Method Article
Procedure for Human Saphenous Veins Ex Vivo Perfusion and External Reinforcement
* These authors contributed equally
In This Article
Summary
The mechanisms leading to the development of intimal hyperplasia (IH) and vein graft failure are still poorly understood. This study describes an ex vivo system to perfuse human veins under controlled flow and pressure. Furthermore the efficiency of external mesh reinforcement to limit the development of IH was evaluated.
Abstract
The mainstay of contemporary therapies for extensive occlusive arterial disease is venous bypass graft. However, its durability is threatened by intimal hyperplasia (IH) that eventually leads to vessel occlusion and graft failure. Mechanical forces, particularly low shear stress and high wall tension, are thought to initiate and to sustain these cellular and molecular changes, but their exact contribution remains to be unraveled. To selectively evaluate the role of pressure and shear stress on the biology of IH, an ex vivo perfusion system (EVPS) was created to perfuse segments of human saphenous veins under arterial regimen (high shear stress and high pressure). Further technical innovations allowed the simultaneous perfusion of two segments from the same vein, one reinforced with an external mesh. Veins were harvested using a no-touch technique and immediately transferred to the laboratory for assembly in the EVPS. One segment of the freshly isolated vein was not perfused (control, day 0). The two others segments were perfused for up to 7 days, one being completely sheltered with a 4 mm (diameter) external mesh. The pressure, flow velocity, and pulse rate were continuously monitored and adjusted to mimic the hemodynamic conditions prevailing in the femoral artery. Upon completion of the perfusion, veins were dismounted and used for histological and molecular analysis. Under ex vivo conditions, high pressure perfusion (arterial, mean = 100 mm Hg) is sufficient to generate IH and remodeling of human veins. These alterations are reduced in the presence of an external polyester mesh.
Introduction
Cardiovascular diseases are the leading cause of morbidity and mortality in Western countries1. Despite advances made in endovascular treatments, bypass surgery remains the mainstay of contemporary therapies, thus over half a million vein grafts are performed annually in the United States. However, despite decades of research, 30-60% of lower extremity vein grafts fail within the first years due to intimal hyperplasia (IH)2. Mechanical forces, particularly low shear stress (SS) and high wall tension, are pivotal in the initiation and development of this hyperplastic response3,4. To address this issue, an ex vivo veins perfusion system (EVPS) was generated to study, under strictly controlled hemodynamic conditions (pressure and shear stress), the behavior of human saphenous veins. In this study, following insertion into the arterial-like circulation, high pressure (mean = 100 mm Hg) was sufficient to stimulate proliferation and migration of smooth muscle cells into the intimal layer (IH)5.
Mammalian studies have suggested the use of external reinforcement as an efficient method to support the “arterialized vein” and counteract the acute hemodynamic changes the vein faces once implanted into an arterial milieu. The mesh prevented over-distension, increased shear stress, and reduced wall tension and consequently IH6-10. However, the underlying mechanisms and its applicability to human veins in improving bypass patency have not been fully characterized. Our EVPS was used to compare, in condition mimicking the alterations a vein faces once inserted into an arterial regimen (high shear stress and pressure), the behavior of human saphenous veins in the absence and presence of an external macroporous polyester tubular mesh. By preventing pathological remodeling and IH, the mesh provided evidence of its potential clinical efficiency11.
This study 1) introduces a model of ex vivo human saphenous veins perfusion under controlled pressure and shear stress 2) demonstrates that external macro-porous polyester mesh reduces IH and provides crucial information for its potential clinical application.
Protocol
The Ethical Committee of the University of Lausanne approved the experiments, which are in accordance with the principles outlined in the Declaration of Helsinki of 1975, as revised in 1983 for the use of human tissues.
1. Human Great Saphenous Vein Harvest
- Obtain surplus segments of non-varicose human saphenous veins from patients undergoing lower limb bypass surgery for ischemia. In the operating room, disinfect the entire leg with an iodine solution and drape the patient to expose the leg from the groin to the foot.
- Make a median incision from the groin to the knee (leaving the interrupted skin portion).
- Harvest the great saphenous vein with a pedicle of surrounding tissue (no-touch technique). Secure side branches of the veins with 4-0 silk ties. Immediately store a minimum of 9 cm long surplus segment of the greater saphenous vein, with an external diameter of 2.5-4 mm at 4 °C in a RPMI-1640 Glutamax medium, supplemented with 12.5% fetal calf serum and bring it to the laboratory.
2. EVPS Design
- Assemble the general equipment shown in Figure 1. Autoclave all equipment and keep all components under sterile conditions. In addition, ensure that the system is waterproof and does not leak chemicals into the medium. Use polymethacrylate methyl (PMMA-GS) for the cover. Steel (X5 Cr Ni 18 10) and polyoxymethylene plastic (POM) as the vein support.
- Design the perfusion chamber to the desired geometry to allow the placement of the vein and its connection. Make sure the depth (or radius if using cylindrical construction) is at least 2.5 cm so it allows minimal flexion and dilatation of the vessel along with constant coverage by the culture media (Figure 1). Sealing is a major issue and is the reason rectangular PMMA-GS construction is used.
- Design the vein support to the desired geometry. To avoid vein kinking or over distension, allow length adjustment by pushing or pulling (screw cannot be used to that purpose, as the vein would be twisted along with the screw).
NOTE: A full steel rod connected by 2 sliding L-shaped pieces that support the 2 vein cylinders (5 mm diameter to fit the vessel) and the vein (Figure 1B and Figure 2) is used here. - Design the pressure column, such that the “resting pressure” applied to the system is: p = 0-10 = h x ρ x g, where p = pressure (N/m2, Pa) h = height of fluid column (m) ρ = density of liquid (kg/m3) and g = the gravitational constant (9.81 m/sec2). Design four connection ducts, from top to bottom: to apply pressure, for the outflow (from the vein), the inflow (to the vein) and to allow medium change.
- Prepare the medium. Based on previous studies5,11-14, choose RPMI-1640, supplemented with Glutamax, 12.5% fetal calf serum, and 1% antibiotic-antimycotic solution (10,000 U/ml penicillin G, plus 10 mg/ml streptomycin sulfate, plus 25 mg/ml amphotericin B, plus 0.5 μg/ml: gentamycin). Shear stress (SS) is given by SS= 4 μQ/π*r3Q is the flow rate (ml/sec), r the radius (cm) of the vein segment, and μ is the viscosity of the perfusion medium.
- Modulate SS by adjusting the viscosity through addition of 70 kDa dextran. Measure the viscosity with a viscometer. Here, add 8% 70 kDa dextran to set SS to 9-15 dyn/cm2.
- Set the gearing pump to induce a pulsatile cardioid signal of 60 pulses/min and constant amplitude generating a unidirectional flow of 150 ± 15 ml/min, independent from the pressure applied in the system and controlled by a computer. Ensure that the driving software integrates constant acquisition and monitoring of pressures, flow velocity, pulse rate, and signal. If desired, use a second pump (non synchronized) to produce a non-laminar, turbulent flow.
3. EVPS Assembly (Figure 1)
- Before starting, make sure all the equipment is sterile. Perform all the following steps under asepsis in a laminar flow hood.
- Place the vein in a Petri dish filled with medium. Use a surgical blade and divide the vein into 3 equal segments.
- Immediately rinse one segment in PBS. Divide the segment in 3 parts, fix one in formalin for morphometry. Freeze the other two for quantitative transcript (RT-PCR) and protein (western blot) analysis. Consider these segments as a control, non-perfused vein.
- Use the 2 remaining segments for perfusion.
- Very gently inject medium into the vein and determine the normal flow direction; in presence of valves the vein is reversed.
- Sealing the veins is of utmost importance to experimental success. Check for leaks through collaterals. Secure any leaks with 6-0 silk sutures.
- Connect the vein segment between the two metallic cylinders, one end at a time (2.3, Figure 1). Secure the cylinders with Ethibon 3-0 around the indentations (Figure 1A and B).
- Place the entire venous segment into the perfusion chamber previously filled with medium. Repeat the same procedure for the second segment.
NOTE: Failure to properly seal the vein to the cylinder will be a source of leak, require reintervention, and significantly increase the risk of infection and experimental failure.
- Place the entire venous segment into the perfusion chamber previously filled with medium. Repeat the same procedure for the second segment.
- To reinforce (mesh) the second segment, release the two cylinders (with the vein attached) from the L-shaped pieces (2.3 and Figure 1).
- Be gentle and do not touch the vein with any instruments. Slide the mesh first on the cylinder then onto the vein. A push/pull jostling will get the mesh on the vein.
- Once the mesh covers the entire surface of the vein secure the jacketed vein to the cylinders with Ethibon 3-0.
- Reassemble the vein/cylinder compound to the L-shaped support and transfer it to the perfusion chamber, previously filled with medium.
- Connect each metallic cylinder (in-and outflow) to a Y-splitter using peroxide-treated silicone tubing with an internal diameter of 3.2 mm.
- Connect the outflow splitter to a second Y-splitter using the same type of tubing. From this Y-splitter, use one tube to measure the perfusion pressure through both vessels. Connect the other one back to the column to form a closed loop system (Figure 2).
- Inside the incubator, use a long (one-meter length) tube to connect the pressure column to the pump head.
- Complete the set up by connecting the pump head to the inflow Y-splitter with another long length tube (Figure 1).
4. Veins Perfusion
- After the EVPS assembly has been completed, fill the column with medium (stay below the vein outflow duct to allow refilling). Add more medium into the column until the system is full. Move all the system into the incubator maintained at 37 ± 0.1 °C with a pH kept constant at 7.40 ± 0.01 (using a CO2/pH algorithm based on the Henderson-Hasselbach equation).
- Bring the gear pump head outside the incubator and connect it to the gear pump drive. Screw the rods to secure the assembly.
- Switch on the pump power, make sure it is activated on the driving software and allow 5 min for the medium to be equally distributed in every compartment.
- To monitor the pressure, use an arterial line monitoring. Connect the EVPS pressure output (it corresponds to the arterial catheter) to the pressure transducer linked to the computer.
- Make sure the tube is entirely filled with medium and does not contain any bubbles. De-bubble the culture system through the “arterial line” tube (Figure 2). Pay attention to the display and look for a pulsatile cardioid signal of 60 pulses/min of constant amplitude. At this point, the average pressure is between 0-10 mm Hg. If the pressure is < 0 and the column progressively empties look for a leak (vein collateral or inadequate seal between the vein and the tube).
- Set the minimal pressure to 6 mm Hg for a venous test or at 90 mm Hg for an arterial test. Under these conditions, an air injector applies the required pressure to the column and system.
- Change the medium every 2 days by using the tube connected to the pressure column. To prevent pressure change damage, open the column plug first.
5. Completion of the Perfusion
- After 3 or 7 days of perfusion: take the EVPS out of the incubator and dismount the veins. Discard the 5 mm proximal and distal vein ends attached to the equipment. Cut a central, 5 mm thick rings from the remaining segment and fix in formalin (morphometry). Freeze the remaining fragments and reduce into powder for further molecular analyses.
Results
The EVPS provides a valuable tool to independently assess the hemodynamic forces on human saphenous vein grafts remodeling and IH.
Figure 1 shows the perfusion chamber and the vein support. In Figures 1A and B, the vein support before (Figure 1A) and after (Figure 1B) assembly, respectively, is pictured. It is composed (from the top to the bottom) of 1 plain stainless steel tube measuring 9 cm, which serves as a support for 2 L-shaped pieces that can easily slide (from the left to the right) and provides a reliable technique to adjust the support size to the vein. Each of these pieces holds a POM disc to fit a steel cylinder (vein connector) fixed in place by integrated screw (arrowhead). Figure 1C-D shows the perfusion chamber alone (C) and after insertion of the vein support (D). On the perfusion chamber, depressions are designed to hold the vein support in place (top) and to avoid kinking of the connecting tube coming in and out from the vein (bottom).
Figure 2 shows real time pictures (Figure 2A) and a schematic representation (Figure 2B) of the EVPS. The perfusion chamber, veins and its supports, as well as the pressure column, are maintained in a controlled environment (temperature, CO2 and O2) whereas the pump, pressure injector, and control devices all remain outside the incubator. The figure illustrates the gearing pump (1) that generates a pulsatile signal controlled by a computer (2), which monitors the flow velocity (3), pressure (4), and controls the minimal diastolic pressure (5); two segments of a same saphenous vein are connected in parallel to the perfusion pump inside separate perfusion chambers (6a and 6b) placed in a cell culture incubator.
In Figure 3, histomorphometric analysis shows that external reinforcement prevents intimal hyperplasia and pathological media remodeling otherwise observed after 7 days under high pressure (arterial regimen, mean = 100 mm Hg) perfusion. In Figure 3A, representative histological sections stained for Hematoxylin-eosin (HE) reveals the lining of the lumen by nuclei of endothelial cells and the nuclei of SMCs in the media layer in all conditions. Figures 3B-C shows representative Van Gieson Elastic Lamina (VGEL) stained sections. In Figure 3B, the intima is thickened (IH) in veins perfused at high pressure (mean = 100 mm Hg) for 7 days compared to control non-perfused veins, a phenomenon largely decreased in the presence of an external mesh. Figure 3C illustrates the pathological outward remodeling and media thinning in veins subjected to 7 days of arterial pressure. This is largely prevented by the external reinforcement. Furthermore, in Figure 3D, Masson’s trichrome staining (blue=connective tissue, red=muscle) associates this pathological remodeling with the persistence of only one of the three muscle layers and accumulation of smooth muscle cells in the inner layer (intima). The external reinforcement preserves the distribution of the SMCs and media structure.
Figure 4 illustrates a current clinical application of the external mesh. An illustrative example is provided by external reinforcement of an aneurysmal arterio-venous fistula (hemodialysis access). Figure 4A shows a time-course representation of the vein reinforcement (from the top to the bottom). First, the mesh was placed around a rigid tube while the vein’s extremity is fixed to a mandrel (upper panel). Then, the vein was pulled through the tube thanks to the mandrel. Once the vein was in place, the tube was slowly retracted, leaving the mesh around the vein (upper and middle panel). In this particular case, the procedure was repeated on both sides of the veins, and the veins segments and mesh reinforcements were assembled by an end-to-end anastomosis (lower panel). Figure 4B provides a larger view of the arterio-venous end-to-side anastomosis, showing that the mesh is wrapped around the anastomosis by tying it along the posterior wall of the artery.

Figure 1. The vein support and perfusion chamber. A. The vein support is composed of 1 plain tube, 2 L-shaped pieces, discs and cylinders (form the top to the bottom). B. The vein support once assembled. C. View of the perfusion chamber. D. The perfusion chamber is designed to hold in place the vein support and allows its connection to the vein and connecting tubes.

Figure 2. The ex vivo perfusion system. A. The completely assemble EVPS in the cell-culture incubator. B. Schematic representation. 1) the pump generating a pulsatile cardioid wave; 2) the computer controlling the pressure, flow (type, rate and amplitude); 3) the flow meter; 4) the pressure transducer – arterial line; 5) the pressure injector; 6) two segments of a very same saphenous vein are perfused without (6a) or with external mesh reinforcement (6b).

Figure 3. The external reinforcement prevents intimal hyperplasia. A. Representative histological sections stained with Hematoxylin-eosin (HE). Bar represents 50 µm. B-C. Representative histological sections stained for elastin (VGEL). l = lumen m = media, IH = intimal hyperplasia. Bar represents 50 µm. D. Representative histological sections stained with Masson’s trichrome. Bar represents 50 µm.
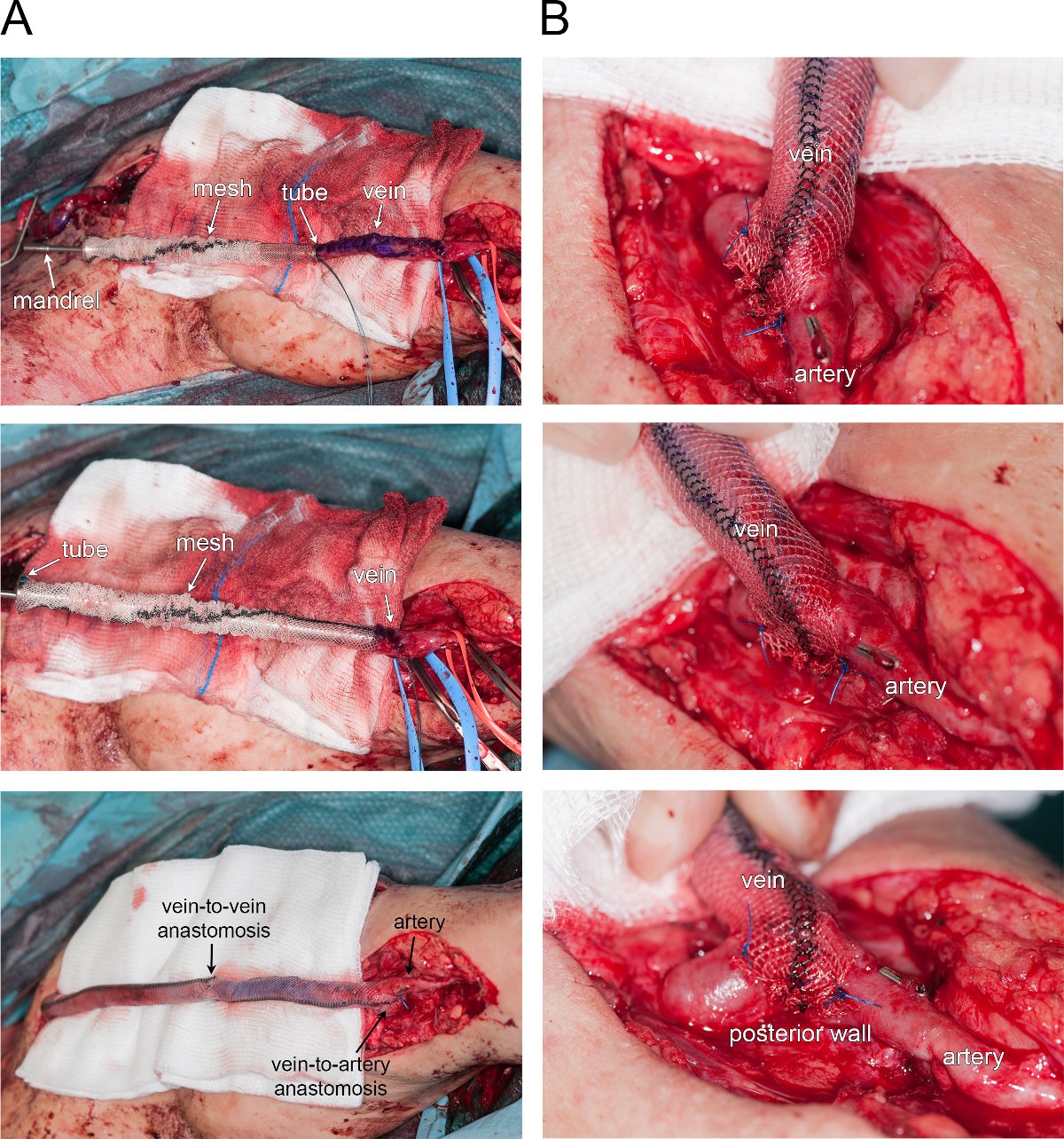
Figure 4. External reinforcement of an aneurysmal fistula. A. Time-course photographs of vein reinforcement (from top to bottom). B. Larger view of the arterio-venous end-to-side anastomosis.
Discussion
This study uncovers an ex vivo vein perfusion system (EVPS) to perform extensive hemodynamic studies in human veins. This system allows saphenous veins perfusion under defined hemodynamic parameters in the absence of aggravating inflammatory and growth factors released by circulating cells in vivo. Thus, it provides a better understanding of the underlying pathways involved in the control of IH in human veins grafts5,11,12,15.
Reproducible and quantifiable hemodynamic perturbations are limited in vivo. Several complex murine microsurgical procedures have been described. Using a bypass isograft model via interposition of a vena cava from a donor mouse into the right common carotid artery and the additional creation of an outflow branch ligation, mid graft or common carotid stenosis, flow and SS acutely decreased and enhanced IH3. Outward versus inward remodeling can further be interrogated using a mid-focal versus distal common carotid stenosis16. In large animals (sheep, pig, and baboon), bypass grafts are technically easier and represent an attractive approach to test pre-clinical human sized devices such as the mesh used in the present study8-10. However, its cost and the paucity of validated molecular tools limit the use of these strategies. Finally, these flow manipulations constantly alter wall tension and fail to interrogate one single constituent. In addition, the intricate relationships between the hemodynamics and the immune and endocrine systems further limit the analysis of a single actor.
Several issues arise with the use of the EVPS. 1) Low-grade bacterial contamination often accompanied human vein harvest and the ex-vivo absence of circulating cells stand as an important cause of infection. This is mainly prevented by hand washing the pieces separately then autoclaving all material prior to use. Furthermore the assembly is performed in less than 90 minutes and under rigorous asepsis. 2) Sealing that endures repeated sterilization. For this reason, a rectangular PMMA-GS construction was used, avoiding the use of joints and limiting deformation. 3) SS and wall tension are calculated at defined time points, based on the vessel lumen radius (histology), the flow and viscosity being constant. The integration of a longitudinal imaging (high definition camera, laser or Doppler) that continuously monitors the vein diameter and/or flow will provide more detailed information on local flow variations and allow cyclic strain calculations. 4) The two parallel vein segments may have uncontrolled differences in their wall compliance and radius. Thus, we only compare segments from a same vein and assume that under the same pressure, the flow rate pattern is similar in both segments.
In this study, veins were submitted to pulsatile laminar flow; however, 50% of intimal hyperplastic lesions occur in the end-to-side perianastomotic areas of the vein graft, where laminar flow is disrupted. Turbulent conditions can be modeled with the addition of a second pump, non-synchronized with the first pump. Future studies will be performed to specifically assess the impact of laminar flow disruption on IH and the potentially beneficial effect of mesh reinforcement. Interestingly, the flexible structure of the mesh allows circumferential wrapping at the anastomosis sites, as already performed to repair aneurysmal fistulas17 (Figure 4). Thus, the mesh could prove useful to limit perianastomotic dilatation, laminar flow disruption and consequently reduce IH at the anastomosis sites. This may be particularly beneficial in distal bypass graft, frequently affected by diameter mismatch between the vein and the tibial or peroneal artery.
In summary, the setup shown here allows parallel perfusion of human veins, under identical hemodynamic conditions. These data demonstrate that the use of an external macroporous tubular polyester mesh is an efficient method to limit the development of IH in vein grafts inserted into an arterial environment11. This system can profit several areas of research. Especially, it emerges as a powerful tool to perform pre-clinical studies testing the feasibility and efficiency of various approaches to reduce IH in human material, and is a valuable addition to in vivo animal models. Other prosthetic supports or meshes coated with pharmaceutical agents will be evaluated using this method6-10. In addition, one could envision to test locally applied pharmacological molecules to prevent IH in human tissue, under near physiological state. Gene therapy interventions are also achievable, transducing a vein segment to overexpress or silence target genes of interest.
In conclusion, our system will increase our understanding of the hemodynamic contribution to human vein graft diseases. It provides an innovative platform to test new therapeutic strategies and may arise as a “bench to beside translation tool.”
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported by grants from the SNF [31003A-138528], the Octav and the Marcella Botnar Foundation, the Novartis Foundation and the Emma Muschamp Foundation. We thank Martine Lambelet, and Jean-Christophe Stehle for their excellent technical assistance.
Materials
| Name | Company | Catalog Number | Comments |
| Name | Company | Catalog Number | Comments |
| RPMI 1640 - Glutamax | Life Technologies | 61870-010 | |
| Penicilline/Streptomycine/Fungizone | Bioconcept | 4-02F00-H | |
| Dextran from Leuconostoc spp. 500 gr. | Sigma-Aldrich | 31390 | |
| Tampon PBS CHUV pH 7.1-7.3 1 lt. | Laboratorium und Grosse Apotheke Dr. G. Bichsel AG | 100 0 324 00 | |
| Cryosectionning embedding medium - Tissue-Tek OCT Compound | Fisher Scientific | 14-373-65 | |
| Silicon Tubing (Peroxide) L/S 16 (96400-16 ) - 7.5m | Idex Health & Science GMBH | MF0037ST | |
| Y-splitter | Idex Health & Science GMBH | Y-connector | |
| 35 mm Culture dish | Sigma-Aldrich | CLS430165-100EA | |
| 15 ml Falcon tube | BD Bioscence | 352096 | |
| 50 ml Falcon tube | BD Bioscence | 352098 | |
| Gearing pump - Reglo-Z | Idex Health & Science GMBH | SM 895 | App-Nr 03736-00194 |
| Pump Head | Idex Health & Science GMBH | MI0008 | |
| Monitoring Kit TRANSPAC IV | icumedical | 011-0J736-01 | |
| 20 mL Syringes | B. Braun Medical SA | 4612041-02 | |
| Etibon 3-0 FS-2 | Ethicon- Johnson&Johnson | EH7346H | |
| Mesh ProVena 6-8mm | B. Braun Medical SA | 1105012-14 | |
| NaCl: Sodium Chlorure Solution perfusion 0.9% (100 ml) | B. Braun Medical SA | 534534 | |
| Masterflex L/S Standard Drive | Cole-Parmer Instrument Co | 7521-10 | |
| Acquisition card | National Instruments | PCI-6024 E | |
| Flowmeter module | Transonic Systems Inc. | TS410 and T402 | |
| Stopcock with 3-ways | BD Connexta Luerlock | 394600 | |
| Millex Filter | Milian | SE2M229I04 |
References
- Sal Go, A., et al. Executive summary: heart disease and stroke statistics--2014 update: a report from the american heart association. Circulation. 129, 399-410 (2014).
- Sal Conte, M., et al. Results of PREVENT III: a multicenter, randomized trial of edifoligide for the prevention of vein graft failure in lower extremity bypass surgery. Journal of Vascular Surgery. 43, 742-751 (2006).
- Yu, P., Nguyen, B. T., Tao, M., Bai, Y., Ozaki, C. K. Mouse vein graft hemodynamic manipulations to enhance experimental utility. The American Journal of Pathology. 178, 2910-2919 (2011).
- Davies, M. G., Hagen, P. O. Reprinted article "Pathophysiology of vein graft failure: a review". European journal of vascular and endovascular surgery : the official journal of the European Society for Vascular Surgery. 42, S19-S29 (2011).
- Berard, X., et al. Role of hemodynamic forces in the ex vivo arterialization of human saphenous veins. Journal of Vascular Surgery. 57, 1371-1382 (2013).
- Vijayan, V., et al. Long-term reduction of medial and intimal thickening in porcine saphenous vein grafts with a polyglactin biodegradable external sheath. Journal of Vascular Surgery. 40, 1011-1019 (2004).
- Jeremy, J. Y., et al. On the biology of saphenous vein grafts fitted with external synthetic sheaths and stents. Biomaterials. 28, 895-908 (2007).
- Zilla, P., et al. Constrictive external nitinol meshes inhibit vein graft intimal hyperplasia in nonhuman primates. The Journal of Thoracic and Cardiovascular Surgery. 136, 717-725 (2008).
- Zilla, P., et al. Utilization of shape memory in external vein-graft meshes allows extreme diameter constriction for suppressing intimal hyperplasia: a non-human primate study. Journal of Vascular Surgery. 49, 1532-1542 (2009).
- Yeoman, M. S., et al. A constitutive model for the warp-weft coupled non-linear behavior of knitted biomedical textiles. Biomaterials. 31, 8484-8493 (2010).
- Longchamp, A., et al. The use of external mesh reinforcement to reduce intimal hyperplasia and preserve the structure of human saphenous veins. Biomaterials. 35, 2588-2599 (2014).
- Saucy, F., et al. Ex vivo pulsatile perfusion of human saphenous veins induces intimal hyperplasia and increased levels of the plasminogen activator inhibitor 1. European Surgical Research. Europaische Chirurgische Forschung. Recherches Chirurgicales Europeennes. 45, 50-59 (2010).
- Dubuis, C., et al. Atorvastatin-loaded hydrogel affects the smooth muscle cells of human veins. The Journal of pharmacology and experimental. 347, 574-581 (2013).
- Deglise, S., et al. Increased connexin43 expression in human saphenous veins in culture is associated with intimal hyperplasia. Journal of Vascular Surgery. 41, 1043-1052 (2005).
- Muto, A., Model, L., Ziegler, K., Eghbalieh, S. D., Dardik, A. Mechanisms of vein graft adaptation to the arterial circulation: insights into the neointimal algorithm and management strategies. Circulation Journal : Official Journal of the Japanese Circulation Society. 74, 1501-1512 (2010).
- Tao, M., et al. A simplified murine intimal hyperplasia model founded on a focal carotid stenosis. The American Journal of Pathology. 182, 277-287 (2013).
- Berard, X., et al. Salvage treatment for venous aneurysm complicating vascular access arteriovenous fistula: use of an exoprosthesis to reinforce the vein after aneurysmorrhaphy. European Journal of Vascular and Endovascular Surgery : the Official Journal of the European Society for Vascular Surgery. 40, 100-106 (2010).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved