Method Article
Effects of Allogeneic Platelet-Rich Plasma (PRP) on the Healing Process of Sectioned Achilles Tendons of Rats: A Methodological Description
In This Article
Summary
This protocol describes the evaluation process of healing tendons in rats that have been injected with allogeneic platelet rich plasma (PRP) or saline solution after removing part of the Achilles tendon. The progress of tendon healing is evaluated at several time points using different types of analyses.
Abstract
This article describes the experimental procedures used to observe if PRP can positively affect tendon healing. There are 4 main steps to follow: induce a lesion in the Achilles tendon; prepare PRP and inject it (or the saline solution); remove the tendon; and perform biomechanical, molecular, and histological evaluations. At each step, all the procedures and methods are described in detail, so they can be reproduced easily.
Achilles tendons have been surgically sectioned (removal of a 5-mm long section). Afterwards, PRP or saline solution was injected to study whether PRP has a positive effect on the healing of the tendon. Three groups of 40 animals (a total of 120 rats were used in this study) were subdivided into 2 subgroups: PRP injection group and a saline injection control group. Rats were sacrificed at increasing time points (Group A: 5 days; Group B: 15 days; Group C: 30 days) and tendons were removed. 90 tendons underwent biomechanical testing before performing transcriptomic analysis and the 30 remaining tendons were submitted to histological analysis.
Introduction
Coagulation, inflammatory processes, and the immunity modulation roles of platelets are well known1. More recently, it has been demonstrated that they also have restorative properties2,3. Indeed, various cytokines and growth factors (VEGF, PDGF, TGF-B, IGF-I, and HGF) are released by platelets during degranulation. These growth factors promote angiogenesis, tissue remodeling, and wound healing (bone, skin, muscle, tendon)2. Centrifuging autologous blood produces platelet rich plasma (PRP) which contains high platelet concentrations depending on the isolation method (between 3 and 10 times blood baseline concentrations). Indeed, various PRP preparation techniques cannot provide an identical final product. Up to now, there has been no international general agreement on this issue. Overall, PRP could be an attractive therapeutic option for treating chronic musculoskeletal conditions, such as tendinopathy, plantar fasciitis, osteoarthritis, and nonunion4. It was used for the first time in oral surgery and implantology4 to improve and accelerate bone healing after placing a dental implant. In this study, we describe a reproducible method that allows the acquisition of PRP for animal experimentation4.
Since lesions of tendons are frequently observed in sportsmen and physical workers, enhancing the healing process and thus reducing the time for recovery are of great interest5. New treatment methods that are developing often involve the use of growth factors, and the administration of PRP is a simple and minimally invasive way to deliver a blend of endogenous growth factors4.
Several in vitro or animal studies have demonstrated that the administration of plasma containing a high level of platelets, by releasing biological mediators, can stimulate tendon and ligament repair by releasing biological mediators6,7,8,9. Furthermore, other studies have shown that PRP can stimulate type I and III collagen synthesis in tendon cells9,10,11. It has also been suggested that PRP can decrease the activation of matrix metalloproteinases (MMPs) and therefore reduce the degradation of the matrix. Cells that are involved in the inflammation process can produce MMP-9, which plays a role in tissue remodeling (physiologic and pathologic) induced by inflammation12.
Based on this information, we hypothesized that a single PRP injection into the sectioned Achilles tendons of rats could improve the recovery process and the mechanical strength of the repaired tissue. This is tested by measuring the biomechanical properties of the healing tendons during the recovery process and by performing histological and molecular analyses to evaluate collagen remodeling in the newly formed tissue. The aim of the study was to observe if a single injection of allogeneic PRP could influence the healing of sectioned Achilles tendons.
Protocol
Care and handling of the animals were performed in accordance with the Guide for the Care and Use of Laboratory Animals prepared by the National Academy of Sciences and published by the National Institutes of Health (USA). European and national legislation were followed carefully.
1. Animal Preparation
- Use 132 2-month old male Sprague-Dawley rats weighing 320-450 g (120 rats for experimentation and 12 rats for blood sampling, Figure 1). Based on Dell13, 15 rats for each group was enough for a power of 0.8 (80% chance of finding statistical significance, if the specified effect exists).
- House all rats in classical cages under isolation conditions in a conventional facility equipped with a changing station. Instigate breeding in an IVC (individually ventilated cage) rack, with SPF (specific pathogen free) rats being specifically purchased.
- Use the following housing conditions: temperature range from 19-24 °C; relative humidity: 40-60%; ventilation: frequency of air renewal: 10-15 per h; light/dark cycle: 12 h-12 h.
- Sterilize all caging equipment and use irradiated bedding. Systemically implement cage enrichment by placing sterile cardboard trays and towels inside the cages.
- Allow access to the zone under restricted conditions: new lab coat, mask, gloves, footwear, and hair bonnet at the entrance.
- Carry out colony health monitoring on sentinel animals housed on dirty bedding on an annual basis, according to the Felasa14 recommendations for conventional facilities. Keep four to five animals per cage, with the appropriate irradiated diet and provide acidified water ad libitum. Monitor daily.
- Transfer rats elected for surgical intervention in filtering top cages to the surgical rooms 2 h before surgery.
2. Surgical Procedure
- Prepare the surgical instruments: fine scissors, a clamp, two hemostatic clamps, and a needle holder. During all the procedures, wear gloves, a mask, a hood, and a lab coat.
- Randomly divide the rats into two groups (PRP and saline solution).
- Take the rat out of its cage and weigh it. Use numbered ear tags to identify the rats. To avoid desiccation of the eyes, place waterdrops on the cornea.
- Anesthetize the rat intraperitoneally with xylazine (10 mg/kg of body weight) and ketamine (80 mg/kg of body weight). To confirm anesthetization, place the animals under cardio-respiratory monitoring and check for any eye reflexes.
- Inject 50 µL of buprenorphine subcutaneously (0.01-0.05 mg/kg every 8-12 h) in the neck area as a painkiller.
- Shave the left hind limb with an electric shaver, disinfect it with 3 alternating scrubs of iso-betadine/alcohol (diluted 1:10) solution and place the rat on a warm pad (20 °C) under a dissecting microscope. Place the rat on lateral decubitus with the leg to be operated on in a superior position. Hold the paw with surgical forceps.
- Using the scissors, make a small lateral incision (20-25 mm) in the skin surrounding the left Achilles tendon, and dissect the fascia using fine scissors to expose the Achilles tendon complex (Figure 2a).
- Remove the plantaris tendon with a pair of scissors (Figure 2b).
- Cut the Achilles tendon transversally 5 mm proximal to its calcaneal insertion and remove a 5-mm long portion using scissors. Do not suture the tendon (Figure 2c, 2d).
- Suture the fascia and then the skin with resorbable yarn doing an overjet suture (continuous suture). Monofilament sutures can be used as well to prevent wicking from the skin into the surgical incision.
- Place the rat under a heating lamp until awake and then place the rat back in a cage (58 x 38 cm) (Nota bene: No immobilization is imposed).
3. PRP Preparation15
- Anesthetize the donor rats with xylazine (10 mg/kg of body weight) and ketamine (80 mg/kg of body weight) by intraperitoneal injection. The PRP injection is carried out 2 h postoperatively without any re-anesthetic.
- Inject 50 µL of buprenorphine subcutaneously as a painkiller.
- Collect whole blood (20 mL per rat, final bleeding) by cardiac puncture into sterile tubes containing 3.2% buffered sodium citrate (0.109 M, anticoagulant). Then euthanize the rat by intracardiac injection.
- To obtain PRP, centrifuge the blood for 10 min at 150 x g and room temperature. This initial low speed centrifugation step yields two distinct phases: a lower phase consisting of red blood cells (RBCs) that accounts for about 80-90% of the total volume, and an upper phase consisting of platelet-rich plasma (PRP) (typically 10-20% of the blood sample).
- Gently collect the PRP (upper phase) in a secondary plastic tube using a plastic transfer pipette. As the interface between PRP and red blood cells is very loose, do not pipette too close to the interface. When handled properly, contaminating RBCs in PRP should be below 0.05 x 103 cells/µL. Discard the remaining lower phase and the primary blood collection tube.
- Determine the volume of collected PRP and measure the platelet count on a hematology analyzer. These values are required for calculations to adjust platelet concentration in the next step. A blood cell count is also useful to evaluate potential contamination with RBCs and WBCs. Platelet concentration in PRP at this stage is typically 1 – 1.5 x106/µL.
- Perform a second centrifugation of the PRP for 10 min at 1,000 x g and room temperature. This high-speed centrifugation step yields a loose platelet pellet and a supernatant consisting of autologous platelet-poor plasma (PPP). Using the values determined in step 3.6, calculate the volume of supernatant to be discarded to concentrate the PRP and reach a final concentration of 2.5 x106/µL.
- Gently collect the supernatant (PPP) in a secondary plastic tube using a plastic transfer pipette, leaving about two thirds of the final volume calculated in step 3.7. As the pellet is loose, a significant quantity of platelets is lost when PPP is discarded, hence potentially reducing the final desired concentration.
- Carefully resuspend the platelet pellet with the remaining supernatant by gentle repeated pipetting. Measure the platelet count on this concentrated PRP. If required, add the appropriate volume of autologous PPP to reach the final target concentration.
NOTE: Use the PRP within 3 h of preparation. - Add 50 µL of CaCl2 (11 mEq 10 mL) per mL of PRP to activate the platelets.
- Inject 50 µL of fresh PRP or saline solution with a 21G needle directly into the sutured operation site about 1 h after preparation. During this time, keep PRP at room temperature.
- Monitor the functional recovery of the rats closely during the days after surgery and before the removal of the Achilles tendon.
4. Removal of Achilles tendon and Biomechanical Testing16
- 5, 15, or 30 days later5, weigh the rat. Then, anesthetize with xylazine (10 mg/kg of body weight) and ketamine (80 mg/kg of body weight) by intraperitoneal injection.
- Inject 50 µL of buprenorphine subcutaneously as a painkiller. Remove the tendon before sacrificing the animal to keep the physiological conditions for as long as possible.
- Shave the left hind limb and place the rat under a dissecting microscope. Place the rat on lateral decubitus with the leg to be operated on in a superior position. Hold the paw with fingers.
- Make a small incision (10 mm) in the previous operation site and extend until the triceps suralis is exposed.
- To remove the healing Achilles tendon, cut the calcaneal bone transversally 5-10 mm distal to its Achilles tendon attachment. Then dissect a part of the triceps suralis, which is attached to the Achilles tendon (Figure 3b). The muscle part has to be big enough to fit in the cryo-jaw (Figure 3c-d). Place the sample immediately into the cryo-jaw using forceps (Figure 3e).
- After removing the muscle-tendon-bone complex, euthanize the rat by intracardiac injection with Nembutal (200 mg/kg). Confirm death by assessing the absence of heart rhythm and respiration for 15 min.
- Place the muscle unit into the upper jaw (Figure 3e), close it and place it vertically into a universal testing machine (106.2 kN, Figure 3a). Then fix the bone between the lower clamps of the machine (Figure 3f).
- Add liquid nitrogen into both of the upper jaw basins to freeze the muscle, so that it is an order of magnitude stiffer than the tendon and does not deform during the tensile test.
- When the freezing zone reaches the metal clamp border, start the tensile test, so the tendon will preserve its structure. Set the displacement rate of the machine at a constant speed of 1 mm/s until rupture. Record the ultimate tensile strength (UTS) given in Newtons (N) on a computer.
- To calculate the cross-sectional area, place two cameras perpendicular to each other, forming an elliptical shape, and take pictures.
- To account for the difference in the cross-sectional area of the healing tendons, normalize the UTS to a unit area (N per square millimeter), which represents the mechanical stress experienced by the tissue.
5. Histological Analysis
NOTE: 15 tendons of each group underwent histological analysis.
- After its removal, submerge the Achilles tendon immediately in 4% paraformaldehyde to preserve its structure (1 mm of tissue is fixed per hour, so the time of fixation varies depending on the size of the tendon).
- Replace the paraformaldehyde by 70% ethanol, and leave the sample for at least one night in this solution.
- Place the tendon in a little plastic container with holes and place that container in a fresh 70% ethanol solution for 1 h.
- Place the container in 95% ethanol for 1 h. Repeat.
- Place the container in 100% ethanol for 1 h. Repeat.
- Place the container in 100% xylene for 1 h. Repeat.
- Now place the plastic container, which contains the tendon, in liquid paraffin (56 °C) and leave it overnight.
- Using an embedding station (equipped with molten wax, a hot plate, and a cold plate), choose a metallic mold that best fits the tissue sample, and fill it with a little liquid paraffin so that the base is covered.
NOTE: Orientate the sample to obtain vertical sections at the microtome. - When the sample is correctly positioned, fill up the mold with liquid paraffin and place it onto the refrigerating machine. Let it cool down for at least 15 min.
- Remove from the paraffin block and place it on ice for at least 30 min.
- Prepare several clean glass slides and put some droplets of deionized water on them.
- At the microtome, cut the blocks in sections of 5 µm (only use sections from the middle part of the tendon) and place the section on a slide.
- Place the slides in a heating oven at 65 °C for several min, so that the paraffin just starts to melt to bond the tissue to the glass.
- Place the slides containing paraffin sections in a slice holder.
- Now deparaffinize and rehydrate the tissue by placing it in the following successive baths:
8 min in Xylene (Bath 1)
4 min in Xylene (Bath 2)
2 min in 100% ethanol
2 min in 95% ethanol
2 min in 70% ethanol
2 min in deionized water - For Hematoxylin-Eosin staining, place the tissue in the following solutions.
- Immerse the tissue for 8 min in hematoxylin solution (1 g hematoxylin monohydrate, 0.2 g KIO3, 50 g AIK (SO4)2.12H2O; 200 mL glycerin; adjust to 1 L with distilled water).
- Immerse the tissue for 8 min under running tap water.
- Immerse the tissue for 30 s in eosin solution (0.25% eosin in 100 mL of distilled water containing 200 µL of undiluted acetic acid).
- Immerse the tissue for 2 min under running tap water.
- For the Masson's Trichrome Staining, follow these steps.
- After rehydration, place the sections in a container with iron alum (0.5 g (NH4)Fe(SO4)2.12H2O in 10 mL H2O) and close the container. Heat it up for 3 min at 280 W in a microwave.
- Let it cool down for a minute and then rinse it with deionized water.
- Place the sections in a closed container with Regaud's hematoxylin solution (0.5 g hematoxylin dissolved in 50 mL H2O at 50 °C, then add 5 mL 95% ethanol and 5 mL glycerin), heat it up for 90 s at 280 W.
- Rinse it with deionized water.
- Place the sections in a picric acid solution (at saturation in methanol) for 3 min, rinse for 5 min under running tap water and then place it in deionized water for a few seconds.
- Now place the sections in acid fuchsin (0.5 g in 50 mL H2O containing 0.25 mL of undiluted acetic acid) for 5 s and rinse under running tap water until the dye disappears.
- Incubate the sections in phosphomolybdic acid solution (0.5 g in 50 mL H2O) for 5 min, place it for a few seconds in deionized water.
- Place the sections in light green (0.5 g in 50 mL H2O containing 0.25 mL of undiluted acetic acid) solution for another 5 min and place it under running tap water until the dye disappears.
- Now dehydrate the tissue again.
- Immerse the section for 1 min in 70% ethanol.
- Immerse the section for 1 min in 95% ethanol.
- Immerse the section for 1 min in 100% ethanol.
- Immerse the section for 1 min in Xylene (Bath 1).
- Immerse the section for 1 min in Xylene (Bath 2).
- Remove the sections without removing too much xylene and put a drop of mounting medium on the sample.
- Put a coverslip on top (without provoking any bubbles) and let it harden for at least 2 h.
- Hydrolyze 5 histological sections (each of a different tendon) of each group in 6 N HCl for 3 h and determine the concentration of collagen by measuring hydroxyproline as an index in 5 µm unstained sections.
- Normalize the results by the section area using the manufacturer's software.
- Stain some of the sections with Light Green Stain to visualize and quantify fibrillar collagen by computer assisted analysis.
- Scan the sections and convert the pictures in nuances of grey using the IrfanView software. Use the software for semi-quantification (e.g., Quantity One).
6. Molecular Evaluation
- After tendon rupture, which occurs during mechanical testing, take the samples, snap freeze them in liquid nitrogen, and store them at -80 °C (PRP and saline solution)17.
- Use a commercial Total RNA kit to isolate total RNA17.
- Measure the expression of collagen (Col I and Col III), Matrix Metalloproteinases (MMP-2, MMP-3 and MMP-9), and tenomodulin (TNMD) by RT-PCR17,18.
- Normalize the expression levels of mRNA to the levels of 28S19.
Results
The results are expressed as the mean ± standard deviation of the mean and were compared with analysis of variance (ANOVA). A two-way ANOVA and post hoc test de Scheffé, which is a parametric test, were used.
The ultimate tensile strength (UTS) required to provoke a rupture of the non-injured Achilles tendons of rats was 42.0 ± 5.7 N (n = 10). The tensile strength increased significantly (p<0.0001) in both groups after day 5. Comparing both groups, the UTS was higher in the PRP group at any time measured, especially at day 15 and 30. By measuring the cross-sectional area of the tendons that were removed before undergoing biomechanical evaluation (11.4 ± 5.5 mm2 for non-injured tendons), it was found that at day 5, it was larger in the saline treated group. But at day 15 and 30, the cross-sectional area was larger in the PRP group, although this quantity was more variable when compared to the saline treated group (Figure 4 and Figure 5).
The histological evaluation of the tendons, using hematoxylin-eosin staining, showed a high cellularity at day 5, which decreased afterwards (no significant difference was observed between the saline solution and PRP group). The Masson's Trichrome staining showed a higher presence of fibrillar collagen in the PRP group at day 5 and 15. However the intensity of the staining was similar in both groups 30 days after injection (Figure 6). The semi-quantification obtained by staining the samples with Light Green showed that at day 5 and 15, the intensity was higher in the PRP group (not statistically significant) but at day 30, there was no difference observed between the two groups.
The data was normalized by measuring the amount of an amino acid, hydroxyproline, which is specific to collagen, confirming the histological semi-quantification (day 5: larger value in PRP group). The collagen concentration was not stable in the PRP group but was stabilized at day 30 in the two groups. The volume of the callus was measured and was found to be significantly larger in PRP-treated animals during the first stages of the healing process. Taken together, these results imply that the PRP injection into the injured tendon causes an important amount of fibrillar collagen to deposit.
Measuring the expression level of several molecules showed that in non-injured tendons, Col III and TNMD were present in a lesser amount (2.5-3.0 times) than in healing tendons at day 15, however there was no difference in Col I expression between the groups. MMPs were present at a 12-fold higher concentration in the healing tendons. In addition, at day 30, there was a significant increase of COL1A1 in the PRP group, and a positive correlation between COL1A1 expression and the UTS was found. In both groups, it was found that Col III was expressed in a high quantity from day 1 until day 14, before it decreased (same for both groups). MMP-9 concentration did not change in both groups but MMP-2 and MMP-3 were present at higher concentrations during the healing period. At day 5, TNDM was expressed in a higher amount in the PRP (p<0.03) but then decreased between day 15 and 30.

Figure 1: Experimental design of the study. Please click here to view a larger version of this figure.

Figure 2: Surgical Procedure.
(a) The tendon complex after removal of the surrounding fascia.
(b) Removal of the plantaris tendon.
(c) Removal of a section of the Achilles tendon.
(d) The 2 tendons that have been removed. Please click here to view a larger version of this figure.

Figure 3: Biomechanical Testing
(a) Universal testing machine (106.2 kN).
(b) The muscle-tendon-bone complex that will be fixed into the cryo-jaw.
(c) Upper and lower cryo jaw
(d) Upper and lower cryo-jaw
(e) The complex put into the cryo jaw
(f) The closed cryo-jaw containing the muscle-tendon-bone complex fixed into the machine. Please click here to view a larger version of this figure.

Figure 4: Biomechanical Testing Results
Tensile strength expressed in Newtons (N) in control and PRP groups at the different time points after surgery. There was an increase of UTS in both groups over time, with the PRP group showing significantly higher values at 15 and 30 days after surgery.
Error bar defines the standard deviation (SD). Please click here to view a larger version of this figure.
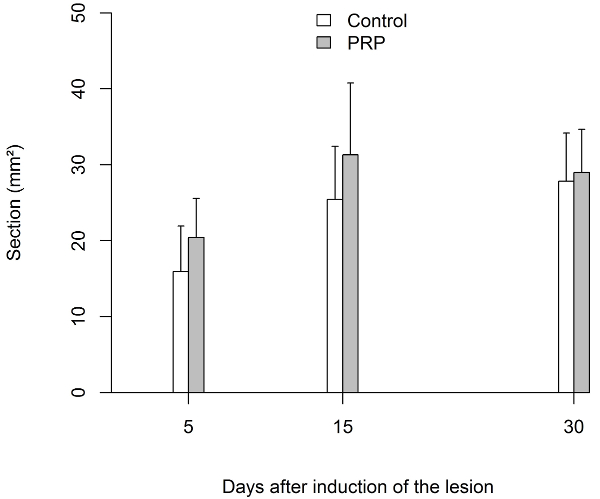
Figure 5: Biomechanical Testing Results
Transverse area of the tendon expressed in square millimeters (mm2). The cross-sectional area was significantly greater in the PRP group until day 15. Afterwards, the section was similar in both groups.
Error bar defines the standard deviation (SD). Please click here to view a larger version of this figure.

Figure 6: Histological results
Representative longitudinal sections of the Achilles tendon from control and PRP groups, stained with Masson's Trichrome. Scale bar = 100 µm. There is a stronger green staining in the tendon of the PRP group at day 5. Please click here to view a larger version of this figure.
Discussion
Platelets are essential for the early inflammatory phase of the tendon healing process. When these platelets are exposed to binding tissue or factors that induce coagulation, they will release growth factors that are stocked in α granules. Due to this interaction, extracellular matrix macromolecules are synthetized and mesenchymal cells proliferate. Platelets also have a chemotactic activity on progenitor cells in the blood circulation, enhancing angiogenesis and stimulating cellular differentiation6,20.
Biomechanical testing was done using a clamping device specially made for ex vivo tensile testing of the Achilles tendon in rats15. The cryo-jaw allows the freezing of the muscle by adding liquid nitrogen, and the calcaneal bone, which is the lower part of the specimen, is directly fixed into the machine. It is very important to start the tensile test when the muscle is completely rigid, but the tendon is still flexible. This technique avoids tissue damage and preserves the mechanical integrity. Also this technique is simple, secure, non-compressive, and demonstrably reproducible, as several studies have used this method4,15.
To support the mechanical results, some tendons underwent histological evaluation. The hematoxylin-eosin staining shows a great overview of the healed tissue and gives general information about the quantity of cells and collagen present. Also, the measurement of hydroxyprolin is useful as it is an amino acid only found in collagen and allows the objectivation of the histological data. However, it is necessary to do a Masson's Trichrome staining, as it shows much more detailed information about the deposition and concentration of collagen fibers4.
Although the level of collagen III mRNA in the PRP treated tendons was not altered, at day 30 mRNA of Col I was present in a higher concentration in the injured tendons treated with PRP. Previously it had been shown that PRP interferes with the proliferation of macrophages and the production of IL-121, which thus could avoid an excessive inflammatory reaction during the early stages of the healing process11. It is possible that PRP stimulates the proliferation, the activation of metabolic pathways, and the transformation of mesenchymal cells into tenocytes that are active.
High concentrations of TNDM in the tendons of the PRP group indicate that the injected molecules could attract cells circulating in the blood stream and induce a differentiation toward the tenocyte phenotype11. Taken together, these results demonstrate that only one injection of PRP into a ruptured Achilles tendon has a positive effect on the early healing phase and leads to a higher mechanical strength.
Several pre-clinical studies have already shown that PRP improves the healing process, and that the different growth factors have specific actions during this process22. Mazzocca and his team demonstrated that PRP stimulates cell proliferation in muscles, bones, and tendons. Out of different preparations, strongly concentrated PRP without any white blood cells proved to be the most effective treatment23. McCarrell et al. did a similar experiment, testing several preparations of PRP containing different concentrations of PRP and white blood cells on horse tendons. Preparations containing an intermediate concentration of platelets and a high concentration of white blood cells led to a higher release of pro-inflammatory cytokines, such as IL-1ß and TNFa, and lower collagen synthesis. Mixtures with a high concentration of platelets and white blood cells also led to an increase of inflammatory cytokines but inhibited collagen synthesis. In conclusion this means that if the platelet concentration is too high, collagen synthesis and cell metabolism are slowed down24. Boswell et al. confirmed these findings25. The most efficient PRP preparation would thus contain a platelet concentration lower than 106 platelets/µL and no white blood cells.
A major advantage of using PRP is autogenity. Although in our study, we used allogeneic PRP by sacrificing donor rats to have a sufficient amount of blood. Furthermore, taking blood from the operated rats would weaken them too much. Another limitation of this study is that all the ruptures are acute and performed on healthy tendons, which is not always the case in humans, as the tendons often rupture because of prior degeneration. This model being based on sharp tendon injuries, no definitive conclusions can be drawn for degenerative tendinopathies.
Using this method, there are some critical steps to remember, the first one being the preparation of PRP, which should be as reproducible as possible. Another critical step is the surgical procedure: the removal of the tendon and muscle-tendon complex should be done in a reproducible way to avoid any bias. Lastly, the preparation for biomechanical testing: liquid nitrogen is added to freeze the muscle, and it is quite important that the tendon is not frozen in this process because it could lead to biased results, as the tendon stiffness would be altered. This is also a limitation of the study, since there is no standardized protocol to ensure that the tendon's elasticity is not modified.
We based our method on the method developed by Virchenko et al. 26, but adapted it using the cryo-jaw which protects the tendon against exterior aggressions induced by clamps. The major advantage of this method is that it is reproducible even if it is not yet standardized. It gives an idea about how tendon injuries could be treated in humans, though experiments with rats don't always translate well to treating human injuries. It is likely that an adapted version of this method can be useful in treating those injuries in the future, supported by the fact that it's easy to use, has a relatively low cost, and is less invasive than other methods.
Disclosures
No conflicts of interest declared.
Acknowledgements
This study was supported by Standard de Liège and Lejeune- Lechien grants of the Leon Frédéricq Funds.
Materials
| Name | Company | Catalog Number | Comments |
| Xylazine (Xyl-M) | VMD | none | anesthetic |
| Ketamin (Jétamine 1000 CEVA) | CEVA Santé Animale | none | anesthetic |
| Buprenorphin (Vetergésic Multidosis) | ALSTOE | none | Painkiller |
| iso-Betadine | MEDA-Pharma | none | Desinfectant |
| resorbable yarn Vicryl 6/0 | Johnson & Johnson | ||
| Nembutal | CEVA Santé Animale | none | Anesthetic |
| Paraformaldehyde | Sigma-Aldrich | P6148 | Preserves structure of the tissue |
| Isopropanol 100% | VWR | 20,922,364 | |
| Ethanol 95% | VWR | 20,823,362 | |
| Xylene | VWR | 28973.363 | |
| Paraffin | VWR | LEIC3950.1006 | |
| Hematoxylin | Millipore | 1.15938.0025 | Colorant |
| Eosin | Millipore | 1.15935.0100 | Colorant |
| Eukitt | Sigma-Aldrich | 3989 | Mounting Medium |
| CaCl2 |
References
- Kaux, J., Degrave, N., Crielaard, J. Platelet rich plasma traitement des tendinopathies chroniques? Revue de la littérature. Platelet rich plasma treatment of chronic tendinopathies? Review of literature. J. Traumatol. du Sport. 24, 99-102 (2007).
- Anitua, E., et al. Autologous preparations rich in growth factors promote proliferation and induce VEGF and HGF production by human tendon cells in culture. J. Orthop. Res. 23, 281-286 (2005).
- Bosch, G., et al. Effects of platelet-rich plasma on the quality of repair of mechanically induced core lesions in equine superficial digital flexor tendons: A placebo-controlled experimental study. J. Orthop. Res. 28, 211-217 (2010).
- Kaux, J. F., Drion, P., Croisier, J. L., Crielaard, J. M. Tendinopathies and platelet-rich plasma (PRP): From pre-clinical experiments to therapeutic use. J. Stem Cells Regen. Med. 11, P7-P17 (2015).
- Maffulli, N., Wong, J., Almekinders, L. C. Types and epidemiology of tendinopathy. Clin. Sports Med. 22, 675-692 (2003).
- Molloy, T., Wang, Y., Murrell, G. The roles of growth factors in tendon and ligament healing. Sports Med. 33, 381-394 (2003).
- Lyras, D. N., et al. The effect of platelet-rich plasma gel in the early phase of patellar tendon healing. Arch. Orthop. Trauma Surg. 129, 1577-1582 (2009).
- Aspenberg, P., Virchenko, O. Platelet concentrate injection improves Achilles tendon repair in rats. Acta Orthop. Scand. 75, 93-99 (2004).
- Visser, L. C., et al. Growth Factor-Rich Plasma Increases Tendon Cell Proliferation and Matrix Synthesis on a Synthetic Scaffold: An In Vitro Study. Tissue Eng. Part A. 16, 1021-1029 (2010).
- Zhang, J., Wang, J. H. -. C. Platelet-Rich Plasma Releasate Promotes Differentiation of Tendon Stem Cells Into Active Tenocytes. Am. J. Sports Med. 38, 2477-2486 (2010).
- Kajikawa, Y., et al. Platelet-rich plasma enhances the initial mobilization of circulation-derived cells for tendon healing. J. Cell. Physiol. 215, 837-845 (2008).
- Pasternak, B., Aspenberg, P. Metalloproteinases and their inhibitors-diagnostic and therapeutic opportunities in orthopedics. Acta Orthop. 80, 693-703 (2009).
- Dell, R. B., Holleran, S., Ramakrishnan, R. Sample size determination. ILAR J. 43, 207-213 (2002).
- Mähler Convenor, M., et al. FELASA recommendations for the health monitoring of mouse, rat, hamster, guinea pig and rabbit colonies in breeding and experimental units. Lab. Anim. 48, 178-192 (2014).
- Kaux, J. -. F., et al. Étude comparative de cinq techniques de préparation plaquettaire (platelet-rich plasma). Pathol. Biol. 59, 157-160 (2011).
- Wieloch, P., Buchmann, G., Roth, W., Rickert, M. A cryo-jaw designed for in vitro tensile testing of the healing Achilles tendons in rats. J. Biomech. 37, 1719-1722 (2004).
- Kaux, J. -. F., et al. Vascular Endothelial Growth Factor-111 (VEGF-111) and tendon healing: preliminary results in a rat model of tendon injury. Muscles. Ligaments Tendons J. 4, 24-28 (2014).
- Docheva, D., Hunziker, E. B., Fässler, R., Brandau, O. Tenomodulin is necessary for tenocyte proliferation and tendon maturation. Mol. Cell. Biol. 25, 699-705 (2005).
- Lambert, C. A., Colige, A. C., Munaut, C., Lapière, C. M., Nusgens, B. V. Distinct pathways in the over-expression of matrix metalloproteinases in human fibroblasts by relaxation of mechanical tension. Matrix Biol. 20, 397-408 (2001).
- Nurden, A. T., Nurden, P., Sanchez, M., Andia, I., Anitua, E. Platelets and wound healing. Front. Biosci. 13, 3532-3548 (2008).
- Woodall, J., Tucci, M., Mishra, A., Benghuzzi, H. Cellular effects of platelet rich plasma: a study on HL-60 macrophage-like cells. Biomed. Sci. Instrum. 43, 266-271 (2007).
- Taylor, D. W., Petrera, M., Hendry, M., Theodoropoulos, J. S. A systematic review of the use of platelet-rich plasma in sports medicine as a new treatment for tendon and ligament injuries. Clin. J. Sport Med. 21, 344-352 (2011).
- Mazzocca, A. D., et al. The positive effects of different platelet-rich plasma methods on human muscle, bone, and tendon cells. Am. J. Sports Med. 40, 1742-1749 (2012).
- McCarrel, T. M., Minas, T., Fortier, L. A. Optimization of leukocyte concentration in platelet-rich plasma for the treatment of tendinopathy. J. Bone Joint Surg. Am. 94 (1-8), e143 (2012).
- Boswell, S. G., et al. Increasing platelet concentrations in leukocyte-reduced platelet-rich plasma decrease collagen gene synthesis in tendons. Am. J. Sports Med. 42, 42-49 (2014).
- Virchenko, O., Aspenberg, P. How can one platelet injection after tendon injury lead to a stronger tendon after 4 weeks?: Interplay between early regeneration and mechanical stimulation. Acta Orthop. 77, 806-812 (2006).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved