Method Article
Murine Left Anterior Descending (LAD) Coronary Artery Ligation: An Improved and Simplified Model for Myocardial Infarction
In This Article
Summary
We provide a reliable method for left anterior descending artery (LAD) ligation in a mouse model. This method is comparatively less invasive than other methods, involving endotracheal intubation, a left-sided thoracotomy approach, and thoracentesis. This method can be used as a model for both acute and chronic myocardial infarction (MI).
Abstract
Ischemic heart disease (IHD), or acute coronary syndrome (ACS), is one of the leading causes of death in the United States. IHD is characterized by reduced blood supply to the heart, resulting in the loss of oxygen to and the ensuing necrosis of the heart muscle. The MI model has gained popularity for its use as a short-term ischemia-reperfusion model and a long-term permanent ligation model. Below, we describe a reliable method for the permanent ligation of the LAD. With mouse genetic engineering technology becoming more advanced, and with an increasing availability of quality murine surgical instruments, the mouse has become a popular model for MI surgeries. Our surgical model incorporates the use of an easily reversible anesthetic for the rapid recovery of the mouse; a minimally invasive endotracheal intubation without involving a tracheotomy; and a thoracentesis through the original thoracotomy site without creating an additional incision in the chest, as is done in some other methods, to effectively remove excess blood and air from the chest cavity. This method is comparatively less invasive than other methods, which dramatically reduces surgical and post-surgical complications and mortality and improves reproducibility.
Introduction
Coronary disease, or ACS, is the most prevalent cardiovascular event and will be considered the main cause of morbidity and mortality worldwide in 20201. The cause of ACS is the presence of a myocardial thrombosis due to the rupture of a coronary atherosclerotic plaque that blocks or reduces blood flow to the heart tissue2. Therefore, there are clinical signs consistent with the presence of acute myocardial ischemia, such as myocardial infarction (MI)3,4. MI leads to a loss in mass of the cardiomyocytes and a progression to pathological ventricular remodeling, which can lead to ventricular dysfunction and heart failure5,6.
One of the most effective ways to study IHD has been to mimic human myocardial infarction in an animal model. This is achieved by occluding the LAD in mice. Using this model, we study how the heart can be protected from the damage resulting from IHD.
Over the last decade, researchers have shifted from using larger animal models to smaller animals, including the shift from rats to mice. The smaller mouse model is starting to be preferred for many reasons, including their small size, large litter size, low cost to maintain, and short gestation period, as well as for the expansive availability of transgenic and gene knockout models7. Although mice are small in size, new surgical instruments specifically designed for them have aided in this development. Our method utilizes these new surgical instruments.
While several methods implement an invasive tracheotomy, we use a less invasive method of endotracheal intubation. Using overhead illumination of the oropharynx, we intubate without creating any incisions, providing a safer and less traumatic experience for the animal. The mouse is then placed on a ventilator and kept on isoflurane during the entire procedure. Due to the short duration of anesthesia produced by the drug, it only takes a few minutes for the animal to recover from the anesthetic once it is discontinued. Our surgical model also includes a minimally invasive thoracentesis. The careful removal of blood and excess air from the chest cavity using thoracentesis through the original thoracotomy incision has addressed a common post-operative complication of the LAD ligation: the tension pneumothorax. This method, which eliminates the need for the two additional incisions used in other methods-one for the tracheotomy and another for the thoracentesis-has yielded fewer post-surgical complications and has drastically reduced mortality.
Protocol
This animal protocol has been reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) at Rhode Island Hospital.
1. Anesthesia and Intubation
- Weigh the mouse to calculate the dosage of post-operative pain medication.
- Place the mouse in an induction chamber and deliver 4% isoflurane for 9 - 10 min, monitoring the animal throughout. Turn on a hot bead sterilizer so that the apparatus can preheat to approximately 250 °C. Preheating will take 15 - 20 min.
- Once the mouse reaches a deep plane of anesthesia, with a breathing rate of approximately 32 breaths/min, place the mouse supine on a Styrofoam board and use an elastic band secured under the top incisors to hold the mouth open. Confirm sedation by performing a toe pinch. Position a high-intensity illuminator above the mouse so that the oropharynx can be visualized.
- Use curved forceps to open the jaw and another pair of forceps to lift the tongue out of the way. Be sure to intubate while positioned at or slightly below eye-level with the body of the mouse. The use of surgical loupes is recommended.
- Visualize the opening and closing of the vocal cords. When open, insert a 20-gauge, 1-in intravenous (IV) catheter with a blunt-tip needle introducer. Use the needle to guide the catheter to the tracheal opening, but avoid inserting the needle into the trachea. Verification of correct placement can be done using a plastic transfer pipette.
- Transfer the intubated mouse to an operating surface equipped with a heating device. Connect the mouse to a small rodent ventilator set to a stroke volume of 150 µL/stroke and a stroke rate of 130 strokes/min.
- Deliver 2.5% isoflurane. Verify the intubation by checking for bilateral chest rise. Verify anesthesia by performing a toe pinch. The mouse may need 5 - 10 min on the ventilator to become fully anesthetized.
2. Preparing the Mouse
- Tape down the intubation tube at the connecting site between the ventilator and the IV catheter. Tape down the extremities. Place sterile lubricating drops on the eyes.
- Trim the ventral left side of the thorax with an electric razor. Dust off the shaved fur with dry wipes and apply a small layer of hair removal cream using a sterile cotton swab. The cream should remain in contact with the hair follicles for approximately 30 - 45 s.
- While the cream processes, place three sterile cotton swabs in three 1.5-mL tubes filled with Betadine to soak. Using wipes moistened with distilled water, gently wipe away the cream and fur.
- Clean the surgical field three times, alternating Betadine and sterile 70% isopropanol prep pads, cleaning in a circular motion moving from center to periphery. Place a sterile drape with a quarter-sized hole over the surgical field of the mouse.
- Clean the area surrounding the mouse with 70% ethanol. Verify anesthesia once more with a toe pinch.
3. LAD Ligation
- Place the autoclaved surgical instruments in hot bead sterilizer preheated to 250 °C for approximately 20 s. Place the sterilized instruments on sterile autoclaved surgical drape. Don surgical gloves.
- Use fine-tip forceps to gently lift the skin at a point approximately 5 mm to the left of the prominent xiphoid cartilage. Use a surgical scalpel with a No. 10 blade to create a vertical incision in the skin from this point upwards, to the level of the manubrium.
- Use curved forceps to gently separate the skin and muscle layers. Open the muscle layer, following the skin incision. Insert two 5-0 polypropylene sutures through the muscle layer, one on either side of the incision, and secure the sutures temporarily with clamps to hold the muscle layer open.
- Identify and make an incision in the third intercostal space, following the natural angle of the ribcage. Remove the tape from the left extremities of the mouse and secure its left rear foot to its right rear foot with tape. Cut a longer piece of tape and secure its left front foot to the operating surface in a slightly elevated position. Clean the gloves with 70% ethanol.
- Use a retractor to gently spread apart the 3rd and 4th ribs. Cut a small section of sterile gauze, approximately 1 in x ½ in, and dip it in sterile 0.9% saline. Squeeze out the excess saline and use forceps to gently insert the gauze against the left lung to prevent accidental lung damage during the procedure.
- Gently remove the thin pericardium with forceps.
- Tear a small amount of cotton off a sterile cotton swab and roll it into a small ball. Dip this cotton ball into sterile 0.9% saline and gently swab over the surface of the heart to appreciate the arteries. Gently push the left auricle upwards and locate the coronary arteries underneath.
- Identify the LAD and pass an 8-0 nylon suture under the LAD; complete two throws to secure the ligation. If the ligation is successful, the left ventricle distal from the ligature will blanch.
- Using forceps, remove the gauze inserted earlier, and then gently remove the retractor. Insert a 6-in, 25-gauge flexible tube attached to a 25-gauge needle into the chest cavity through the thoracotomy opening. Advance approximately 1 - 2 in of tubing into the space above the left lung. Return the mouse to a supine position and clean the gloves with 70% ethanol.
- Use 5-0 polypropylene sutures in a simple interrupted pattern to close the ribcage, keeping the chest tube in place. Remove the two sutures holding the muscle layer open. Use 5-0 polypropylene sutures in a simple continuous pattern to close the muscle layer, again keeping the chest tube in place.
- Attach a 1-mL syringe to the 25-gauge needle on the chest tube. Gently pull upwards on the plunger while simultaneously gradually extracting the chest tube from the chest cavity with forceps. Extract the tubing slowly, as this step removes excess air and blood, which would otherwise become trapped in the chest cavity and result in a pneumothorax.
- Once the syringe is full, detach the syringe from the needle and dispose of the waste in a waste beaker or sink. Continue this process until the chest tube is completely extracted. Ensure that the chest is tightly sealed.
- Decrease the isoflurane to 1.5%. Close the skin with 4-0 polypropylene sutures in a simple interrupted pattern. Turn the isoflurane vaporizer off.
- Administer 0.1 mg/mL buprenorphine in 0.9% saline via an intraperitoneal (IP) injection. Topically apply 2 mg/mL Lidocaine with 2 mg/mL Bupivacaine in 0.9% saline to the incision. Administer between 200 - 500 µL of 0.9% saline via a subcutaneous injection, scaling the saline amount to the weight of the mouse.
- Wait 5 min after administering the pain medications to remove the mouse from the intubation tube. This aids in the transition off the ventilator.
- If the mouse does not have a bilateral chest rise once off the ventilator, perform needle decompression. To do this, introduce a 25-gauge sterile needle and a 1-mL syringe between the 3rd and 4th ribs until it enters the thoracic cavity, denoted by a sudden decrease in resistance. Pull up gently on the plunger to remove excess air.
- When the mouse demonstrates an adequate bilateral breathing rate and depth and responds to a toe pinch, place the mouse in a clean recovery cage under a heat lamp. Provide the mouse with moist food and a water bottle, monitoring in a laminar flow hood for 15 - 20 min. Monitor for an exaggerated breathing effort, excessive bleeding, or other potentially life-threatening complications.
- For the next three days, administer 0.1 mg/mL buprenorphine pain medication via an IP injection twice daily. Monitor the mouse daily.
Results
The mice are euthanized twenty-eight days after surgery, and the hearts are harvested and examined. The mice are anesthetized with 50 - 75 mg/kg ketamine and 5 - 10 mg/kg xylazine. When the animal is under adequate anesthesia, the thoracic cavity is opened, and using a 23-gauge needle, cold potassium chloride (KCL, 30 mM) is injected into the posterior basal region of the heart. The heart is arrested in diastole. For further validation of the ligation, the heart is removed from the animal and is injected with 4% paraformaldehyde and then 1% Evan's Blue dye. Figure 1 shows the lack of Evan's Blue in the ischemic left ventricle. Figure 2 demonstrates a proper endotracheal intubation setup. Figure 3 displays the placement of the chest tube for the thoracentesis in the initial incision site, with the muscle layer sutured closed around the tube before air extraction from the chest cavity. Trichrome staining shows an increase in collagen in the infarcted region (Figure 4).

Figure 1: Evan's Blue Injection. The Evan's Blue injection reveals a lack of dye in ischemic tissue, localized to the left ventricle infarcted region. Please click here to view a larger version of this figure.

Figure 2: Endotracheal Intubation. Demonstration of a proper endotracheal intubation setup. The operator, wearing surgical loupes, is seated at eye-level with the mouse. A high-intensity illuminator is focused downward onto the tracheal region, transilluminating the oropharynx. An elastic band is hooked behind the upper incisors, allowing the operator to open the mouth with the curved forceps. The curved forceps are used to hold the tongue to the side for clear visualization. An intravenous catheter intubation cannula with a blunt-tip needle introducer is advanced at a slight upward angle while the opening and closing of the vocal cords is visualized. Visualization of the vocal cords opening and closing before the intubation attempt is one of the critical points for a successful intubation. Please click here to view a larger version of this figure.
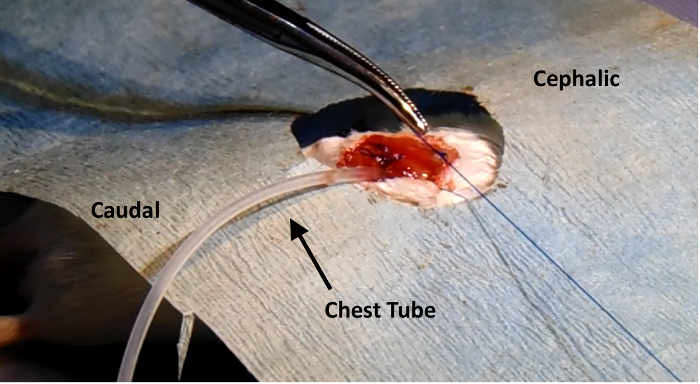
Figure 3: Thoracentesis. Placement of the chest tube used for thoracentesis, inserted in the original incision site. The muscle layer is sutured closed around the tube before the air within the chest cavity is extracted with a syringe, and then the chest tube is removed. Please click here to view a larger version of this figure.
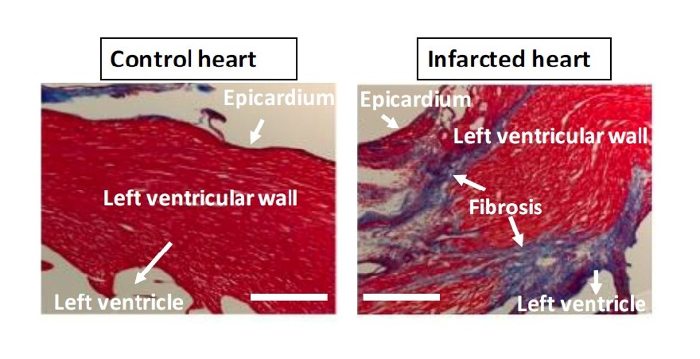
Figure 4: Trichrome Staining. Left panel: Control, non-LAD-ligated heart. Right panel: LAD-ligated, infarcted heart. Trichrome stain (Masson) employing Biebrich scarlet-acid fuchsin solution, phosphotungstic/phosphomolybdic acid solution, and aniline blue reveals increased collagen (blue) as a marker for fibrosis in the cross-sectioned left ventricle infarcted region. Bar = 500 µm. Please click here to view a larger version of this figure.
Discussion
With an increasing use of the MI model in laboratories, the described procedure seeks to increase the efficiency and survival rate of the mice while minimizing their post-operative pain and discomfort. This protocol strives to minimize mortality by making numerous improvements to various aspects of the LAD ligation procedure. There are a few distinctions. Some murine intubation studies that utilize ketamine and xylazine along with isoflurane for induction, due to the benefit of their longer duration of anesthesia, have shown increased mortality8. Our method uses only isoflurane for induction, greatly reducing the potential for drug-related complications. Another similar LAD ligation protocol includes a tracheotomy, producing an extended recovery time and an increased need for precise training9. The procedure we describe here utilizes a non-invasive method of endotracheal intubation, resulting in lower mortality and higher reproducibility of results. Also, rather than only using an electric razor, our procedure also utilizes hair removal cream for fur removal, providing a completely clear visualization of the sterile field in less than 1 min.
Another key distinction is the repositioning of the mouse, which occurs after, rather than before, the initial incisions. Making the incisions while the mouse is supine allows for a more direct and accurate visualization of landmarks such as the xiphoid cartilage, thus resulting in higher reproducibility of results. Our method also uses sterile cotton swabs rather than a cauter for bleeding management, decreasing iatrogenic burn injuries and the risk of infection. Apart from these differences, the chest tube insertion for the thoracentesis is especially of note, as our method does not involve creating a new incision for the tube. Rather, it involves inserting the tube into a previous incision, again decreasing mortality. The procedure we describe also includes: (1) the use of a retractor, enabling a more accurate and stable visualization of the coronary arteries; (2) the insertion of sterile gauze into the chest cavity during the procedure, thereby reducing the risk of iatrogenic lung injuries; and (3) the administration of saline following the procedure, which has been shown to both shorten the recovery time and prevent hypothermia.
Although we describe a permanent ligation model, this procedure can also be modified for an acute MI model. The acute MI model, also described as ischemia and reperfusion, refers to 30 - 60 min of ischemia followed by reperfusion in the heart tissue7. An alternative method to assess the infarct size or the area at risk after ischemia and reperfusion is the staining of 2% triphenyl tetrazolium chloride (TTC)10. TTC staining is based on the ability to stain viable tissue after ischemic insult due to dehydrogenases present in cardiac tissue. These enzymes convert a soluble component into an insoluble red component, thereby delineating the infarcted region11. The acute MI model can mimic the mechanisms that occur in human heart disease and thus may be a useful tool for elucidating the events of myocardial ischemia10. The LAD ligation can be verified by observing an immediate change in tissue color, providing an advantage for this method of inducing an MI. Another method to check for successful ligation is the use of electrocardiograms, although this involves the use of expensive equipment and may not be feasible for all labs.
As described above, there are several easy and affordable molecular techniques to confirm the ligation after harvesting the heart. The two techniques shown above are Evan's Blue staining and trichrome staining. Evan's Blue dye is directly injected into the arch of the aorta, indicating where there is a lack of blood flow. This is a quick and efficient method employed immediately after harvesting the heart to test whether the model was successful and to measure the extent of coronary blockage. For trichrome staining, the heart must be sectioned and then subjected to immunohistochemistry. Trichrome staining can indicate post-ischemic fibrotic areas or cardiac areas affected by chronic ischemia. The injection of post-operative mice 2 - 24 h prior to sacrifice with 5-ethynyl-2′-deoxyuridine (EdU), an analogue for thymidine, is an effective method for indicating areas of DNA replication and cell proliferation following ischemia, especially in studies involving vascular regeneration12.
In general, the limitations of the LAD ligation study include the incidence of post-operative mortality, caused mainly by the presence of cardiac arrhythmias, bleeding, and pneumothorax. An effective thoracentesis, without additional chest incisions (described in the current method), and proper post-operative care are necessary to avoid morbidity and mortality in the animals. The very careful monitoring of post-operative hypothermia is also critical. The reduction in the number of neck and chest incisions (for tracheotomy and thoracentesis) described in the current method will help improve survival rates. The avoidance of injectable pre-operative anesthetics described herein will also improve the post-operative recovery of the animals.
In order to obtain high reproducibility, the LAD ligation model requires rigorous training and experience. The operator needs to perform several weeks of surgeries to gain the ability to reproducibly make infarcts at the desired sites on the heart. Training and experience are two critical factors for a successful LAD ligation survival surgery.
Disclosures
The authors declare that they have no competing financial interests.
Acknowledgements
This model was developed with the support of the National Institute of General Medical Sciences (NIGMS)/the National Institute of Health (NIH) grant 1P20GM103652 (Project# 3) (to MRA) and the American Heart Association (AHA) Grant-in-Aid 14GRNT20460291 (to MRA); the Brazilian government grant CAPES (to KR and FR); and a Brown University LINK award (to IM). We also acknowledge the outstanding technical support from our veterinarians and animal facility staff.
Materials
| Name | Company | Catalog Number | Comments |
| High-Intensity Light Source | Harvard Apparatus | 72-0215 | |
| SurgiSuite Operating Platform | Kent Scientific Corporation | SurgiSuite | Uses a rechargeable, battery-operated far infrared warming pad. Charge overnight before surgery. |
| SurgiSuite LED Lighting Kit | Kent Scientific Corporation | SURGI-5003 | |
| Hot Bead Sterilizer | Fine Science Tools | 18000-45 | Preheating takes 15 - 20 min. Instruments take 20 s to sterilize. |
| Small Rodent Anesthesia System | VetEquip Inc. | 901810 | |
| Isofluorane | Piramal Enterprises | 66794-017-10 | |
| Buprenorphine | Rhode Island Hospital Pharmacy | NDC 12496-0757-1, 12496-0757-5 | |
| Surgical Loupes | Roboz | RS-6687 | |
| Small Rodent Ventilator | Harvard Apparatus | 73-0043 | |
| Lubricating Drops | Thermo Fisher Scientific | 19-898-350 | |
| Electric Razor | Kent Scientific Corporation | CL 9990-1201 | |
| Hair Removal Cream | Nair | ||
| Medical Tape | Thermo Fisher Scientific | 18-999-380 | |
| Betadine | Thermo Fisher Scientific | 19-027136 | |
| 70% Isopropanol Wipes | Thermo Fisher Scientific | 22-363-750 | |
| Surgical Drapes | Braintree | SP-TS | |
| Surgical Gloves | Thermo Fisher Scientific | 18999102D | |
| 5-0 Polypropylene Sutures | Ethicon | 8630G | |
| 8-0 Nylon Sutures | Fine Science Tools | 12051-08 | |
| Platinum-Cured Tubing | Harvard Apparatus | 72-1042 | 0.3 mm inside diameter x 0.6 mm outside diameter |
| 0.9% Saline | Thermo Fisher Scientific | 19-310-207 | |
| 4-0 Polypropylene Sutures | Ethicon | 8631G | |
| 1 CC Syringe with 25-Gauge Needle | Thermo Fisher Scientific | 14-826-100 | |
| Scissors | Kent Scientific Corporation | INSS600225 | |
| Forceps | Kent Scientific Corporation | INS700100 | |
| Cotton Swabs | Thermo Fisher Scientific | 23-400-118 | |
| IV Catheter, 20-Gauge | Thermo Fisher Scientific | NC9892181 | |
| Retractor | Kent Scientific Corporation | INS 750369 | |
| Forceps | Fine Science Tools | 11003-12 | |
| Dissecting Forceps, Straight | Kent Scientific Corporation | INS 700101 | |
| Dissecting Forceps, Curved | Kent Scientific Corporation | INS 700103 | |
| Hemostatic Forceps, Straight | Kent Scientific Corporation | INS 750451 | |
| Hemostatic Forceps, Curved | Kent Scientific Corporation | INS 750452 | |
| Tissue Forceps | Kent Scientific Corporation | INS 700131 | |
| Needle Holder | Kent Scientific Corporation | INS 600109 | |
| Scissors | Kent Scientific Corporation | INS 600225 |
References
- Hausenloy, D. J., Yellon, D. M. New directions for protecting the heart against ischaemia-reperfusion injury: targeting the Reperfusion Injury Salvage Kinase (RISK)-pathway. Cardiovasc Res. 61 (3), 448-460 (2004).
- Roffi, M., et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 37 (3), 267-315 (2015).
- Kumar, A., Cannon, C. P. Acute Coronary Syndromes: Diagnosis and Management, Part I. Mayo Clin Proc. 84 (10), 917-938 (2009).
- Eitan, A., Nikolsky, E. Antithrombotic therapy in patients with acute coronary syndromes: how to make the right choice. Minerva Med. 104 (4), 357-381 (2013).
- Abbate, A., Bussani, R., Amin, M. S., Vetrovec, G. W., Baldi, A. Acute myocardial infarction and heart failure: role of apoptosis. Int J Biochem Cell Biol. 38 (11), 1834-1840 (2006).
- Zheng, Z., et al. Nebivolol protects against myocardial infarction injury via stimulation of beta 3-adrenergic receptors and nitric oxide signaling. PLOS ONE. 9 (5), 98179(2014).
- Tarnavski, O., et al. Mouse cardiac surgery: comprehensive techniques for the generation of mouse models of human diseases and their application for genomic studies. Physiol Genomics. 16 (3), 349-360 (2004).
- Buitrago, S., Martin, T. E., Tetens-Woodring, J., Belicha-Villanueva, A., Wilding, G. E. Safety and Efficacy of Various Combinations of Injectable Anesthetics in BALB/c Mice. J Am Assoc Lab Anim Sci. 47 (1), 11-17 (2008).
- Kolk, M. V., et al. LAD-Ligation: A Murine Model of Myocardial Infarction. J Vis Exp. (32), e1438(2009).
- Wu, Y., Yin, X., Wijaya, C., Huang, M. H., McConnell, B. K. Acute myocardial infarction in rats. Journal of visualized experiments : J Vis Exp. (48), (2011).
- Ferrera, R., Benhabbouche, S., Bopassa, J. C., Li, B., Ovize, M. One hour reperfusion is enough to assess function and infarct size with TTC staining in Langendorff rat model. Cardiovasc Drugs Ther. 23 (4), 327-331 (2009).
- Zeng, C., et al. Evaluation of 5-ethynyl-2'-deoxyuridine staining as a sensitive and reliable method for studying cell proliferation in the adult nervous system. Brain Res. 1319, 21-32 (2010).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved