Method Article
A Pulmonary Trunk Banding Model of Pressure Overload Induced Right Ventricular Hypertrophy and Failure
In This Article
Summary
We present a surgical method to induce right ventricular hypertrophy and failure in rats.
Abstract
Right ventricular (RV) failure induced by sustained pressure overload is a major contributor to morbidity and mortality in several cardiopulmonary disorders. Reliable and reproducible animal models of RV failure are therefore warranted in order to investigate disease mechanisms and effects of potential therapeutic strategies. Banding of the pulmonary trunk is a common method to induce isolated RV hypertrophy but in general, previously described models have not succeeded in creating a stable model of RV hypertrophy and failure.
We present a rat model of pressure overload induced RV hypertrophy caused by pulmonary trunk banding (PTB) that enables different phenotypes of RV hypertrophy with and without RV failure. We use a modified ligating clip applier to compress a titanium clip around the pulmonary trunk to a pre-set inner diameter. We use different clip diameters to induce different stages of disease progression from mild RV hypertrophy to decompensated RV failure.
RV hypertrophy develops consistently in rats subjected to the PTB procedure and depending on the diameter of the applied banding clip, we can accurately reproduce different disease severities ranging from compensated hypertrophy to severe decompensated RV failure with extra-cardiac manifestations.
The presented PTB model is a valid and robust model of pressure overload induced RV hypertrophy and failure that has several advantages to other banding models including high reproducibility and the possibility of inducing severe and decompensated RV failure.
Introduction
The right ventricle (RV) can adapt to a persistent pressure overload. In time, however, adaptive mechanisms fail to sustain cardiac output, the RV dilates and eventually the RV fails. RV function is the main prognostic factor of several cardiopulmonary disorders including pulmonary arterial hypertension (PAH), thromboembolic pulmonary hypertension (CTEPH), and various forms of congenital heart disease with a pressure (or volume) overload of the RV. Despite intense treatment, RV failure remains a predominant cause of death in these conditions.
As a consequence of the unique properties1,2 and embryological development3 of the RV, knowledge derived from left heart failure cannot simply be extrapolated to right heart failure. Animal models of right heart failure are therefore needed in order to investigate the mechanisms of RV failure and potential pharmacological treatment strategies.
There are experimental models of pulmonary hypertension induced by SU5416 combined with hypoxia (SuHx)4 or monocrotaline (MCT)5, which induce RV failure secondary to disease in the pulmonary vasculature. These models are used to evaluate therapeutic effects of drugs that target the pulmonary vasculature. Both the SuHx and the MCT model are non-fixed afterload models of RV failure. Consequently, it is not possible to conclude if an improvement in RV function after an intervention is secondary to afterload reducing pulmonary vascular effects or if it is caused by direct effects on the RV. In addition, the MCT model has several extra-cardiac effects.
In experimental pulmonary trunk banding models, the afterload of the RV is fixed due to a mechanical constriction of the pulmonary trunk. This allows for the investigation of direct cardiac effects of an intervention on the RV independent from any pulmonary vascular effects6,7,8,9. Usually, the banding is performed by placing a needle along the pulmonary trunk. Then a ligature is placed around the needle and the pulmonary trunk and tied with a knot, and the needle is removed leaving the suture around the pulmonary trunk. Depending on the gauge of the needle, different degrees of constrictions can be applied, but despite this approach being widely used, it has some disadvantages. First, the diameter of the banding is not exactly the same as the outer diameter of the needle as the ligature is tied around both the needle and the pulmonary trunk. Second, there may be significant variation to how tightly the knot is tied making it difficult to reproduce a certain degree of banding. This will lead to a variation in banding diameter and thereby a larger dispersion. Finally, the knot may come loose over time.
One study applies a half-closed tantalum clip around the pulmonary trunk10. They compressed the clip around the pulmonary trunk to an inner area of 1.10 mm2 and compared it to rats subjected to banding with a suture using an 18 G needle. Overall, banding with the clip was associated with less peri-surgical complications and data variance.
Based on the principles described by Schou et al.11, we further developed and characterized the pulmonary trunk banding (PTB) model of RV hypertrophy and failure. Here, we present our experience using this model based on results from previous studies12,13. For this model, a titanium clip is compressed around the pulmonary trunk to an exact preset inner diameter, which may be adjusted in order to induce distinct RV failure phenotypes.
Protocol
All rats were treated according to Danish national guidelines described in the Danish law on animal experiments and Ministerial order on animal experiments. All experiments were approved by the Institutional Ethics Review Board and conducted in accordance with the Danish law for animal research (authorization number 2012-15-2934-00384, Danish Ministry of Justice).
1. Adjustment of the Ligating Clip Applier
NOTE: The banding of the pulmonary trunk is performed with a modified open ligating clip applier with an angled jaw. The applier is modified with an adjustable stop mechanism to stop the compression when the jaws reach an exact distance from each other. When a small titanium ligating clip is compressed with the modified applier, a lumen persists between the legs of the clip with a specific diameter according to the adjustment of the stop mechanism (Figure 1).
- Choose the diameter of the desired banding, e.g., 0.6 mm.
- Adjust the ligating clip applier until the distance between the jaws is 1.0 mm when fully compressed. This leaves a lumen of 0.6 mm as the two clip legs have a thickness of 0.2 mm each.
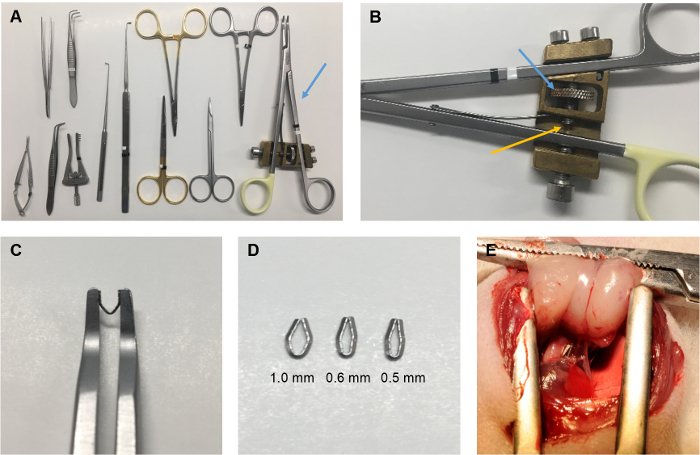
Figure 1: The PTB procedure. (A) The surgical instruments used for the PTB procedure including the ligating clip applier (blue arrow). (B) The adjustable stop mechanism of the ligating clip applier. Turning the cogwheel (blue arrow) will adjust the position of the pin (yellow arrow), which stops the closing of the applier when the jaws reach a certain distance from each other. The distance corresponds to twice the thickness of the legs of the clip plus the inner diameter of the clip, when the clip is compressed, and can be calibrated by using for example a needle with a known outer diameter. (C) The applier compresses a titanium clip to an exact inner diameter pre-specified by adjustment of the applier. (D) The inner diameter of the compressed clip can be adjusted in order to induce different severities of RV hypertrophy and failure. For the data presented, an inner diameter of 1.0 mm was used to induce mild RV hypertrophy, an inner diameter of 0.6 mm was used to induce moderate RV failure, and an inner diameter of 0.5 mm was used to induce severe RV failure. (E) The clip after application around the pulmonary trunk. Please click here to view a larger version of this figure.
2. Preparation of the Rat
NOTE: Other regimens of analgesics may be applied.
- Use Wistar rat weanlings weighing approximately 100–120 g. In order to maintain body temperature during the surgery, use a covered heating pad.
- For surgery, use a mechanical ventilator set to a tidal volume of approximately 1.75 mL and respiratory rate of 75 per min.
- Anaesthetize the rat with sevoflurane (7% mix in 1.5 L of O2) in an induction chamber for 5 minutes. Intubate the rat using a 17 G IV cannula, where the distal 2 mm of the needle have been cut off in order for the soft catheter to cover the tip. Remove the needle and connect the cannula to the ventilator.
- Place the rat on its back on the heating pad. Make sure that the intubation is correct by observing the movements of the thorax. These should be without side differences and in pace with the ventilator.
NOTE: Absence of movements of the thorax, abdominal contractions and inflation of the stomach in the upper left abdomen are signs of a misplaced tube. Remove the cannula, put the rat back in the induction chamber and re-intubate. - After correct intubation, reduce the sevoflurane to maintenance concentration (3.5% mix in O2, 1.5 L/min) and fix the paws of the rat to the heating pad.
- Confirm prober anesthetization by checking withdrawal reflexes of the extremities using a forceps to squeeze the paws of the rat.
- Inject the rats with buprenorphine (0.1 mg/kg subcutaneously (s.c.)) and carprofene (5 mg/kg s.c.) to relieve post-operative pain.
- Shave the chest and disinfect with chlorhexidine.
3. Isolation of the Pulmonary Trunk
- With a pair of scissors, make a 2 cm incision in the skin along the middle part of the sternum. Identify the major pectoral muscle and cut its sternal attachment. Identify the 2nd, 3rd, and 4th costa below.
- Optionally, grab the 2nd costa with a fixation forceps, put a suture (4-0, multifilament, absorbable) around the 2nd costa from the 1st intercostal space to the lower medial part of the 2nd intercostal space. Tie a firm knot in order to ligate the anterior thoracic artery.
NOTE: This can be useful if bleeding from the anterior thoracic artery is a recurrent issue. - Cut the 4th, 3rd, and 2nd costa close to the sternum with a pair of scissors and carefully dissect the intercostal muscles until a complete left thoracotomy has been performed. If any bleeding from the anterior thoracic artery occurs, compress with a pean and ligate the artery.
- Insert a retractor between the sternum and the costae and open it to get a good operating field. At the top of the field is the thymus covering the aorta and the pulmonary trunk. Carefully lift the thymus using a pean and flip it upwards in order to expose the aorta and the pulmonary trunk below.
- Guide the tip of a small surgical hooklet with a 85° angle through the transverse pericardial sinus located behind the left atrial appendage. Pull it halfway back through the sinus and guide the tip of the ear hook upwards until it appears between the ascending aorta and the pulmonary trunk.
- Remove any connective tissue covering the tip with an iris scissor in order to separate the pulmonary trunk from the ascending aorta.
- Repeat the step with a larger hook (optional).
- Guide an angled muscle forceps around the pulmonary trunk through the passage made with the hook(s). Grab the end of an approximately 10 cm ligature (4-0, multifilament) and pull half of the ligature back through the passage. Now the pulmonary trunk is separated from the ascending aorta and can be controlled by the ligature around it.
4. Application of the Clip
- Load the adjusted ligating clip applier with a clip. Carefully guide one of the jaws and one leg of the clip though the passage around the pulmonary trunk. Use the ligature to gently pull the pulmonary trunk upwards and into the fork of the clip.
- When the pulmonary trunk is in the fork of the clip and the two tips of the clip legs are free of any connective tissue, compress the clip with the applier to apply the banding.
- Observe how the RV immediately dilates in response to the banding and remove the ligature.
5. Closing of the Thorax
- Remove the pean from the thymus and reposition the thymus to its natural position. Remove the retractor.
- Close the thorax in three layers: the intercostal layer, the major pectoral muscle, and the skin with suture (4-0, multifilament, absorbable). Inject 2 mL of saline s.c. to replace fluid lost during surgery.
- Turn the sevoflurane off and and keep the rat on the ventilator (1.5 L of O2) until it starts breathing spontaneously. Then, extubate the rat.
- Treat the rats with buprenorphine in the drinking water for the following three days14 or apply a similar analgesic protocol. After three days, the rats have recovered and are without discomfort.
- In the following weeks, the well-being of the rats and possible adverse effects should be evaluated on a daily basis. The healing of the wound from the thoracotomy should receive special attention during the first week in order to detect any signs of infection or insufficiency of the cicatrices. If the rats show signs of failure to thrive including bristly fur, impaired mobility, respiratory problems, and weight loss, they should be monitored closely and euthanized if they lose more than 20% of their body weight or develop fulminant respiratory insufficiency.
6. Sham Surgery
- Perform a sham surgery by following all of the steps above except for the application of the clip (step 4).
Results
Using the described PTB procedure in previous studies from our group12,13, we induced RV hypertrophy (PTB mild) by banding with a 1.0 mm clip, a moderate degree of RV failure (PTB moderate) by banding with a 0.6 mm clip and a severe degree of RV failure (PTB severe) by banding with a 0.5 mm clip. The rats subjected to the severe banding developed extra-cardiac manifestations of RV failure including liver failure and ascites (Figure 5E). All rats were evaluated seven weeks after the PTB and sham rats underwent the same procedure only without the application of the clip. Perioperative mortality was less than 1 in 6. Seven weeks survival rate was 80% for rats subjected to severe banding and close to 100% in rats subjected to mild or moderate banding or sham surgery.
For evaluation of the effects of the PTB procedure, we used echocardiography together with cardiac magnetic resonance imaging (MRI) to assess RV volumes and cardiac output. Tricuspid annular plane systolic excursion (TAPSE) was measured as the distance of the tricuspid annular plane with the RV contraction in the apical four-chamber view. An average of three cycles outside respiration was used as a representative value. RV end-diastolic volume (EDV) and end-systolic volume (ESV) were assessed by drawing of the endocardium in a series of short axis images through the RV obtained by MRI for each rat, and RV ejection fraction (EF) calculated as EF = (EDV-ESV)/EDV. Cardiac output was measured between the pulmonary valves and the clip using a phase-contrast MRI sequence. Digital recordings of RV pressures were obtained by a micro tip catheter installed in the RV before euthanasia. Further details of the methods have been described previously12. RV hypertrophy was evaluated as the ratio of the RV weight divided by the weight of the left ventricle (LV) plus septum and as the weight of the RV divided by the length of the tibia to correct for the size of the rat. All methods were applied as described previously12.
In one week, elevated RV pressures and RV dysfunction evident by a decrease in cardiac output and TAPSE had developed in the PTB rats compared to sham operated rats. Accordingly, interventions or pharmacological treatments can be initiated already at this time point if one aims to investigate effects on established RV failure. After additional six weeks, RV pressure had further increased. The differences of the moderate vs the severe RV failure phenotype were even more pronounced shown by a stepwise decrease in both cardiac output and TAPSE with increased severity of the banding (Figure 2 and Figure 3). Detailed hemodynamic differences between PTB mild rats and PTB severe rats 4 weeks after surgery have been published by our group previousely15.
The PTB procedure also caused RV dilatation evident by an increase in both RV EDV and RV ESV in the moderate PTB rats compared to sham operated rats and in the severe PTB rats compared to both moderate PTB and sham. A stepwise decrease in RV EF was also seen (Figure 4).
The development of RV hypertrophy was associated to the magnitude of the pressure overload applied by the clip. The ratio of the RV over the LV plus septum weight increased stepwise from rats with a mild banding with a clip of 1.0 mm over rats banded with a moderate 0.6 mm clip to rats banded with a severe 0.5 mm clip. Similar results were seen for the RV weight corrected for the size of the rat by dividing with the length of the tibia. The hypertrophy was also seen as an increase in cardiomyocyte cross sectional area in the PTB rats compared to sham operated rats. Apart from hypertrophy of the cardiomyocytes, the pressure overload also induced other morphological changes of the RV associated with RV failure including RV fibrosis. In rats subjected to severe banding, decompensated RV failure was induced. This phenotype was characterized by signs of backward failure including hepatic congestion seen as a dark discoloration of the liver. Hepatic congestion was usually accompanied by ascites (Figure 5).

Figure 2: Effects of PTB one week and seven weeks after the procedure. (A) Right ventricular (RV) systolic pressure (B) cardiac output and (C) tricuspid annular plane systolic excursion (TAPSE) measured one week after sham or PTB operation with a moderate or a severe banding respectively. (D, E and F) The same measures seven weeks after the procedures and further development of RV failure12. Data presented as mean ± SEM. One-way ANOVA with post hoc Bonferroni analysis. **p < 0.01, ***p < 0.001, and ****p < 0.0001 PTB vs sham and PTB severe vs PTB moderate. Please click here to view a larger version of this figure.

Figure 3: Effects of the PTB procedure assessed by echocardiography. (A) A representative four chamber view and (B) measurements of velocity time integral (VTI) in the pulmonary trunk (upper panel) and tricuspid annular plane systolic excursion (TAPSE) (lower panel) in a sham operated rat. (C and D) Similar images for a PTB rat subjected to moderate banding. All images are seven weeks after sham operation or banding. Please click here to view a larger version of this figure.

Figure 4: Magnetic resonance imaging of PTB rats. Cardiac magnetic resonance imaging (MRI) of PTB and sham operated rats. (A) Representative four chamber images (upper panel) and short axis views (lower panel) of sham rats (left) and PTB rats with a moderate degree of RV failure (right). In the PTB rat, the high RV pressures caused septum bulging (blue asterisk). (B) The PTB procedure induced RV dilatation evident by an increase in both RV end diastolic volume (EDV) and RV end systolic volume (ESV). (C) RV ejection fraction (EF) decreased12. Data presented as mean ± SEM. One-way ANOVA with post hoc Bonferroni analysis. *p < 0.05, **p < 0.01, and ****p < 0.0001 PTB vs sham and PTB severe vs PTB moderate. Please click here to view a larger version of this figure.
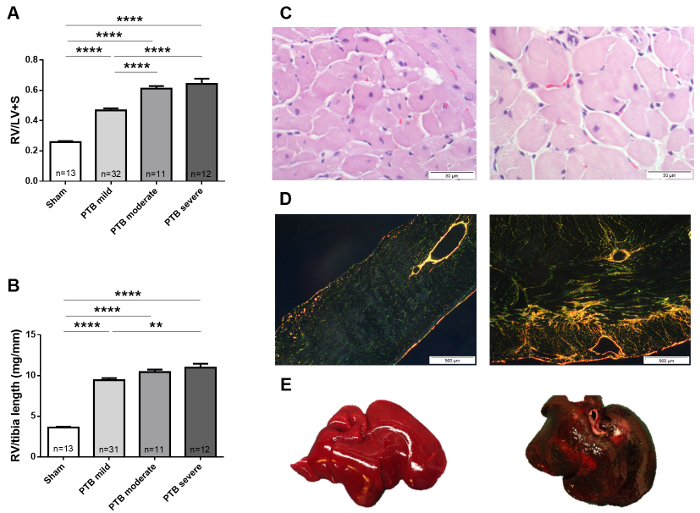
Figure 5: Anatomical data and histology. RV hypertrophy measured as (A) RV divided by the LV plus septum and (B) RV weight divided by tibia length from rats with mild RV hypertrophy, moderate RV failure, and severe RV failure seven weeks after the PTB procedure. (C) Representative images of histological sections stained with hematoxyline eosine for measurement of cardiomyocyte cross sectional area and (D) picrosirius red analyzed under polarized light for fibrosis from sham rats (left) and PTB rats with moderate RV failure (right). (E) A healthy liver (left) and a discolored liver with congestion (right) from a PTB rat with severe RV failure12,13. Data presented as mean ± SEM. One-way ANOVA with post hoc Bonferroni analysis. **p < 0.01 and ****p < 0.0001 PTB vs sham, PTB moderate vs PTB mild and PTB severe vs PTB moderate. Please click here to view a larger version of this figure.
Discussion
We describe an accessible and highly reproducible method of pulmonary trunk banding using a modified ligating clip applier to compress a titanium clip around the pulmonary trunk. By adjusting the applier to compress the clip to different inner diameters, distinct phenotypes of RV hypertrophy and failure can be induced including severe RV failure with extra-cardiac manifestation of decompensation.
Although simple, the protocol contains a few critical steps. Importantly, the rats cannot be too big when they undergo the PTB procedure. In our experience, Wistar rat weanlings weighing 100–120 g are suitable for the procedure. In larger rats, application of a severe banding may lead to acute RV failure and death. Other studies6,7,8,9,10,16 have mainly used larger rats (160–260 g) but also larger diameters of their respective bandings (1.27–1.65 mm).
The application of banding of less severity may also explain the relative modest increases in RV pressures reported by other groups. Banding with an 18 G needle (1.27 mm) leads to RV systolic pressures in the range of 70–90 mmHg6,7,8,9. In one study6, this was not sufficient to cause RV fibrosis or reduce cardiac output. Here, we report RV pressures of approximately 90 mmHg for a moderate banding and 110 mmHg for a severe banding. With a severe banding, we have also been able to create a phenotype of decompensated RV failure with extra-cardiac manifestations including hepatic congestion and ascites12. Pulmonary trunk banding by ligation using a 20G needle (0.902 mm) caused liver fibrosis, nutmeg-like liver congestion and ascites in Sprague Dawley rats16 despite the relatively mild constriction compared to our study. This may be explained by different rat strains responding differently to the banding. There are significant differences with regards to metabolism17, adrenergic tone and heart rate18 between rat strains. Even within the same rat strain, various characteristics including growth rate may vary with different vendors19. This should always be taken into account. For the specific rat strain used, it is therefore critical that well-designed pilot studies are performed in order to determine the banding diameter and the follow-up time needed for the desired RV failure phenotype to develop. The clip model can potentially be used in rat neonates as opposed to the ligating technique previously used20, but we have no experience with this and the same considerations as mentioned above applies before initiating a study.
The PTB model has a few limitations. Firstly, the very proximal occlusion by the clip around the pulmonary trunk represents the conditions of pulmonic stenosis or CTEPH more than the distal narrowing of the smaller pulmonary arteries seen in PAH. The adaptation of the RV to the increased afterload may vary depending on the location of the obstruction(s)21. Secondly, the application of the clip during surgery causes a very sudden increase in RV afterload different from the gradual increase in pulmonary vascular resistance in PAH. The procedure is, however, performed in rat weanlings (100–120 g) giving rise to a progressively increased RV afterload relative to body weight with growth of the animals. During the seven weeks period after surgery, the body weight of the rats increases approximately fourfold and hence relative RV afterload increases proportionally inducing a progressive disease development6.
Using a modified ligating clip applier and a titanium clip for banding of the pulmonary trunk, we were able to induce RV failure. The method has several advantages including high reproducibility and the possibility of creating different disease severities from mild RV hypertrophy to decompensated RV failure by adjusting the diameter of the banding clip. Changing the diameter by 0.1 mm resulted in distinct RV failure phenotypes ranging from moderate and compensated RV failure to severe and decompensated RV failure demonstrating accuracy of this pulmonary trunk banding method.
Disclosures
The authors have nothing to disclose
Acknowledgements
This work was supported by The Danish Council for Independent Research [11e108410], the Danish Heart Foundation [12e04-R90-A3852 and 12e04-R90-A3907], and The Novo Nordisk Foundation [NNF16OC0023244].
Materials
| Name | Company | Catalog Number | Comments |
| 17 G IV Venflon Cannula | Becton Dickinson, US | 393228 | Distal 2 mm of the needle have been cut off |
| 1 mL syringe + 26 G needle | Becton Dickinson, US | 303172 & 303800 | |
| 4-0 absorbable multifilament suture | Covidien, US | GL-46-MG | Polysorb, violet, 5x18" |
| 4-0 multifilament ligature | Covidien, US | LL-221 | Polysorb, violet, 98" |
| Buprenorphine | Indivior UK Limited | Local procurement, Temgesic 0.3 mg/mL | |
| Carprofene | ScanVet, DK | 27693 | Norodyl 50 mg/mL |
| Chlorhexidine | Faaborg Pharma, DK | Local procurement | |
| Contractor | Aesculap, Germany | BV010R | Blunt, self retaining, 70 mm |
| Ear Hooklet | Lawton, Germany | 66-0261 | Small, 14 cm, tip modified to an angle of 85° |
| Eye gel | Decra, UK | Lubrithal, Local procurement | |
| Forceps, Delicate Tissue | Lawton, Germany | 09-0020 | |
| Forceps, Dissecting | Lawton, Germany | 09-0013 | 1 regular, 1 with tip modified to an angle of 100° |
| Gas Anesthesia System | Penlon Limited, UK | SD0217SL | Sigma Delta Vaporizer |
| Hair trimmer | Oster | 76998-320-051 | |
| Horizon Open Ligating Clip Applier | Teleflex, US | 137085 | Modified with adjustable stop mechanism |
| Horizon Titanium Clips | Teleflex, US | 001200 | Small |
| Induction chamber | N/A | ||
| Iris Scissor | Lawton, Germany | 05-1450 | |
| Iris Scissor | Aesculap, Germany | BC060R | |
| Mechanical ventilator | Ugo Basile, Italy | 7025 | |
| Microscissor | Lawton, Germany | 63-1406 | |
| Microscope | Carl Zeiss, Germany | 303294-9903 | |
| Needle Holder | Lawton, Germany | 08-0011 | TITEGRIP |
| Pean | Lawton, Germany | 06-0100 | Halsted-Mosquito, straight |
| Pro-Optha | Lohmann & Rauscher, Germany | 16515 | Tampon |
| Saline 9 mg/mL | Fresenius Kabi, DK | 209319 | |
| Sevoflurane | AbbVie, US | Sevorane, Local procurement | |
| Surgical hook | Lawton, Germany | 51-0665 | Cushing, 19 cm, tip modified to an angle of 90° |
| Surgical Tape | 3M, US | 1530-0 | Micropore |
| Temperature Controller | CMA Microdialysis; Sweden | 8003760 | CMA 450 |
| Weighing machine | VWR, US | ||
| Wistar rat weanlings | Janvier Labs, France | RjHan:WI, 100-120 g |
References
- Kaufman, B. D., et al. Genomic profiling of left and right ventricular hypertrophy in congenital heart disease. Journal of Cardiac Failure. 14 (9), 760-767 (2008).
- Zungu-Edmondson, M., Suzuki, Y. J. Differential stress response mechanisms in right and left ventricles. Journal of Rare Diseases Research & Treatment. 1 (2), 39-45 (2016).
- Zaffran, S., Kelly, R. G., Meilhac, S. M., Buckingham, M. E., Brown, N. A. Right ventricular myocardium derives from the anterior heart field. Circulation Research. 95 (3), 261-268 (2004).
- de Raaf, M. A., et al. SuHx rat model: partly reversible pulmonary hypertension and progressive intima obstruction. The European Respiratory Journal. 44 (1), 160-168 (2014).
- Gomez-Arroyo, J. G., et al. The monocrotaline model of pulmonary hypertension in perspective. American Journal of Physiology-Lung Cellular and Molecular Physiology. 302 (4), L363-L369 (2012).
- Bogaard, H. J., et al. Chronic pulmonary artery pressure elevation is insufficient to explain right heart failure. Circulation. 120 (20), 1951-1960 (2009).
- Borgdorff, M. A., et al. Sildenafil enhances systolic adaptation, but does not prevent diastolic dysfunction, in the pressure-loaded right ventricle. European Journal of Heart Failure. 14 (9), 1067-1074 (2012).
- Mendes-Ferreira, P., et al. Distinct right ventricle remodeling in response to pressure overload in the rat. American Journal of Physiology-Heart and Circulatory Physiology. 311 (1), H85-H95 (2016).
- Piao, L., et al. The inhibition of pyruvate dehydrogenase kinase improves impaired cardiac function and electrical remodeling in two models of right ventricular hypertrophy: resuscitating the hibernating right ventricle. Journal of Molecular Medicine. 88 (1), 47-60 (2010).
- Hirata, M., et al. Novel Model of Pulmonary Artery Banding Leading to Right Heart Failure in Rats. BioMed Research International. 2015, 753210 (2015).
- Schou, U. K., Peters, C. D., Kim, S. W., Frøkiær, J., Nielsen, S. Characterization of a rat model of right-sided heart failure induced by pulmonary trunk banding. Journal of Experimental Animal Science. 43 (4), 237 (2007).
- Andersen, S., et al. Effects of bisoprolol and losartan treatment in the hypertrophic and failing right heart. Journal of Cardiac Failure. 20 (11), 864-873 (2014).
- Holmboe, S., et al. Inotropic Effects of Prostacyclins on the Right Ventricle Are Abolished in Isolated Rat Hearts With Right-Ventricular Hypertrophy and Failure. Journal of Cardiovascular Pharmacology. 69 (1), 1-12 (2017).
- Jessen, L., Christensen, S., Bjerrum, O. J. The antinociceptive efficacy of buprenorphine administered through the drinking water of rats. Lab Anim. 41 (2), 185-196 (2007).
- Andersen, A., Povlsen, J. A., Botker, H. E., Nielsen-Kudsk, J. E. Right ventricular hypertrophy and failure abolish cardioprotection by ischaemic pre-conditioning. European Journal of Heart Failure. 15 (11), 1208-1214 (2013).
- Fujimoto, Y., et al. Low Cardiac Output Leads Hepatic Fibrosis in Right Heart Failure Model Rats. PloS one. 11 (2), e0148666 (2016).
- Marques, C., et al. High-fat diet-induced obesity Rat model: a comparison between Wistar and Sprague-Dawley Rat. Adipocyte. 5 (1), 11-21 (2016).
- Osadchii, O., Norton, G., Deftereos, D., Woodiwiss, A. Rat strain-related differences in myocardial adrenergic tone and the impact on cardiac fibrosis, adrenergic responsiveness and myocardial structure and function. Pharmacological Research. 55 (4), 287-294 (2007).
- Brower, M., Grace, M., Kotz, C. M., Koya, V. Comparative analysis of growth characteristics of Sprague Dawley rats obtained from different sources. Laboratory Animal Research. 31 (4), 166-173 (2015).
- Wang, S., et al. A neonatal rat model of increased right ventricular afterload by pulmonary artery banding. The Journal of Thoracic and Cardiovascular Surgery. 154 (5), 1734-1739 (2017).
- Borgdorff, M. A., et al. Distinct loading conditions reveal various patterns of right ventricular adaptation. American Journal of Physiology-Heart and Circulatory Physiology. 305 (3), H354-H364 (2013).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved