Method Article
Creating Matched In vivo/In vitro Patient-Derived Model Pairs of PDX and PDX-Derived Organoids for Cancer Pharmacology Research
In This Article
Summary
A method is described to create organoids using patient-derived xenografts (PDX) for in vitro screening, resulting in matched pairs of in vivo/in vitro models. PDX tumors were harvested/processed into small pieces mechanically or enzymatically, followed by the Clevers’ method to grow tumor organoids that were passaged, cryopreserved and characterized against the original PDX.
Abstract
Patient-derived tumor xenografts (PDXs) are considered the most predictive preclinical models, largely believed to be driven by cancer stem cells (CSC) for conventional cancer drug evaluation. A large library of PDXs is reflective of the diversity of patient populations and thus enables population based preclinical trials (“Phase II-like mouse clinical trials”); however, PDX have practical limitations of low throughput, high costs and long duration. Tumor organoids, also being patient-derived CSC-driven models, can be considered as the in vitro equivalent of PDX, overcoming certain PDX limitations for dealing with large libraries of organoids or compounds. This study describes a method to create PDX-derived organoids (PDXO), thus resulting in paired models for in vitro and in vivo pharmacology research. Subcutaneously-transplanted PDX-CR2110 tumors were collected from tumor-bearing mice when the tumors reached 200-800 mm3, per an approved autopsy procedure, followed by removal of the adjacent non-tumor tissues and dissociation into small tumor fragments. The small tumor fragments were washed and passed through a 100 µm cell strainer to remove the debris. Cell clusters were collected and suspended in basement membrane extract (BME) solution and plated in a 6-well plate as a solid droplet with surrounding liquid media for growth in a CO2 incubator. Organoid growth was monitored twice weekly under light microscopy and recorded by photography, followed by liquid medium change 2 or 3 times a week. The grown organoids were further passaged (7 days later) at a 1:2 ratio by disrupting the BME embedded organoids using mechanical shearing, aided by addition of trypsin and the addition of 10 µM Y-27632. Organoids were cryopreserved in cryo-tubes for long-term storage, after release from BME by centrifugation, and also sampled (e.g., DNA, RNA and FFPE block) for further characterization.
Introduction
Cancers are a collection of diverse genetic and immunological disorders. Successful development of effective treatments is highly dependent on experimental models that effectively predict clinical outcomes. Large libraries of well-characterized patient-derived xenografts (PDXs) have long been viewed as the translational in vivo system of choice to test chemo- and/or targeted therapies due to their ability to recapitulate patient tumor characteristics, heterogeneity and patient drug response1, thus enabling Phase II-like mouse clinical trials to improve clinical success2,3. PDXs are generally considered as cancer stem cell diseases, featuring genetic stability, in contrast to cell line derived xenografts2. Over the last few decades, large collections of PDXs have been created throughout the world, becoming the workhorse of cancer drug development today. Although widely used and with great translational value, these animal models are intrinsically costly, time consuming and low throughput, therefore inadequate for large scale screening. PDX are also undesirable for immuno-oncology (IO) testing due to an immune-compromised nature4. It is thus impractical to take full advantage of the available large library of PDXs.
Recent discoveries, pioneered by the Hans Clevers’ laboratory5, have led to the establishment of in vitro cultures of organoids generated from adult stem cells in most human organs of epithelial origin5. These protocols have been further refined to allow the growth of organoids from assumed CSCs in human carcinomas of various indications6,7. These patient-derived organoids (PDOs) are genomically-stable8,9 and have been shown to be highly predictive of clinical treatment outcomes10,11,12. In addition, the in vitro nature of PDOs enables high-throughput screening (HTS)13, thus potentially offering an advantage over in vivo models and leveraging large organoid libraries as a surrogate of the patient population. PDOs are poised to become an important discovery and translational platform, overcoming the many limitations of PDXs described above.
Both PDO and PDX are patient-derived and CSC-driven models, with the ability to evaluate therapeutics in the context of either personalized treatment or clinical trial format. Existing large libraries of PDXs, like the proprietary collection of >3000 PDXs14,15,16,17, are therefore suitable for the rapid generation of libraries of tumor organoids (PDX-derived organoids, or PDXO), resulting in a matched library of paired PDX and PDXO models. This report describes the procedure to create and characterize colorectal cancer PDXO-CR2110 in relation to its parental PDX-CR2110 model16.
Protocol
All the protocols and amendment(s) or procedures involving the care and use of animals were reviewed and approved by the Crown Bioscience Institutional Animal Care and Use Committee (IACUC) prior to conducting the studies. The care and use of animals was conducted in accordance with AAALAC (Association for Assessment and Accreditation of Laboratory Animal Care) International guidelines as reported in the Guide for the Care and Use of Laboratory Animals, National Research Council (2011). All animal experimental procedures were under sterile conditions at SPF (specific pathogen-free) facilities and conducted in strict accordance with the Guide for the Care and Use of Laboratory Animals from different government institutions (e.g., The National Institutes of Health). The protocols were approved by the Committee on the Ethics of Animal Experiments at the facility institution (e.g., institutional IACUC Committee).
1. Preparation for tumor transplantation
- Animal housing
- House Balb/c nude mice (n=5) in individual ventilated cages, at 20-26 °C, 30-70% humidity, and a lighting cycle of 12-h light/12-h dark, with corn cob bedding changed weekly, and irradiation sterilized dry granule food plus sterile drinking water ad libitum.
- Donor tumor fragment preparation
- Closely monitor tumor-bearing donor mice for body weight (BW, via weighing balance), and tumor volume (TV, by caliper measurement).
- When TV reaches 800-1000 mm3, euthanize the tumor-bearing animals in a biohazard hood.
- Place mice in a euthanasia chamber with a lid that delivers CO2 gas into the chamber. Discharge gas into the chamber at a flow rate that produces rapid unconsciousness with minimal distress to the animal. The optimum flow rate for CO2 is around 2-2.5 Lpm.
- Ensure euthanasia by observing that the animals do not regain consciousness.
- After apparent clinical death, maintain gas flow for >1 min to minimize the possibility that an animal may recover.
- Sterilize the skin around the tumor using iodophor swabs. Collect tumors by removing adjacent non-tumor tissues and placing in a Petri dish containing 20 mL of PBS, pre-chilled (4 °C) prior to euthanasia.
- Wash the tumors with PBS in another Petri dish to remove blood components followed by cutting in half and removing any extra skin, blood vessels, calcification and/or necrosis.
- Place only intact tumor pieces into a sterile 50 mL centrifuge tube with 20 mL of PBS, prior to transporting to a separate animal room for transplantation.
2. Subcutaneous tumor growth
- Subcutaneous inoculation of tumor piece into immunocompromised mice
- Cut the PDX tumor into 2 mm pieces with a scalpel, and place each into trocars for subcutaneous (SC) implantation.
- Anesthetize the recipient animals with 5% isoflurane (maintained by a nose cone at 1%). The animals relax, losing their righting reflex and eventually becoming immobile and unresponsive to pain. Fix the anesthetized mice onto an experiment board in the right lateral position and sterilize with iodophor swabs, particularly the areas surrounding the site of tumor inoculation.
- On left flank skin just cranial to the hip, make a 0.5 cm incision with a scalpel and create a tunnel under the skin towards the forelimb, 2-3 cm, using blunt forceps.
- Aseptically transfer one cube of tumor fragment per inoculation site from the medium and place deep inside the subcutaneous tunnel. Visually confirm position of the fragment prior to closure of wound with wound clips.
- Monitor the tumor implanted mice (5) until they become conscious to maintain sternal recumbence, and then return them to their cage only after their full recovery from anesthesia.
- Tumor-bearing mice health monitoring
- Check water and food consumption daily and record body weight weekly.
- Check the mice during routine monitoring. Record any effects on mobility, breathing, grooming and general appearance, food and water consumption, BW gain/loss, ascites, etc.
- Measure TV twice weekly using calipers and express in mm3 per: TV = 0.5 a × b2, where a and b are the length and width of the tumor, respectively.
- Sacrifice animals to collect samples when any of the following signs appear: BW loss >20%; impaired mobility (not able to eat or drink); unable to move normally due to significant ascites or enlarged abdomen; effort in respiration; death.
3. Necropsy and tumor harvest
- When tumor volume reaches 200-800 mm3, euthanize mice per approved protocols (see step 1.2.2).
- Collect tumor tissues by removing adjacent non-tumor tissues, followed by placing the tissue in a 50 mL plastic tube with AD+++ (on ice) before dissociation.
4. Preparation for PDX-derived organoid culture
NOTE: All the following steps were performed inside a biosafety cabinet per tissue culture standard guidelines. Keep pre-warmed stocks of 96-, 24-, and 6-well plates in a 37 °C incubator before use.
- Reagent preparations
- Prepare basement membrane extract (BME) solution (growth factor reduced, Phenol Red-free). Keep 10 mL of BME in a 4 °C refrigerator overnight. Once thawed, swirl the BME bottle to ensure dispersion.
- Prepare base media/washing buffer AD+++. Add 5 mL of 200 mM L-glutamine, 1 M HEPES and Pen/Strep to Advanced DMEM/F12 media by pipetting with a 5 mL pipette.
- Prepare colon organoid medium as described by Sato et al.18 through supplementing base media with N-Ac (1 mM), A83-01 (500 nM), B27 (1x), EGF (50 ng/mL), Noggin (100 ng/mL), Nicotinamide (10 mM), SB202190 (10 nM), R-spondin (500 ng/mL), L-glutamine (2 mM), HEPES (10 µM), penicillin-streptomycin (1x) and Y-27623 (10 µM)19.
- Prepare AD+++Digestion medium: 10 mL of 1x organoid culture media with 500 µL of collagenase type II (20 mg/mL) and 10 µL of RhoKI Y-27632 (10 mM).
- Tumor Dissociation20
- Transfer the tumor in a 50 mL plastic tube to a 10 cm Petri dish. Take a macroscopic photograph alongside a ruler and record a description of its conditions (i.e., size, fat tissues, vascularisation, necrosis, etc.).
- Remove excess AD+++ by aspiration, and cut the tumor tissue into small pieces by scissors followed by transferral of 1-2 pieces into a 2 mL microtube for snap freezing on dry ice. Store at -80 °C for genomic profiling. Mince the remaining pieces into finer pieces by scissors before transferral to a 50 mL plastic tube using AD+++.
- Wash minced tissue 2-3 times by 35 mL of AD+++, followed by addition of 10 mL of digestion medium (see step 4.1.4) and placement on an orbital shaker at 4 x g for 1 h at 37 °C.
- Homogenize the digested tissue by pipetting up and down using a 5 mL sterile plastic pipette, followed by addition of 20 mL of AD+++ and filtration by 100 µm cell strainer.
- Wash the pass-through twice with AD+++ (spin at 450 x g for 5 min), followed by resuspension in BME (add 4x volume of BME to the pellet for suspension) and keep on ice19.
- Preparation of organoid culture
- Add the BME cell suspension into 6-well plate in multiple drops to a total of 200 µL per well.
- Transfer the plate to a 37 °C incubator. After 30 min, the gel drops solidify.
- Add 2 mL of organoid media to each well, with the representative drops recorded by microscopic photography before transferring to an incubator (37 °C and 5% CO2).
- Maintain the organoid cultures with medium change every 3-4 days, and passage at a 1:2 ratio every 7 days or depending on their growth and density.
5. Histopathology and next generation sequencing (NGS) analysis
- Histopathology
- Collect organoids from the well with the existing medium using a P1000 pipette, followed by centrifugation (spin at 450 x g for 5 min), washing with PBS and fixation in 10% formalin for 1 h.
- Place the fixed organoids in 100% gelatin at the bottom of 50 mL conical tube, followed by routine tissue processing and embedding.
- Perform haematoxylin–eosin (H&E) staining using standard protocols on 4 mm paraffin sections.
- RNAseq and whole exome sequencing
- Collect organoids from the well with the existing culture medium using a P1000 pipette, followed by centrifugation using a microcentrifuge at maximum speed (12,000 x g) for 5 min at 4 °C.
- Collect the pellet by removing the medium supernatant to ensure no visible BME was present, followed by snap freezing in a microtube (dry ice) and then transfer to a -80 °C freezer.
- Extract RNA or DNA using standard procedure from manufacturers and perform NGS analysis for both RNAseq and whole exome sequencing (WES).
6. IC50 assay20
- Organoid seeding in 384-well plate for IC50 assay
- Dissociate organoids in BME drops from each well (digesting BME) by adding 20 µL of 100x dispase solution to each well (6-well plate), which contained 2 mL of organoid medium, followed by 30 min of incubation at 37 °C.
- Pipette digested organoids from all wells through a 70 µm filter into a 50 mL plastic tube to collect the organoids.
- Count the organoids under microscopy for the determination of organoid concentration. Suspend the organoids using culture medium, before adding BME to reach final concentration of 5% (v/v) on ice.
- Add 50 µL of the organoid suspension into each 384-well plate with the liquid dispenser, according to the plate map with a seeding density of 200 CR2110 PDXOs per well in corresponding organoid culture medium.
- Cisplatin and irinotecan treatment
- Use cisplatin (with the highest concentration of 100 µM) and “irinotecan” (with the highest concentration of 10 µM). Add SN-38 (a metabolite of irinotecan, as opposed to irinotecan which is usually used for in vivo studies) to each well according to the drug dilution scheme for 9 doses, in serial dilution by digital dispener.
- Create the platemap using the digital dispenser software tool. Include a negative control vehicle with 100% viability and postive control of 5 µM staurosporine, which showed 0% viability.
- Place the drug-treated 384-well plates back into 37 °C incubator.
- Determination of organoid cell viability after drug treatment
- At the end of the 5 days of drug treatment, determine organoid cell viability using luminescent cell viability reagents as per the manufacturer’s recommended procedure. Add luminescent reagent into each well with the liquid dispener and mix for 5 min on a plate shaker, followed by a 30 min incubation at room temperature in dark.
- Record the luminescent signal on a luminescence multi-well plate reader.
- Calculate the normalized viabilities of each well using the raw readings from the plate reader and create a dose-response curve and IC50 values by nonlinear curve fitting.
Results
Morphology of PDXOs, typical of organoids under light microcopy, and consistent with parental PDX per H&E staining
Under light microscopy, PDXO-CR2110 demonstrates typical cystic morphology (Figure 1A), as described previously for patient-derived organoids (PDO), evidence supporting the similarity between PDXO and PDO under the same culture conditions.
Histopathological examination by H&E staining reveals that the tissue structures and cell types of PDXO-CR2110 (Figure 1B) are reflective of the original PDX-CR2110 (Figure 1C), supporting that the PDX and PDXO were developed from the same origin: CR2110. This observation provides histopathology evidence to support the similarity of PDXO to its parental PDX.
Transcriptome expression and whole exome sequencing demonstrates high correlation between PDXO-CR2110 and the parental PDX-CR2110
PDX-CR2110 tumors have been previously genomically-profiled using transcriptome sequencing (RNAseq, mRNA)16 and whole exome (WES, DNA) sequenced. We now have similarly profiled the corresponding PDXO-CR2110. The genomic profile comparisons of the corresponding matched PDX and PDXO (Figure 2) demonstrate a high correlation of 94.92% in transcriptome (mRNA) expression (epigenetic) and a high concordance of 97.67% of DNA mutations (WES) (genetic), suggesting an overall genomic similarity between this pair of models.
Similarities observed for the pharmacological properties between in vitro PDXO-CR2110 and in vivo PDX-CR2110
Drug sensitivity assays were performed on PDXO-CR2110 in 384-well plates, with results shown in Figure 3A. PDXO-CR2110 was sensitive to irinotecan and resistant to cisplatin, consistent with PDX treatment results (Figure 3B) where TGI (tumor growth inhibition) at dose levels of 100 mg/kg, i.p., Q3Dx3 for irinotecan and 5 mg/kg, i.p., Q4Dx4 for cisplatin is 84.63% and 6.61% respectively. This observed pharmacology consistency supports the potentially biological equivalence of both models, and can be used for complementary pharmacology studies in vitro and in vivo.
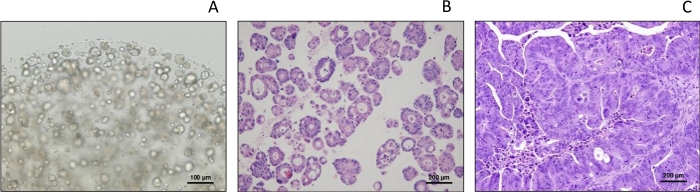
Figure 1: Morphology of PDXO-CR2110. (A) Morphology of under light microscopy (cystic type). (B,C) Histopathology of PDXO-CR2110 and PDX-CR2110, respectively. Please click here to view a larger version of this figure.

Figure 2: Genomic profiles of PDXO-CR2110 vs. PDX-CR2110, WES and RNAseq. Upper panel: global mRNA expression correlation between PDX-CR2110 and PDXO-CR2110 per RNAseq. Lower panel: table of both mRNA expression correlations per RNAseq and DNA mutation concordance per WES: PDX- vs PDXO-CR2110. Please click here to view a larger version of this figure.
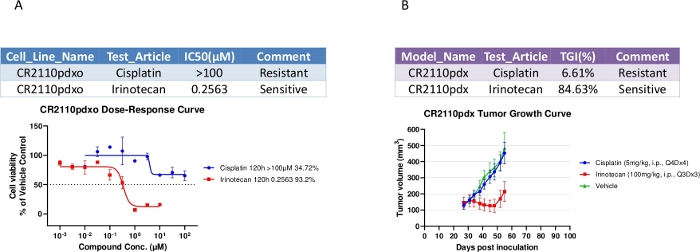
Figure 3: Pharmacological properties of both PDXO-CR2110 in vitro and SC PDX-CR2110 in vivo. (A) PDXO-CR2110 in vitro dose-response to the test compounds. (B) Tumor growth inhibition induced by the same test compounds on CR2110 in vivo. Please click here to view a larger version of this figure.
Discussion
The preliminary data for PDX-/PDXO-CR2110 in this report supports the biological equivalence between PDX and its derivative, PDXO, with regards to genomics, histopathology and pharmacology, since both models represent the disease forms from the original CSC of patient. Both models are patient-derived disease models, potentially predictive of the clinical response of patients10,11,12,21. The matched pair of in vitro and in vivo models can complement each other for in vitro screening and validation in vivo, improving the success rate of drug discovery and potentially reducing attrition rates in clinical development. It is worth noting that PDXO can now enable HTS to take advantage of available large patient-derived organoid libraries, where PDXs fail due to high in vivo costs and longer timelines. Needless to say, a matched PDX-PDXO library would likely become the platform of choice to support drug discovery and translational research in the near future.
Converting the existing library of annotated PDX models could be a fast and productive approach to building a practical organoid library by employing an industrial process. This report converted one PDX to PDXO to explore the feasibility of such a process, and the method used could be a foundation to support large scale process to build an extensive PDXO library. Practically, the methods to create PDXO are generally similar to the widely described method for the generation of PDO18, with the exception of the source of the patient tissue being mice.
There are critical steps to ensure that PDXOs are successfully created: 1) the fresh PDX tumors are fragmented to small pieces; 2) the culture conditions described for organoid culture as described by Clevers and colleagues are faithfully implemented18,22, but may be adjusted for different organoids; 3) different organoids have different growth rates, impacting the duration for organoid culture and health, as well as drug treatment duration; 4) an effective assay to determine mouse content vs. human content is absolutely critical to ensure the cultures are largely human organoid, since some cultures may inevitably have persistent mouse tissue/cell contamination (in the case reported here, there is minimal mouse tissue contamination, data not shown). Mouse contamination could be one of the important limitations in creating PDXO biobanks if effective detection and removal methods are not used.
Disclosures
All authors are or were current full-time employees of Crown Bioscience, Inc.
Acknowledgements
The authors would like to thank Dr. Jody Barbeau, Federica Parisi and Rajendra Kumari for critical reading and editing of the manuscript. The authors would also like to thank the Crown Bioscience Oncology in vitro and in vivo team for their great technical efforts.
Materials
| Name | Company | Catalog Number | Comments |
| Advanced DMEM/F12 | Life Technologies | 12634028 | Base medium |
| DMEM | Hyclone | SH30243.01 | Washing medium |
| Collagenese type II | Invitrogen | 17101015 | Digest tumor |
| Matrigel | Corning | 356231 | Organoid culture matrix (Basement Membrane Extract, growth factor reduced) |
| N-Ac | Sigma | A9165 | Organoid culture medium |
| A83-01 | Tocris | 2939 | Organoid culture medium |
| B27 | Life Technologies | 17504044 | Organoid culture medium |
| EGF | Peprotech | AF-100-15 | Organoid culture medium |
| Noggin | Peprotech | 120-10C | Organoid culture medium |
| Nicotinamide | Sigma | N0636 | Organoid culture medium |
| SB202190 | Sigma | S7076 | Organoid culture medium |
| Gastrin | Sigma | G9145 | Organoid culture medium |
| Rspondin | Peprotech | 120-38-1000 | Organoid culture medium |
| L-glutamine | Life Technologies | 35050038 | Organoid culture medium |
| Hepes | Life Technologies | 15630056 | Organoid culture medium |
| penicillin-streptomycin | Life Technologies | 15140122 | Organoid culture medium |
| Y-27632 | Abmole | M1817 | Organoid culture medium |
| Dispase | Life Technologies | 17105041 | Screening assay |
| CellTiter-Glo 3D | Promega | G9683 | Screening assay (luminescent ATP indicator) |
| Multidrop dispenser | Thermo Fisher | Multidrop combi | Plating organoids/CellTiter-Glo 3D addition |
| Digital dispener | Tecan | D300e | Compound addition |
| Envision Plate reader | Perkin Elmer | 2104 | Luminescence reading |
| Balb/c nude mice | Beijing HFK Bio-Technology Co | ||
| RNAeasy Mini kit | Qiagen | 74104 | tRNA purification kit |
| DNAeasy Blood & Tissue Kit | Qiagen | 69506 | DNA purification kit |
| Histogel | Thermo Fisher | HG-4000-012 | Organoid embedding |
References
- Tentler, J. J., et al. Patient-derived tumour xenografts as models for oncology drug development. Nature Reviews Clinical Oncology. 9 (6), 338-350 (2012).
- Gao, H., et al. High-throughput screening using patient-derived tumor xenografts to predict clinical trial drug response. Nature Medicine. 21 (11), 1318-1325 (2015).
- Yang, M., et al. Overcoming erlotinib resistance with tailored treatment regimen in patient-derived xenografts from naive Asian NSCLC patients. International Journal of Cancer. 132 (2), 74-84 (2013).
- Li, Q. X., Feuer, G., Ouyang, X., An, X. Experimental animal modeling for immuno-oncology. Pharmacology & Therapeutics. 173, 34-46 (2017).
- Sato, T., et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 459 (7244), 262-265 (2009).
- Drost, J., Clevers, H. Organoids in Cancer Researchearch. Nature Reviews Cancer. 18 (7), 407-418 (2018).
- Muthuswamy, S. K. Organoid Models of Cancer Explode with Possibilities. Cell Stem Cell. 22 (3), 290-291 (2018).
- Sachs, N., et al. A Living Biobank of Breast Cancer Organoids Captures Disease Heterogeneity. Cell. 172 (1-2), 373-386 (2018).
- Weeber, F., et al. Preserved genetic diversity in organoids cultured from biopsies of human colorectal cancer metastases. Proceedings of the National Academy of Sciences of the United States of America. 112 (43), 13308-13311 (2015).
- Vlachogiannis, G., et al. Patient-derived organoids model treatment response of metastatic gastrointestinal cancers. Science. 359 (6378), 920-926 (2018).
- Yao, Y., et al. Patient-Derived Organoids Predict Chemoradiation Responses of Locally Advanced Rectal Cancer. Cell Stem Cell. 26 (1), 17-26 (2020).
- Ganesh, K., et al. A rectal cancer organoid platform to study individual responses to chemoradiation. Nature Medicine. 25 (10), 1607-1614 (2019).
- van de Wetering, M., et al. Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell. 161 (4), 933-945 (2015).
- Yang, J. P., et al. A novel RNAi library based on partially randomized consensus sequences of nuclear receptors: identifying the receptors involved in amyloid beta degradation. Genomics. 88 (3), 282-292 (2006).
- Zhang, L., et al. A subset of gastric cancers with EGFR amplification and overexpression respond to cetuximab therapy. Scientific Reports. 3, 2992 (2013).
- Chen, D., et al. A set of defined oncogenic mutation alleles seems to better predict the response to cetuximab in CRC patient-derived xenograft than KRAS 12/13 mutations. Oncotarget. 6 (38), 40815-40821 (2015).
- Guo, S., et al. Molecular Pathology of Patient Tumors, Patient-Derived Xenografts, and Cancer Cell Lines. Cancer Research. 76 (16), 4619-4626 (2016).
- Sato, T., et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett's epithelium. Gastroenterology. 141 (5), 1762-1772 (2011).
- Tiriac, H., French, R., Lowy, A. M. Isolation and Characterization of Patient-derived Pancreatic Ductal Adenocarcinoma Organoid Models. Journal of Visualized Experiments. (155), (2020).
- Kopper, O., et al. An organoid platform for ovarian cancer captures intra- and interpatient heterogeneity. Nature Medicine. 25 (5), 838-849 (2019).
- Corcoran, R. B., et al. Combined BRAF and MEK Inhibition With Dabrafenib and Trametinib in BRAF V600-Mutant Colorectal Cancer. Journal of Clinical Oncology. , (2015).
- Huch, M., et al. Long-term culture of genome-stable bipotent stem cells from adult human liver. Cell. 160 (1-2), 299-312 (2015).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved