at the respective chemical shift, 1H-NMR spectroscopy allows precise quantification. As pointed out in the protocol (step 0), the relative quantification of a methyl-arginine derivate compared to arginine is mainly adequate to observe biological processes, e.g., changes of arginine methylation as a response to modulations, such as knock-down/overexpression of genes, changes in nutrients, or treatment with inhibitors.
As shown previously27, L-arginine and ADMA can be well discriminated by their different characteristic chemical shifts at 3.25 and 3.02 ppm, respectively. SDMA and MMA methyl protons reveal overlapping peaks in D2O (the NMR buffer described in step 1.4) at a chemical shift of 2.85 ppm. If a peak is present at the respective position, one might separate SDMA and MMA by dissolving the samples in d6-DMSO as described in step 6.6 of the protocol. Using this approach, the 1H NMR chemical shifts of SDMA and MMA can be separated and quantified with characteristic chemical shifts at 2.76 ppm (SDMA) and 2.74 ppm (MMA)27. Figure 3B shows representative data of SDMA and MMA (both 100 µmol/L) in D2O and d6-DMSO, respectively. Corresponding data of SMDA and MMA detected in various cells and tissues has been reported recently27. As described in steps 6.4-6.5 of the protocol, reference standards of known concentrations can be used for quantification. All reference standards mentioned in the text are commercially available.

Figure 1: Overview of sample preparation. Scheme revealing the significant steps from sample collection to NMR measurement. The black boxes refer to the numbering in the protocol sections. Additionally, the approximate amount of days (varying according to the number of samples) is shown. Inhomogeneous liquid samples might be centrifuged before adding MeOH (step 3.1) to remove the residues (not shown). The supernatant after initial centrifugation (1) containing, e.g., intracellular metabolites or non-protein-bound arginine metabolites may be stored at -20 °C for further analysis. Routinely, the MeOH pellets (2) are further analyzed. Abbreviations: CHCl3, chloroform; HCl, hydrochloric acid; MeOH, methanol; NMR, nuclear magnetic resonance; o/n, overnight; SN, supernatant; SPE, solid-phase extraction. Please click here to view a larger version of this figure.

Figure 2: Handling of protein hydrolysates and preparation for solid-phase extraction. (A) Beaker filled with sand, glass tubes, screw caps with gray polytetrafluoroethylene (PTFE) sealing foil, and samples in 1.5 mL tubes after cutting off the caps. (B) Tube after centrifugation (step 4.6) and (C) after removal of the aqueous supernatant (step 4.7): some residual water (~50 µL) may remain. The black residue contains insoluble, charcoaled organic remnants. Still, some samples may contain colored substances (D), which do not interfere with L-arginine measurement. This is partly due to solid-phase extraction (SPE) afterward, either using consecutive centrifugation steps (E) or a pipetting robot (F). Please click here to view a larger version of this figure.
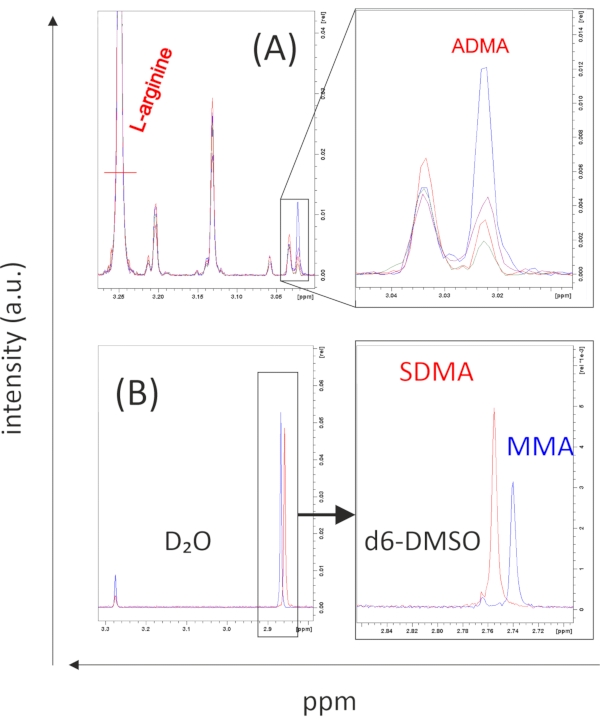
Figure 3: Typical NMR spectra of L-Arginine and its methyl-derivatives. 1H 1D projected J-resolved NMR spectra could readily distinguish the δ-(CH2)-protons of L-arginine (3.25 ppm) and the ω-N-CH3-protons of asymmetric dimethylarginine (ADMA, at 3.02 ppm). Samples from yeast pellets (A) revealing varying amounts of ADMA can be quantified by integrating signal intensities even in the presence of high amounts of arginine (note that the full arginine peak is not shown). (B) Since ω-(NG,N'G)-symmetric dimethylarginine (SDMA) and ω-NG-monomethyl arginine (MMA) can hardly be discriminated in deuterium oxide buffer (D2O), samples must be dissolved in deuterated dimethyl sulfoxide (d6-DMSO), where the peaks (in this case 100 µmol/L of each substance) can easily be distinguished from each other (peaks at 2.75 and 2.76 ppm, respectively) and be quantified. All spectrograms were directly exported from the controlling software of the NMR spectrometer. The software reveals the primary results, which need to be checked before a more detailed analysis, including statistics of many samples, is done. Please click here to view a larger version of this figure.
Discussion
In the following section, the primary focus lies on the method itself; the biological implications of arginine methylation are described in the Introduction section.
Firstly, tissues of different stiffness might need adjustment of sample lysis: cells from cell culture (including bacteria, yeast, etc.) and tissues like brain, young liver, smooth muscle, etc., can quickly be homogenized. For tissues of high stiffness (including liver of elderly subjects, arteries, bones, etc.), the homogenization needs to be done twice (see step 3.2.3). Nevertheless, this process should not be continued more often, even if some insoluble remnants are left. Instead, the samples should be centrifuged for 10 min at 10,000 x g and the supernatants collected for further analysis (to minimize sample degradation).
At each step where samples are frozen (initially, after adding MeOH, steps 3.3, 3.4, or 3.5, respectively) or following lyophilization (steps 4.4 and 6.1), samples can be stored for a few days before further processing. Additionally, the supernatant from step 3.4 (following homogenization; designated as "(1)" in Figure 1) may also be stored for further analysis, e.g., for measurement of metabolites other than methyl-arginines36,37,38.
On the one hand, critical steps involve sample storage, as degradation of proteins may alter the outcome. If protein degradation occurs before MeOH precipitation, arginine and its methyl derivatives may be underestimated. Therefore, storing samples is essential, e.g., before and after cell or tissue lysis at -20 °C, or, whenever possible, at -80 °C. Before or after homogenization (step 3.2), samples should be kept on ice.
On the other hand, methanol pre-treatment of samples to precipitate proteins does not alter the methylation pattern of arginine. This was tested with lysozyme and Escherichia coli protein extracts (which do not contain methylated arginine)27. However, earlier reports have shown that MeOH may methylate the terminal carboxyl groups of glutamate and aspartate39.
For protein hydrolysis, ensure that the sealings of the screw caps are intact (step 4.1). If not, evaporating HCl might affect the drying chamber. The concentration of the analytes might not be critically changed, though, because they do not evaporate, and residual HCl is evaporated later on by lyophilization. Lower concentrations of HCl, e.g., 6 mol/L, can be used but might require a longer hydrolysis time. Following hydrolysis, samples can be treated at room temperature. Proper removal of lipids is essential, though. Lipids may form biphasic mixtures in water (or D2O, respectively) later during NMR measurement. Those inhomogeneities lead to NMR peak broadening and potentially affect the quality and sensitivity of NMR experiments.
If no pipetting robot is available for SPE, it is worth the investment (it can also be used for other purposes). The samples on the SPE pipetting robot are processed sequentially, and in the present setup, one sample takes ~40 min for a complete run. Due to limitations of reagent capacities (i.e., 100 mL), only 19 samples can be processed in one go (taking ~13 h overall). Still, the hands-on time is much less than for the manual SPE procedure and requires less material. During this period, samples can be stored within the robot at room temperature until lyophilization. Equivalent products might replace the used SPE matrices and cartridges. Nevertheless, it is recommended to evaluate the SPE protocol and adjust it if necessary.
The detection limit for ADMA is ~100 nmol/L as evaluated previously by serial dilutions and using the reported NMR measurement times. On the other hand, high arginine concentrations may also overestimate the ratio of ADMA to arginine (which usually is in the range of 5%-15%). This is because above 3 mmol/L of arginine; the SPE column binding capacity may reach saturation27. For checking sensitivity and specificity of the entire workflow in case of unexpected results (e.g., no peaks or overlapping peaks in the 1H-NMR spectra at the reported chemical shifts), standards of ADMA, SDMA, and MMA are available from commercial suppliers (see Table of Materials). If any laboratory has no NMR spectrometer, all steps, including final lyophilization (step 6.1), could be performed in the respective laboratory, and samples might be shipped to an NMR facility for analysis.
Compared to other methods, such as mass spectrometry, no labeling of the analytes is needed, and it can be performed without internal standards for quantification. On the other hand, the sensitivity of NMR is slightly lower, which in most biological cases though plays no role, like arginine and its methyl derivatives can easily be detected in all kinds of eukaryotic cells or tissues. As mentioned before, 3 million cells are enough to quantify protein-bound ADMA - several cells that can readily be achieved with most cell lines. In the case of slowly growing, unmodified primary cells from human origin like adult stem cells40, cells might be grown for a more extended period or pooled from separate experiments. The same is true for tissue samples. ~30 mg of tissue, corresponding to many organs, e.g., of mice (in part or all, e.g., small muscles) or biopsy specimen, is sufficient to prepare enough lysate and protein hydrolysate for NMR measurement27. Other methods for measuring protein arginine methylation include using enzyme-coupled luminescence assays41 or 3H-labeled S-adenosyl-methionine (SAM)42. The sensitivity is high and might be increased by using 14C-labeled SAM but at the expense of increased costs. Additionally, the use of radioactive substances (including the fluids for scintillation counting) causes special waste management, which is not necessary using the current protocol.
A complementary method to the present protocol, using 2D heteronuclear NMR spectroscopy, can reveal site-specific methylation patterns within isolated proteins. It has recently been published, including a detailed description of the detection of methyl-arginines by NMR spectroscopy in general43. The current protocol does not provide information about site-specific arginine methylation within proteins. Still, global methylation is changed on the cellular and physiological level, including cell growth and differentiation, aging, cancer1,2,12, cardiovascular20, and neurodegenerative diseases8,44. The details about the exact mechanisms involved are still not fully understood, and possible drug candidates interfering with protein-arginine methylation11 both in vitro and in vivo need further exploration1. Studying the kinetics of methylation (which our group has recently shown)27 is essential for gaining insights into arginine modification and protein breakdown mechanisms. Global arginine methylation measurements and kinetic experiments (samples can be frozen at any time) ranging from single proteins to samples from whole organisms can be accomplished straightforward using this protocol. It, therefore, provides a robust method, which can easily be adapted for future research.
Disclosures
The authors declare no competing interests.
Acknowledgements
The work was supported by Austrian Science Fund (FWF) grants P28854, I3792, doc.funds BioMolStruct DOC 130, DK-MCD W1226 BioTechMed-Graz (Flagship project DYNIMO), Austrian Research Promotion Agency (FFG) grants 864690 and 870454, the Integrative Metabolism Research Center Graz; Austrian Infrastructure Program 2016/2017, the Styrian Government (Zukunftsfonds) and Startup Fund for High-level Talents of Fujian Medical University (XRCZX2021020). We thank the Center of Medical Research for access to cell culture facilities. F.Z. was trained within the frame of the PhD program Molecular Medicine, Medical University of Graz. Q.Z. was trained within the frame of the PhD program Metabolic and Cardiovascular Diseases, Medical University of Graz.
Materials
| Name | Company | Catalog Number | Comments |
| 15 mL tubes | Greiner Bio One | 188271 | |
| 3-(trimethylsilyl) propionic acid-2,2,3,3-d4 sodium salt (TSP) | Alfa Aesar | A1448 | |
| 5 mL tubes, round bottom | Greiner Bio One | 115101 | |
| Ammonia Solution 32% | Roth | A990.1 | |
| Bruker 600 MHz NMR spectrometer, equipped with a TXI probe head | Bruker | - | |
| Centrifuge, refrigerated, e.g. 5430 R | Eppendorf | 5428000010 | |
| Chloroform ≥99% p.a. | Roth | 3313.1 | |
| Cryocool | Thermo Scientific | SCC1 | heat transfer fluid for SpeedVac System |
| Deuterium Oxide (D2O) | Cambridge Isotope Laboratories | DLM-10-PK | |
| Dimethyl sulfoxide-d6 (d6-DMSO) | Cambridge Isotope Laboratories | DLM-6-1000 | |
| Drying Chamber | Binder | 9090-0018 | |
| DURAN culture tubes, 13 x 100mm, GL 14, 9 mL | VWR International | 212-0375 | |
| Edwards Deep vacuum oil pump RV5 | Thermo Scientific | 16234611 | part of the SpeedVac System |
| Eppendorf 1.5 mL tubes | Greiner Bio One | 616201 | |
| Gilson pipetting robot GX-241 Aspec | Gilson Inc. | 26150008 | |
| L-arginine | AppliChem | A3675 | |
| Methanol ≥99% | Roth | 8388.4 | |
| Milli-Q water aparatus | Millipore | ZIQ7000T0 | |
| Oasis MCX 1cc/30 mg, 1 mL cartridges | Waters | 186000252 | https://www.waters.com/waters/en_US/Waters-Oasis-Sample-Extraction-SPE-Products/ |
| Phosphate Buffered Saline (PBS) | Lonza | LONBE17-512F | |
| Precellys 24 tissue homogenizer | Bertin Instruments | P000669-PR240-A | https://www.bertin-instruments.com/product/sample-preparation-homogenizers/precellys24-tissue-homogenizer/ |
| Precellys tubes (pulping tubes) | VWR International | 432-0351 | |
| Precellyse 1.4 mm zirconium oxide beads | VWR International | 432-0356 | |
| Reacti-Therm/ReactiVap Heating, Stirring, and Evaporation Modules | Thermo Scientific | TS-18820 | https://www.thermofisher.com/order/catalog/product/TS-18820 |
| Rotor for 1.5 mL tubes, FA-45-30-11 | Eppendorf | 5427753001 | |
| Savant Refrigerated Cooling Trap | Thermo Scientific | 15996161 | part of the SpeedVac System |
| Savant SpeedVac vacuum concentrator SPD210 | Thermo Scientific | 15906181 | part of the SpeedVac System; equipped with rotor for 1.5 ml tubes |
| Screw caps for glas vials with PTFE sealing, DN9 | Dr. R. Forche Chromatographie | CT11B3011 | |
| Seasand | Roth | 8441.3 | |
| Short thread glas vials 1.5 mL, ND9 | Dr. R. Forche Chromatographie | VT1100309 | |
| Sodium azide (NaN3) | Roth | K305.1 | |
| Sodium hydroxide (NaOH) | VWR | BDH7363-4 | |
| Sodium phosphate dibasic (Na2HPO4) | VWR | 80731-078 | |
| TopSpin 4.0 (Software) | Bruker | - | https://www.bruker.com |
| ω-NG-asymmetric dimethylarginine (ADMA) | Santa Cruz Biotechnology | sc-208093 | |
| ω-NG-monomethylarginine (MMA) | Santa Cruz Biotechnology | sc-200739A | |
| ω-NG-NG'-symmetric dimethylarginine (SDMA) | Santa Cruz Biotechnology | sc-202235A |
References
- Guccione, E., Richard, S. The regulation, functions and clinical relevance of arginine methylation. Nature Reviews Molecular Cell Biology. 20 (10), 642-657 (2019).
- Bedford, M. T., Clarke, S. G. Protein arginine methylation in mammals: who, what, and why. Molecular Cell. 33 (1), 1-13 (2009).
- Lin, W. J., Gary, J. D., Yang, M. C., Clarke, S., Herschman, H. R. The mammalian immediate-early TIS21 protein and the leukemia-associated BTG1 protein interact with a protein-arginine N-methyltransferase. Journal of Biological Chemistry. 271 (25), 15034-15044 (1996).
- Bachand, F. Protein arginine methyltransferases: from unicellular eukaryotes to humans. Eukaryotic Cell. 6 (6), 889-898 (2007).
- Wang, Y. C., Li, C. Evolutionarily conserved protein arginine methyltransferases in non-mammalian animal systems. FEBS Journal. 279 (6), 932-945 (2012).
- Ahmad, A., Cao, X. Plant PRMTs broaden the scope of arginine methylation. Journal of Genetics and Genomics. 39 (5), 195-208 (2012).
- Fisk, J. C., Read, L. K. Protein arginine methylation in parasitic protozoa. Eukaryotic Cell. 10 (8), 1013-1022 (2011).
- Hofweber, M., et al. Phase Separation of FUS Is Suppressed by Its Nuclear Import Receptor and Arginine Methylation. Cell. 173 (3), 706-719 (2018).
- Chong, P. A., Vernon, R. M., Forman-Kay, J. D. RGG/RG Motif Regions in RNA Binding and Phase Separation. Journal of Molecular Biology. 430 (23), 4650-4665 (2018).
- Nott, T. J., et al. Phase transition of a disordered nuage protein generates environmentally responsive membraneless organelles. Molecular Cell. 57 (5), 936-947 (2015).
- Fong, J. Y., et al. Therapeutic targeting of RNA splicing catalysis through inhibition of protein arginine methylation. Cancer Cell. 36 (2), 194-209 (2019).
- Yang, Y., Bedford, M. T. Protein arginine methyltransferases and cancer. Nature Reviews Cancer. 13 (1), 37-50 (2013).
- Wang, S. M., Dowhan, D. H., Muscat, G. E. O. Epigenetic arginine methylation in breast cancer: emerging therapeutic strategies. Journal of Molecular Endocrinology. 62 (3), 223-237 (2019).
- Gulla, A., et al. Protein arginine methyltransferase 5 has prognostic relevance and is a druggable target in multiple myeloma. Leukemia. 32 (4), 996-1002 (2018).
- Wang, Z., Tang, W. H., Cho, L., Brennan, D. M., Hazen, S. L. Targeted metabolomic evaluation of arginine methylation and cardiovascular risks: potential mechanisms beyond nitric oxide synthase inhibition. Arteriosclerosis, Thrombosis, and Vascular Biology. 29 (9), 1383-1391 (2009).
- Friesen, W. J., Massenet, S., Paushkin, S., Wyce, A., Dreyfuss, G. SMN, the product of the spinal muscular atrophy gene, binds preferentially to dimethylarginine-containing protein targets. Molecular Cell. 7 (5), 1111-1117 (2001).
- Lee, J. H., Park, G. H., Lee, Y. K., Park, J. H. Changes in the arginine methylation of organ proteins during the development of diabetes mellitus. Diabetes Research and Clinical Practice. 94 (1), 111-118 (2011).
- Bulau, P., et al. Analysis of methylarginine metabolism in the cardiovascular system identifies the lung as a major source of ADMA. American Journal of Physiology: Lung Cellular and Molecular Physiology. 292 (1), 18-24 (2007).
- Zakrzewicz, D., Eickelberg, O. From arginine methylation to ADMA: a novel mechanism with therapeutic potential in chronic lung diseases. BMC Pulmonary Medicine. 9, 5 (2009).
- Fulton, M. D., Brown, T., Zheng, Y. G. The biological axis of protein arginine methylation and asymmetric dimethylarginine. International Journal of Molecular Sciences. 20 (13), (2019).
- Aliferis, K. A., Chrysayi-Tokousbalides, M. Metabolomics in pesticide research and development: review and future perspectives. Metabolomics. 7 (1), 35-53 (2011).
- Chiang, K., et al. PRMT5 Is a Critical Regulator of Breast Cancer Stem Cell Function via Histone Methylation and FOXP1 Expression. Cell Reports. 21 (12), 3498-3513 (2017).
- Blanc, R. S., Vogel, G., Chen, T., Crist, C., Richard, S. PRMT7 preserves satellite cell regenerative capacity. Cell Reports. 14 (6), 1528-1539 (2016).
- Lim, Y., Lee, E., Lee, J., Oh, S., Kim, S. Down-regulation of asymmetric arginine methylation during replicative and H2O2-induced premature senescence in WI-38 human diploid fibroblasts. Journal of Biochemistry. 144 (4), 523-529 (2008).
- Bhatter, N., et al. Arginine methylation augments Sbp1 function in translation repression and decapping. FEBS Journal. 286 (23), 4693-4708 (2019).
- Lee, Y. H., Stallcup, M. R. Minireview: protein arginine methylation of nonhistone proteins in transcriptional regulation. Molecular Endocrinology. 23 (4), 425-433 (2009).
- Zhang, F., et al. Global analysis of protein arginine methylation. Cell Reports Methods. 1 (2), (2021).
- Zinellu, A., Sotgia, S., Scanu, B., Deiana, L., Carru, C. Determination of protein-incorporated methylated arginine reference values in healthy subjects whole blood and evaluation of factors affecting protein methylation. Clinical Biochemistry. 41 (14-15), 1218-1223 (2008).
- Weiss, M., Manneberg, M., Juranville, J. F., Lahm, H. W., Fountoulakis, M. Effect of the hydrolysis method on the determination of the amino acid composition of proteins. Journal of Chromatography A. 795 (2), 263-275 (1998).
- Davids, M., et al. Simultaneous determination of asymmetric and symmetric dimethylarginine, L-monomethylarginine, L-arginine, and L-homoarginine in biological samples using stable isotope dilution liquid chromatography tandem mass spectrometry. Journal of Chromatography B: Analytical Technologies in the Biomedical and Life Sciences. 900, 38-47 (2012).
- Stryeck, S., Birner-Gruenberger, R., Madl, T. Integrative metabolomics as emerging tool to study autophagy regulation. Microbial Cell. 4 (8), 240-258 (2017).
- Vignoli, A., et al. High-throughput metabolomics by 1D NMR. Angewandte Chemie International Edition. 58 (4), 968-994 (2019).
- Carr, H. Y., Purcell, E. M. Effects of diffusion on free precession in nuclear magnetic resonance experiments. Physical Review. 94 (3), 630-638 (1954).
- Meiboom, S., Gill, D. Modified spin-echo method for measuring nuclear relaxation times. Review of Scientific Instruments. 29 (8), 688-691 (1958).
- Nagayama, K., Wuthrich, K., Bachmann, P., Ernst, R. R. Two-dimensional J-resolved 1H n.m.r. spectroscopy for studies of biological macromolecules. Biochemical and Biophysical Research Communications. 78 (1), 99-105 (1977).
- Stryeck, S., et al. Serum concentrations of Citrate, Tyrosine, 2- and 3- Hydroxybutyrate are associated with increased 3-month mortality in acute heart failure patients. Scientific Reports. 9 (1), 6743 (2019).
- Zhang, F., et al. Tissue-specific landscape of metabolic dysregulation during ageing. Biomolecules. 11 (2), (2021).
- Zhang, F., et al. Growing human hepatocellular tumors undergo a global metabolic reprogramming. Cancers. 13 (8), (2021).
- Pahlich, S., Zakaryan, R. P., Gehring, H. Protein arginine methylation: Cellular functions and methods of analysis. Biochimica et Biophysica Acta. 1764 (12), 1890-1903 (2006).
- Habisch, H. J., et al. Neuroectodermally converted human mesenchymal stromal cells provide cytoprotective effects on neural stem cells and inhibit their glial differentiation. Cytotherapy. 12 (4), 491-504 (2010).
- Ibanez, G., McBean, J. L., Astudillo, Y. M., Luo, M. An enzyme-coupled ultrasensitive luminescence assay for protein methyltransferases. Analytical Biochemistry. 401 (2), 203-210 (2010).
- Hevel, J. M., Price, O. M. Rapid and direct measurement of methyltransferase activity in about 30min. Methods. 175, 3-9 (2020).
- Altincekic, N., et al. Site-specific detection of arginine methylation in highly repetitive protein motifs of low sequence complexity by NMR. Journal of the American Chemical Society. 142 (16), 7647-7654 (2020).
- Kaneb, H. M., Dion, P. A., Rouleau, G. A. The FUS about arginine methylation in ALS and FTLD. The EMBO Journal. 31 (22), 4249-4251 (2012).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved