Method Article
Generation of a Rat Model of Acute Liver Failure by Combining 70% Partial Hepatectomy and Acetaminophen
In This Article
Summary
The acute liver failure animal model developed in the current study presents a feasible alternative for the study of potential therapies. The current model employs the combined effect of physical and drug-induced hepatic injury and provides a suitable time window to study the potential of novel therapies.
Abstract
Acute liver failure (ALF) is a clinical condition caused by various etiologies resulting in the loss of metabolic, biochemical, synthesizing, and detoxifying functions of the liver. In most irreversible liver damage cases, orthotropic liver transplant (OLT) remains the only available treatment. To study the therapeutic potential of a treatment for ALF, its prior testing in an animal model of ALF is essential. In the current study, an ALF model in rats was developed by combining 70% partial hepatectomy (PHx) and injections of acetaminophen (APAP) that provides a therapeutic window of 48 h. The median and left lateral lobes of the liver were removed to excise 70% of the liver mass and APAP was given 24 h postsurgically for 2 days. Survival in ALF-induced animals was found to be severely decreased. The development of ALF was confirmed by altered serum levels of the enzymes alanine amino transferase (ALT), aspartate amino transferase (AST), alkaline phosphatase (ALP); changes in prothrombin time (PT); and assessment of the international normalized ratio (INR). Study of the gene expression profile by qPCR revealed an increase in expression levels of genes involved in apoptosis, inflammation, and in the progression of liver injury. Diffused degeneration of hepatocytes and infiltration of immune cells was observed by histological evaluation. The reversibility of ALF was confirmed by the restoration of survival and serum levels of ALT, AST, and ALP after intrasplenic transplantation of syngeneic healthy rat hepatocytes. This model presents a reliable alternative to the available ALF animal models to study the pathophysiology of ALF as well as to evaluate the potential of a novel therapy for ALF. The use of two different approaches also makes it possible to study the combined effect of physical and drug-induced liver injury. The reproducibility and feasibility of current procedure is an added benefit of the model.
Introduction
Acute liver failure (ALF) is defined by the American Association for the Study of Liver Diseases as rapid development of acute liver injury without any prior signs of damage and is characterized by severe impairment of the synthetic, metabolic, and detoxifying functions of the liver1. ALF differs from chronic liver failure where the failure occurs as a result of liver injury caused over a long period of time and from acute chronic liver failure (ACLF), where abrupt liver damage takes place as a result of chronic liver diseases2,3,4. The only available cure for ALF is orthotopic liver transplant (OLT), or death may occur. Due to the shortage of liver donors, the rate of mortality in patients suffering from ALF is very high.
To study the potential of alternative therapeutic approaches and to better understand the pathophysiology of ALF, animal models that can reflect the ALF occurring in human beings are needed. Many of the already available ALF animal models have several shortcomings. Acetaminophen (APAP) effects are difficult to reproduce but have the closest similarities in terms of temporal, clinical, biochemical, and pathological parameters. APAP- induced animal models frequently encounter problems due to the presence of methemoglobinemia caused by the oxidation of hemoglobin by APAP and its intermediates5,6,7. Another problem is the lack of reproducibility reflected by unpredictable dose responses and the time of death. The ALF animal models produced using carbon tetra chloride (CCl4) have poor reproducibility8,9,10,11. Concavalin A (Con A) and lipoplysaccharide (LPS)-induced ALF animal models do not reflect the clinical pattern of the human disease, though they have advantages in the study of cellular mechanisms involved in autoimmune liver diseases and in the study of sepsis respectively12,13,14,15. Similarly, thioacetamide (TAA) also requires biotransformation to an active metabolite thioacetamide sulfoxide and shows species variation16,17,18,19. D-galactosamine (D-Gal) produces some biochemical, metabolic, and physiological changes similar to ALF but is not able to reflect the whole ALF pathological condition20,21,22,23. There have been very few attempts to combine two or more of these methods to develop an ALF model that is able to reflect the ALF syndrome in a better manner13. Therefore, further studies are required to develop a model that can reflect the disease parameters, has better reproducibility, and provides enough time to study the effects of a therapeutic intervention.
In the current study, an alternative ALF model in rats has been created by combining the effects of partial hepatectomy (PHx) and lower doses of a hepatotoxic reagent. APAP has a well-established role in causing liver injury5,24,25. It is a widely used analgesic and is toxic to the liver at supratherapeutic doses by forming toxic metabolites. APAP is the cause of many deaths in developed countries. Physical injury caused by partial hepatectomy initiates activation of various processes involved in inflammation as well as liver regeneration. Injection of the hepatotoxic agent APAP causes a hostile environment in the liver, preventing the proliferation of hepatocytes. This reduces the stress period on the animal, which when combined with smaller doses of hepatotoxin, leads to better reproducibility of the procedure. Therefore, using this model, a combinatory effect of two types of liver injuries has been studied. To characterize the developed ALF animal model, physiological and biochemical parameters have been studied. Successful reversibility of ALF was confirmed by transplantation of syngeneic healthy rat hepatocytes.
Protocol
The procedure described below has been approved by the Institutional Animal Ethics Committee of National Institute of Immunology, New Delhi. The serial reference number of the approval is IAEC#355/14.
1. Preparation
- Prepare for the surgical procedure as described earlier by Das B et al.26.
- Use 6–8-week-old inbred Wistar rats with a body weight of 200–250 g.
- House the animals under standard animal care conditions and feed them with rat chow and libitum before and after the procedure.
- When performing 70% PHx, use a standard cocktail mix of ketamine hydrochloride (100 mg/kg body weight) and xylazine (10 mg/kg body weight), which is injected intraperitoneally.
NOTE: The type of anesthetic used can have postoperative effects on the mortality and morbidity. - During the cell transplant, use inhalant anesthesia Isoflurane (2-chloro-2-(difluoromethoxy)-1,1,1-trifluoro-ethane) to reduce the time of recovery of the animal after the transplantation surgery.
- Induce and maintain the inhalational anesthesia using a customized anesthesia system. Maintain the oxygen flow at 4 L/min. For induction use isoflurane at 4% and for maintenance use 2–3% during the surgical procedure.
2. Preoperative procedures
- Anesthetize the rat by injecting the ketamine-xylazine mixture described in step 3.1 intraperitoneally. Confirm the complete anesthetization by pinching the toe of the animal. Further procedures are carried out only when there is no pedal reflex.
- To prevent corneal desiccation, apply a carboxy methyl-cellulose based eye drop to both eyes.
- Restrain the anesthetized animal to a surgical board using white tape. Place the animal with the abdominal side facing up, ensuring the mouth is on the distal side from the person performing the operation.
- Remove hair from the upper right abdominal surgical area using an electric clipper.
- Disinfect the surgical site by three alternating scrubs of povidone iodine and 70% ethanol using sterilized cotton pads in circular motion.
3. Partial hepatectomy (PHx) to remove 70% of the mass of the liver
NOTE: Perform the entire surgical procedure under a sterile environment in a laminar flow hood. Use only sterile surgical instruments to minimize the risk of infection postsurgically. Removal of 70% of the liver mass, named 70% partial hepatectomy (70% PHx), was performed as described by C. Mitchell and H. Willenbring, 200827.
- Prior to the start of the surgery, confirm the complete anesthetization of the animal by pinching its toe. Further procedures are carried out only when there is no pedal reflex.
- Mark the skin to be cut just beneath the sternum, perpendicular to the xiphoid, and parallel to the ribcage.
- Place a sterile drape sheet having an opening of around 3 cm x 1 cm over the marked skin.
- Perform a transverse incision of around 2–3 cm along the marked line with a scalpel. Use surgical blade No. 22. Gently remove the attachment of the skin to the underlying muscle layer in the vicinity of the incised area using sterile moistened cotton tips.
- Next, make a transverse incision through the peritoneal layer just beneath the xiphoid process.
- With the help of two saline moistened cotton tips, expose the left lobe of the liver by applying gentle pressure on the thorax. Place one cotton tip on the diaphragmatic region of the incised portion and the other cotton tip below the incised region to lift the liver lobe up.
- Slip an 8–10 cm long sterile nylon thread loop (size 4–0, 0.15 mm diameter) around the exposed liver lobe. Take the loop to the base of the lobe close to the hilum with the help of microdissecting forceps or moistened cotton buds.
- With the help of the microsurgery needle holder and microforceps, tie the two ends of the loop, placing the knot as close to the base of the lobe as possible to constrict the blood vessel and reduce bleeding after the liver lobe is removed. Tie two additional knots on the other side.
- Take precaution not to tie the knot too close to the nearby blood vessels, which may otherwise cause venous obstruction (stenosis).
- Use microsurgery scissors to cut the tied lobe just above the knot, which leaves a discolored mass of tissue called an ischemic stump in place of the lobe.
NOTE: The rat liver, like those of mice, is divided into four distinct lobes: the median lobe, right lateral lobe, left lateral lobe, and caudate lobe, which represent about 40%, 20%, 30%, and 7% of the total liver mass, respectively. Any combination of these lobes can be removed to excise 70% liver mass. In the current study, the median lobe and left lateral lobes were removed. - Carefully locate the median lobe without damaging the remaining stump of left lateral lobe. Gently pull it out of the abdominal cavity, and at the base of the lobe tie an 8–10 cm long nylon thread (size 4–0) knot as mentioned earlier. Tie two additional knots on the other side. Carefully excise and remove the tied median lobe taking all the precautions mentioned.
- After removing the lobes, suture the peritoneum using an absorbable chromic 4–0 suture with continuous stitches followed by skin suturing with an interrupted suture.
- Apply povidone iodine on the skin surrounding the sutures to prevent infection.
- Remove the drape sheet and remove the animal from the surgery board.
4. Postoperative care in animals
- Intraperitoneally inject the animal with a dose of 12 mg cefotaxime antibiotic in 1 mL of 5% glucose solution with a 1 mL syringe to protect it from the risk of postoperative infection.
- Administer a subcutaneous injection of analgesic meloxicam (1 mg/kg body weight) for pain relief after surgery and follow it up by two more doses, keeping the regimen as one dose per day.
- House the operated animals under standard conditions of 12 h light/dark cycle and monitor at regular intervals.
5. Injection of drug in partially hepatectomized animals to induce liver failure
- After 24 h postsurgery, when the animals have successfully recovered from 70% PHx, measure the body weight of the animal followed by the injections.
- Inject 750 mg/kg body weight of APAP intraperitoneally in partially hepatectomized animals 24 h after the 70% PHx following the animals' successful recovery from the surgical procedure. Repeat the dose again after 24 h.
NOTE: Two doses of APAP are administered intraperitoneally to the animal (i.e., 24 h and 48 h post the 70% PHx procedure, respectively). - At each time point after the injection of APAP, measure the body weight of the recovering animal.
NOTE: APAP (biocetamol) is injected in animals as a 150 mg/mL solution in 2% benzyl alcohol.
6. Transplantation of healthy hepatocytes in ALF animal models
NOTE: To study the reversibility of ALF in rats, transplant healthy syngeneic rat hepatocytes intrasplenically in the ALF-induced animals along with the 1st dose of APAP. In the current study, to provide ample time to the transplanted cells for homing and engraftment, the transplantation was done just after giving the 1st dose of APAP. Rat hepatocytes are isolated by a protocol first published by Berry and Friends et al.28 and later adapted in various other studies29,30,31 with some modifications. For intrasplenic transplantation of cells in the ALF animal model, follow the steps mentioned below.
- Place the rat into a poly (methyl methacrylate) chamber for induction of anesthesia with 4% isoflurane and 4 L/min oxygen flow for a rat of 250–350 g body weight. Check for the depth of anesthesia by the lack of pedal reflexes when pinching the toe of the animal.
- Place the anaesthetized rat on the surgical board such that its left lateral portion is facing up. Maintain anesthesia at 2–3% isoflurane inhalation through a suitable mouthpiece.
- Shave the skin on the left lateral region and sterilize it by povidone iodine solution.
- Make a transverse incision on the shaved region of skin.
- Make a 1–2 cm cut in the peritoneal layer to expose the spleen.
- Gently take out the spleen of the peritoneal cavity and lift it up with the help of two moistened cotton tips.
- Keep the cells (typically 107 per animal) to be transplanted suspended in 50 µL IMDM media in a 1 mL insulin syringe with a 29 G needle.
- Gently pierce the needle into the spleen cortex and release the cell suspension into the spleen within 2–3 min.
- After the cell transplantation is completed, carefully take out the needle and dab the area of the needle puncture with a moistened cotton tip to avoid leakage of the cell suspension from the site.
- Close the peritoneum and skin by a 4–0 absorbable suture with continuous and discontinuous suturing respectively.
- Apply povidone iodine solution on the skin at the place of the sutures to prevent infection on the operated site.
- Intraperitoneally inject 1 mL volume of 12 mg/mL of antibiotic (e.g., cefotaxime) solution and subcutaneously inject analgesic (e.g., meloxicam) 1 mg/kg body weight to the animal as a part of postoperative care. Move the animal to a warm recovering cage.
- Keep the operated animal in isolation under normal conditions of 12 h light/dark cycle until the surgical wounds are completely healed. This may take 3–4 days.
7. Characterization of ALF development
- Euthanize the animals by overdose of ketamine-xylazine solution 2 h after the 2nd dose of APAP treatment and collect blood and tissue samples.
- Collect serum from blood for biochemical studies32.
- Process liver tissue samples for histological and gene expression studies33,34,35.
Results
Survival percentage in animal models of ALF
The optimum dose of APAP to cause ALF in combination with 70% PHx was standardized as 750 mg/kg body weight. The treatment regimen started 24 h after 70% PHx, when the animals had completely recovered from surgery, and consisted of two APAP doses at 24 h intervals. Mortality was observed at the rate of 80% after the administration of the second dose of APAP, 48 h post-surgery. The survival percentage was analyzed and plotted via the Kaplan-Meier method (Figure 1). The reproducibility in time of death and time period provided by this model makes it a suitable candidate for studying a therapeutic intervention against ALF.
Four groups of animals were considered for the study: Group 1 (control group, only saline treatment), Group 2 (only APAP at 750 mg/kg body weight), Group 3 (saline treatment with 70% PHx) and Group 4 (APAP at 750 mg/kg body weight with 70% PHx). Three animals each were included in all the groups and two representative images are included from each group. Animals from all four groups were euthanized 2 h after the administration of the 2nd dose of the respective treatment. Liver samples taken from all four groups showed distinct morphology from each other. Group 1 showed the reddish-brown morphology of a healthy rodent liver. Liver samples of Group 2 showed no apparent signs of damage at a morphological level and showed similar appearance to the healthy livers of Group 1. Liver samples taken from Groups 3 and 4 did not appear healthy and had discoloration and a patchy appearance. The discoloration corresponds to the damage in the liver tissue and was more apparent in Group 4. Representative data are presented in Figure 2.
The extent of liver injury was determined by checking the serum levels of ALT, AST, and ALP enzymes36,37,38. The levels of ALT, AST, and ALP showed marked differences among the four groups corresponding to the extent of liver damage. Group 4 showed a significant increase in the AST and ALP levels compared to Group 1 (Figure 3).
To compare the gene expression profile in control and ALF-induced groups, q-PCR analysis of genes involved in cell death (Bax2, Caspase3, Fas) in liver tissue samples was performed35,39. These were found to be upregulated in Group 3 in comparison to control Group 1, whereas no difference was seen in Group 2 and Group 4. Genes which are known to be overexpressed in response to liver injury (Mcp1 and Mmp2)33,40 were found to be upregulated in all three groups in comparison to control Group 1. Mmp9 was upregulated in Groups 3 and 4 whereas Group 2 showed no marked difference from control Group 1. Expression levels of Timp1 and Timp234 were found to be higher in Group 3 but showed no marked overexpression in Group 4 (Figure 4).
Genes involved in inflammation of the liver (Tnfa, IL1a, Tgfbr1, and IL1b)41,42,43,44 were found to be overexpressed in Group 3 in comparison to control Group 1 but their expression level was decreased in Group 4. ALR is overexpressed in liver tissue after the induction of liver injury. The downstream cascades of this gene help in liver regeneration. RIP1 protein is necessary in APAP-induced toxicity. ALR and RIP1 were found to be upregulated in Groups 2 and 3 in comparison to control Group 1, whereas their levels remain similar or lower in Group 4. Alpha smooth muscle actin (aSMA), a marker of excessive ECM deposition that further leads to fibrosis, was found to be upregulated in Group 3 and 4 in comparison to control Group 1 (Figure 4). Gene expression data suggests that the above-mentioned biomarkers of inflammation and cell death that are known to be overexpressed during liver injury are upregulated in liver tissue samples of animals in which ALF was-induced by proposed method, thus confirming the occurrence of liver injury at the molecular level.
Hematoxylin and eosin (H&E) staining
Hematoxylin and eosin (H&E) staining was done to assess the severity of liver injury by observing the extent of hepatocyte degeneration. The liver tissue sections stained with H&E were subjected to blind analysis by a third party. In the 70% partial hepatectomy group vesicular fatty degeneration was observed and in the acetaminophen group periportal inflammation and necrosis was seen along with mild sinusoidal dilatation. Vesicular fatty degeneration was observed mostly in the ALF group (Figure 5).
Prothrombin time and blood glucose levels
Prothrombin Time (PT) is a parameter that depends on the activity of the liver-synthesized tissue thromboplastin and is used to characterize the efficiency of the blood coagulation mechanism45. Generally, an increase in PT is observed in the conditions of liver-related diseases. PT was found to be significantly higher in the ALF-induced group in comparison to the control group. The international normalized ratio (INR)45 was also found to be higher, representing a defect in the mechanism of blood coagulation as a result of inefficient liver physiology (Figure 6).
Evaluation of survival after cell healthy syngeneic rat hepatocytes transplantation
An important criterion of an ALF model is the reversibility of liver failure in the presence of a therapeutic intervention. In the current study, 10 million healthy syngeneic rat hepatocytes were transplanted instrasplenically to observe the effect of cell transplant therapy on liver failure. The appropriate number of cells to be transplanted was determined by observing the reversibility of liver failure after transplantation of different cell numbers (data not shown). Cells were transplanted after administration of the 1st dose of APAP, and survival was found to be restored in animals after the transplantation of cells (Figure 7). The serum levels of enzymes ALT, AST, and ALP were found to be normalized after 10 days of cell transplantation (Figure 8).

Figure 1: Survival percentage in the ALF-induced group. Kaplan-Meier Survival Curve showing the survival percentage of animals after induction of ALF with the combination of 70% PHx and APAP dose (750 mg/kg body weight). Time 0 h denotes the time of 70% PHx. The 1st and 2nd doses of APAP were administered 24 and 48 h postsurgically (n = 12, total animals). Please click here to view a larger version of this figure.

Figure 2: Representative images of liver morphology in the four different groups included in the study. Image panels A–D show the morphology of the livers of different groups after being euthanized 2 h after the 2nd dose of the respective treatment. (A) The liver morphology of control Group 1 in which only saline was given. (B) The liver morphology of Group 2 in which only APAP was given at the dose of 750 mg/kg body weight. (C) The liver morphology of Group 3 in which saline was given following 70% PHx. (D) The liver morphology in Group 4 in which APAP was given following 70% PHx at the dose similar to Group 3 (n = 3 animals per group). Please click here to view a larger version of this figure.

Figure 3: A comparison of the biochemical profile of serum in the different groups. Bar graphs represent the mean value of ALT, AST, and ALP in serum samples of the four groups included in the study. Serum was collected 2 h after the 2nd dose of the respective treatment. Error bars = S.E.M; *** = p < 0.001; * = p < 0.05; n = 3. Please click here to view a larger version of this figure.
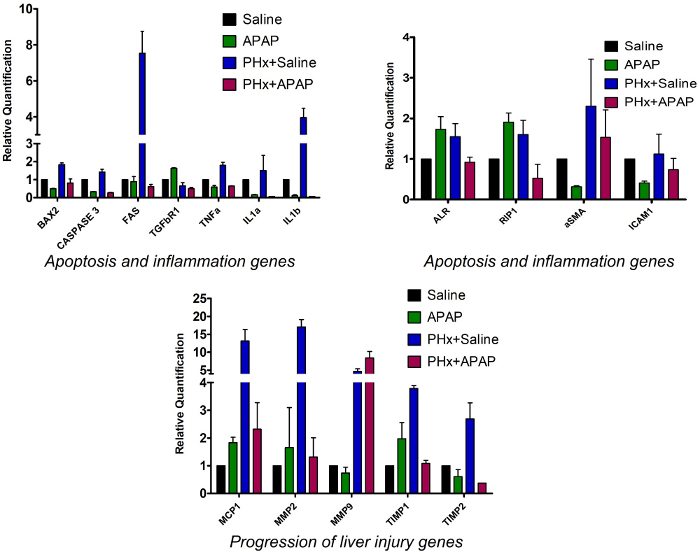
Figure 4: Gene expression profile of liver tissue samples. Bar graphs represent the relative quantification of the mRNA expression of various genes involved in inflammation and cell death in the four groups subjected to different treatments. All genes were normalized against the expression of control Gapdh. Error bar = standard error mean of the three different samples (n = 3). Please click here to view a larger version of this figure.

Figure 5: Histological study of liver tissue samples. Representative hematoxylin and eosin staining images of liver tissue samples from the four groups included in the study, as observed under 200x magnification in bright field microscopy. There were mostly normal hepatocytes in control Group 1 with mild periportal infiltration (green arrows). In Group 3 (70% partial hepatectomy) vesicular fatty degeneration was observed (white arrows) and in the acetaminophen Group 2 periportal inflammation (red arrows) and necrosis were seen along with mild sinusoidal dilatation (yellow arrows). Macrovesicular fatty degeneration was mostly observed in the ALF Group 4 (orange arrows). Scale bar = 50 µm. Please click here to view a larger version of this figure.

Figure 6: Comparison of prothrombin time (PT) and international normalized ratio (INR). (A) Comparison of PT between control Group 1 (saline injection post 70% PHx) and ALF-induced Group 4 (APAP injection post 70% PHx). Blood was collected 2 h after the 2nd dose of the respective treatment and time (in seconds) required for fibrin clot formation is shown on the Y axis (**** = p < 0.0001, n = 5). (B) The INR in the ALF-induced Group 4 in comparison to control Group 1. The mean value of INR in Group 4 was found to be 2.28, which falls in the Warfarin-induced anti coagulating effect (n = 5). Please click here to view a larger version of this figure.

Figure 7: Survival percentage in ALF-induced group after cell transplant. Kaplan-Meier survival curve showing the survival percentage after healthy hepatocyte transplantation in the ALF-induced Group 4 in comparison to control Group 1 (n = 5). Please click here to view a larger version of this figure.
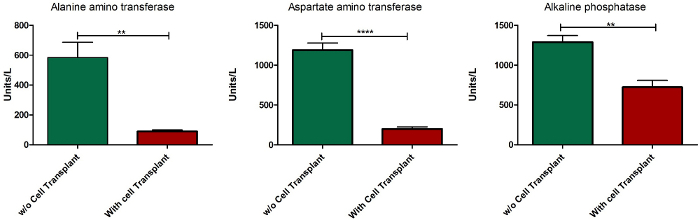
Figure 8: Serum biochemical profile of ALF-induced animals after cell transplant. Serum levels of enzymes ALT, AST, and ALP 10 days after cell transplantation in comparison with the serum levels of ALF-induced animals euthanized after a 2nd dose of APAP administration. Error bar = standard error of mean of five different samples; **** = p < 0.0001; ** = p > 0.01; n = 5. Please click here to view a larger version of this figure.
Discussion
The development of an appropriate animal model for ALF is paramount for the better understanding of pathogenesis and progression of ALF. A well characterized ALF animal model also provides the opportunity for the development and trial of new therapeutic approaches against ALF. Many attempts have been made to develop a clinically relevant model of ALF6,12,21,23,46,47,48. Most of these studies either utilize surgical procedures or induce liver injury by hepatotoxic chemicals.
It is difficult to create an ALF model by PHx alone because PHx would not reflect the pathology of ALF due to the absence of inflammation caused by the necrotic and apoptotic tissues. At therapeutic doses, APAP is metabolized completely by the processes of glucoronidation and sulphation. At higher doses, when these pathways are saturated, APAP is metabolized by cytochrome p450 enzymes which are primarily synthesized by hepatocytes, resulting in the formation of a toxic intermediate, NAPQI (N-acetyl-p-benzoquinone imine). Under normal conditions NAPQI is neutralized by the glutathione (GSH) reserves of cells, whereas at supratherapeutic doses it causes oxidative stress in hepatocytes, resulting in hepatocyte death4,24,25,46. Hence, by combining the physiological effects of 70% PHx and APAP-induced liver injury, an ALF rat model was created in the current study. A smaller therapeutic window reduces the stress period on the animal. When combined with smaller doses of APAP, this leads to better reproducibility of the procedure.
The dose of APAP was selected to provide a suitable therapeutic window during which a therapeutic intervention can be given to the animal (i.e., within 48 h of the 1st dose and 24 h of 2nd dose). Within 24 h after the 2nd dose of APAP, 100% mortality was observed. However, the time of death within this period was variable for each animal. Hence, for the purpose of studying the development of ALF, the animals were euthanized at a standard time point of 2 h after the 2nd dose of APAP.
The time of therapeutic intervention can be decided depending upon the individual study and the type of therapy being studied, which in the current study was transplantation of syngeneic rat hepatocytes just after the 1st dose of APAP, allowing the cells enough time to home and engraft. In the current model, transplantation of at least 10 million cells is required for a successful rescue from ALF.
To ensure the success of the surgical procedure, a few important points should be considered. It is recommended to use different types of anesthetics in different surgical procedures. To perform partial hepatectomy, a cocktail of ketamine-xylazine should be used in the recommended doses because it gives ample time to complete the surgical procedure. During cell transplant, inhalable anesthesia isoflurane should be used because it reduces the physical stress on ALF-induced animals.
In conclusion, due to a uniform mortality rate and a convenient therapeutic window, an APAP and 70% PHx combined model was used to study the reversibility of ALF by cell transplantation. To assess the reversibility of ALF, 10 million healthy syngeneic rat hepatocytes were transplanted intrasplenically in the ALF-induced animals. After transplantation, the survival percentage was found to be increased in ALF-induced animals. Improvement in serum levels of ALT, AST, and ALP was also observed in animals after cell transplant, suggesting the restoration of liver metabolism. This ALF animal model presents an alternative to evaluate the therapeutic potential of cells bridging the gap between acute liver failure and liver transplantation. It also provides the opportunity to study liver damage caused by physical injury and hepatotoxic agent.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported by the core grant received from the Department of Biotechnology, Government of India to National Institute of Immunology, New Delhi.
Materials
| Name | Company | Catalog Number | Comments |
| Acetaminophen (Biocetamol) | EG Pharmaceuticals | No specific Catalog Number (Local Procurement) | |
| Alkaline Phosphatase Kit (DEA) | Coral Clinical System, India | No specific Catalog Number (Local Procurement) | |
| Automated analyser | Tulip, Alto Santracruz, India | Screen Maaster 3000 | Biochemical analyser for liver functional test |
| Betadine (Povidon-Iodine Solution) | Win-Medicare; India | No specific Catalog Number (Local Procurement) | |
| Biological safety cabinet (Class I) | Kartos international; India | No specific Catalog Number (Local Procurement) | |
| Bright Field Microscope | Olympus, Japan | LX51 | |
| Cefotaxime (Taxim®) | AlKem; India | cefotaxime sodium injection, No specific Catalog Number (Local Procurement) | |
| Cell Strainer | Sigma; US | CLS431752 | |
| Collagenase Type I | Gibco by Life Technologies | 17100-017 | |
| Cotton Buds | Pure Swabs Pvt Ltd; India | No specific Catalog Number (Local Procurement) | |
| Drape Sheet | JSD Surgicals, Delhi, India | No specific Catalog Number (Local Procurement) | |
| DPX Mountant | Sigma; US | 6522 | |
| Eosin Y solution, alcoholic | Sigma; US | HT110132 | |
| Forceps | Major Surgicals; India | No specific Catalog Number (Local Procurement) | |
| Gas Anesthesia System | Ugo Basile; Italy | 211000 | |
| Glucose | Himedia, India | GRM077 | |
| Hair removing cream (Veet®) | Reckitt Benckiser, India | No specific Catalog Number (Local Procurement) | |
| Hematoxylin Solution, Mayer's | Sigma; US | MHS16 | |
| Heparin sodium salt | Himedia; India | RM554 | |
| Hyaluronidase From Sheep Testes | Sigma; US | H6254 | |
| I.V. Cannula (Plusflon) | Mediplus, India | Ref 1732411420 | |
| Insulin Syringes | BD; US | REF 303060 | |
| Isoflurane (Forane®) | Asecia Queenborough | No B506 | Inhalation Anaesthetic |
| Ketamine (Ketamax®) | Troikaa Pharmaceuticals Ltd. | Ketamine hydrochloride IP, No specific Catalog Number (Local Procurement) | |
| Meloxicam (Melonex®) | Intas Pharmaceuticals Ltd; India | No specific Catalog Number (Local Procurement) | |
| Micro needle holders straight & curved | Mercian; England | BS-13-8 | |
| Micro needle holders straight & curved | Mercian; England | BS-13-8 | |
| Microtome | Histo-Line Laboratories, Italy | MRS3500 | |
| Nylon Thread | Mighty; India | No specific Catalog Number (Local Procurement) | |
| Paraformaldehyde | Himedia; India | GRM 3660 | |
| Percoll® | GE Healthcare | 17-0891-01 | |
| Refresh Tears/Eyemist Gel | Allergan India Private Limited/Sun Pharma, India | P3060 | No specific Catalog Number |
| RPMI | Himedia; India | No specific Catalog Number (Local Procurement) | |
| Scalpel | Major Surgicals; India | No specific Catalog Number (Local Procurement) | |
| Scissors | Major Surgicals; India | No specific Catalog Number (Local Procurement) | |
| SGOT (ASAT) KIT | Coral Clinical System, India | No specific Catalog Number (Local Procurement) | |
| SGPT (ALAT) KIT | Coral Clinical System, India | No specific Catalog Number (Local Procurement) | |
| Shandon Cryotome E Cryostat | Thermo Electron Corporation; US | No specific Catalog Number | |
| Sucrose | Sigma; US | S0389 | |
| Surgical Blade No. 22 | La Medcare, India | No specific Catalog Number (Local Procurement) | |
| Surgical Board | Locally made | No specific Catalog Number (Local Procurement) | |
| Surgical White Tape | 3M India; India | 1530-1 | Micropore Surgical Tape |
| Sutures | Ethicon, Johnson & Johnson, India | NW 5047 | |
| Syringes (1ml, 26 G) | Dispo Van; India | No specific Catalog Number (Local Procurement) | |
| Trimmer (Clipper) | Philips | NL9206AD-4 DRACHTEN QT9005 | |
| Weighing Machine | Braun | No specific Catalog Number (Local Procurement) | |
| William's E Media | Himedia; India | AT125 | |
| Xylazine (Xylaxin®) | Indian Immunologicals Limited | Sedative, Pre-Anaesthetic, Analgesic and muscle relaxant |
References
- Polson, J., Lee, W. M. AASLD position paper: the management of acute liver failure. Hepatology. 41, 1179-1197 (2005).
- Chung, R. T., et al. Pathogenesis of liver injury in acute liver failure. Gastroenterology. 143, 1-7 (2012).
- Fyfe, B., Zaldana, F., Liu, C. The Pathology of Acute Liver Failure. Clinical Liver Disease. 22, 257-268 (2018).
- Lefkowitch, J. H. The Pathology of Acute Liver Failure. Advances in Anatomic Pathology. 23, 144-158 (2016).
- Mitchell, J. R., et al. Acetaminophen-induced hepatic necrosis. I. Role of drug metabolism. Journal of Pharmacology and Experimental Therapeutics. 187, 185-194 (1973).
- Rahman, T. M., Hodgson, H. J. Animal models of acute hepatic failure. International Journal of Clinical and Experimental Pathology. 81, 145-157 (2000).
- Rahman, T. M., Selden, A. C., Hodgson, H. J. A novel model of acetaminophen-induced acute hepatic failure in rabbits. Journal of Surgical Research. 106, 264-272 (2002).
- Dashti, H., et al. Thioacetamide- and carbon tetrachloride-induced liver cirrhosis. European Surgical Research. 21, 83-91 (1989).
- Demirdag, K., et al. Role of L-carnitine in the prevention of acute liver damage-induced by carbon tetrachloride in rats. Journal of Gastroenterology and Hepatology. 19, 333-338 (2004).
- Sheweita, S. A., Abd El-Gabar, M., Bastawy, M. Carbon tetrachloride-induced changes in the activity of phase II drug-metabolizing enzyme in the liver of male rats: role of antioxidants. Toxicology. 165, 217-224 (2001).
- Sinicrope, R. A., Gordon, J. A., Little, J. R., Schoolwerth, A. C. Carbon tetrachloride nephrotoxicity: a reassessment of pathophysiology based upon the urinary diagnostic indices. American Journal of Kidney Diseases. 3, 362-365 (1984).
- Takada, Y., Ishiguro, S., Fukunaga, K. Large-animal models of fulminant hepatic failure. Journal of Artificial Organs. 6, 9-13 (2003).
- Takada, Y., et al. Increased intracranial pressure in a porcine model of fulminant hepatic failure using amatoxin and endotoxin. Journal of Hepatology. 34, 825-831 (2001).
- Leist, M., Wendel, A. A novel mechanism of murine hepatocyte death inducible by concanavalin A. Journal of Hepatology. 25, 948-959 (1996).
- Mizuhara, H., et al. Strain difference in the induction of T-cell activation-associated, interferon gamma-dependent hepatic injury in mice. Hepatology. 27, 513-519 (1998).
- Bruck, R., et al. Hypothyroidism minimizes liver damage and improves survival in rats with thioacetamide-induced fulminant hepatic failure. Hepatology. 27, 1013-1020 (1998).
- Chieli, E., Malvaldi, G. Role of the microsomal FAD-containing monooxygenase in the liver toxicity of thioacetamide S-oxide. Toxicology. 31, 41-52 (1984).
- Fontana, L., et al. Serum amino acid changes in rats with thioacetamide-induced liver cirrhosis. Toxicology. 106, 197-206 (1996).
- Peeling, J., et al. Cerebral metabolic and histological effects of thioacetamide-induced liver failure. American Journal of Physiology. 265, 572-578 (1993).
- Blitzer, B. L., et al. A model of fulminant hepatic failure in the rabbit. Gastroenterology. 74, 664-671 (1978).
- Diaz-Buxo, J. A., Blumenthal, S., Hayes, D., Gores, P., Gordon, B. Galactosamine-induced fulminant hepatic necrosis in unanesthetized canines. Hepatology. 25, 950-957 (1997).
- Maezono, K., Mawatari, K., Kajiwara, K., Shinkai, A., Maki, T. Effect of alanine on D-galactosamine-induced acute liver failure in rats. Hepatology. 24, 1211-1216 (1996).
- Patzer, J. F., et al. D-galactosamine based canine acute liver failure model. Hepatobiliary & Pancreatic Diseases International. 1, 354-367 (2002).
- Newsome, P. N., Plevris, J. N., Nelson, L. J., Hayes, P. C. Animal models of fulminant hepatic failure: a critical evaluation. Liver Transplantation. 6, 21-31 (2000).
- Yoon, E., Babar, A., Choudhary, M., Kutner, M., Pyrsopoulos, N. Acetaminophen-Induced Hepatotoxicity: a Comprehensive Update. Journal of Clinical and Translational Hepatology. 4, 131-142 (2016).
- Das, B., et al. Intrasplenic Transplantation of Hepatocytes After Partial Hepatectomy in NOD.SCID Mice. Journal of Visualized Experiments. , (2018).
- Mitchell, C., Willenbring, H. A reproducible and well-tolerated method for 2/3 partial hepatectomy in mice. Nature Protocols. 3, 1167-1170 (2008).
- Berry, M. N., Friend, D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. Journal of Cell Biology. 43, 506-520 (1969).
- Fry, J. R., Jones, C. A., Wiebkin, P., Bellemann, P., Bridges, J. W. The enzymic isolation of adult rat hepatocytes in a functional and viable state. Analytical Biochemistry. 71, 341-350 (1976).
- Green, C. J., et al. The isolation of primary hepatocytes from human tissue: optimising the use of small non-encapsulated liver resection surplus. Cell Tissue Bank. 18, 597-604 (2017).
- Ismail, T., et al. Growth of normal human hepatocytes in primary culture: effect of hormones and growth factors on DNA synthesis. Hepatology. 14, 1076-1082 (1991).
- Greenfield, E. A. Sampling and Preparation of Mouse and Rat Serum. Cold Spring Harbor Protocols. 11, (2017).
- Walsh, K. M., Timms, P., Campbell, S., MacSween, R. N., Morris, A. J. Plasma levels of matrix metalloproteinase-2 (MMP-2) and tissue inhibitors of metalloproteinases -1 and -2 (TIMP-1 and TIMP-2) as noninvasive markers of liver disease in chronic hepatitis C: comparison using ROC analysis. Digestive Diseases and Sciences. 44, 624-630 (1999).
- Thiele, N. D., et al. TIMP-1 is upregulated, but not essential in hepatic fibrogenesis and carcinogenesis in mice. Scientific Reports. 7, 714 (2017).
- Li, C. P., Li, J. H., He, S. Y., Li, P., Zhong, X. L. Roles of Fas/Fasl, Bcl-2/Bax, and Caspase-8 in rat nonalcoholic fatty liver disease pathogenesis. Genetics and Molecular Research. 13, 3991-3999 (2014).
- Kim, W. R., Flamm, S. L., Di Bisceglie, A. M., Bodenheimer, H. C. Serum activity of alanine aminotransferase (ALT) as an indicator of health and disease. Hepatology. 47, 1363-1370 (2008).
- Henry, L. Serum transaminase levels in liver disease. Journal of Clinical Pathology. 12, 131-137 (1959).
- Giannini, E. G., Testa, R., Savarino, V. Liver enzyme alteration: a guide for clinicians. Canadian Medical Association Journal. 172, 367-379 (2005).
- Hammam, O., et al. The role of fas/fas ligand system in the pathogenesis of liver cirrhosis and hepatocellular carcinoma. Hepatitis Monthly. 12, 6132 (2012).
- Prystupa, A., et al. Activity of MMP-2, MMP-8 and MMP-9 in serum as a marker of progression of alcoholic liver disease in people from Lublin Region, eastern Poland. The Annals of Agricultural and Environmental Medicine. 22, 325-328 (2015).
- Sekiyama, K. D., Yoshiba, M., Thomson, A. W. Circulating proinflammatory cytokines (IL-1 beta, TNF-alpha, and IL-6) and IL-1 receptor antagonist (IL-1Ra) in fulminant hepatic failure and acute hepatitis. Clinical and Experimental Immunology. 98, 71-77 (1994).
- Schwabe, R. F., Brenner, D. A. Mechanisms of Liver Injury. I. TNF-alpha-induced liver injury: role of IKK, JNK, and ROS pathways. American Journal of Physiology-Gastrointestinal and Liver Physiology. 290, 583-589 (2006).
- Ataseven, H., et al. The levels of ghrelin, leptin, TNF-alpha, and IL-6 in liver cirrhosis and hepatocellular carcinoma due to HBV and HDV infection. Mediators of Inflammation. 2006, 78380 (2006).
- Ambrosino, G., et al. Cytokines and liver failure: modification of TNF- and IL-6 in patients with acute on chronic liver decompensation treated with Molecular Adsorbent Recycling System (MARS). Acta Bio Medica Atenei Parmensis. 74, 7-9 (2003).
- Robert, A., Chazouilleres, O. Prothrombin time in liver failure: time, ratio, activity percentage, or international normalized ratio. Hepatology. 24, 1392-1394 (1996).
- Francavilla, A., et al. A dog model for acetaminophen-induced fulminant hepatic failure. Gastroenterology. 96, 470-478 (1989).
- Terblanche, J., Hickman, R. Animal models of fulminant hepatic failure. Digestive Diseases and Sciences. 36, 770-774 (1991).
- Tunon, M. J., et al. Rabbit hemorrhagic viral disease: characterization of a new animal model of fulminant liver failure. Journal of Laboratory and Clinical Medicine. 141, 272-278 (2003).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved