Method Article
The Soft Agar Colony Formation Assay
In This Article
Summary
The soft agar colony formation assay is a method used to confirm cellular anchorage-independent growth in vitro. The goal of this protocol is to illustrate a stringent method for the detection of the tumorigenic potential of transformed cells and the tumor suppressive effects of proteins on transformed cells.
Abstract
Anchorage-independent growth is the ability of transformed cells to grow independently of a solid surface, and is a hallmark of carcinogenesis. The soft agar colony formation assay is a well-established method for characterizing this capability in vitro and is considered to be one of the most stringent tests for malignant transformation in cells. This assay also allows for semi-quantitative evaluation of this capability in response to various treatment conditions. Here, we will demonstrate the soft agar colony formation assay using a murine lung carcinoma cell line, CMT167, to demonstrate the tumor suppressive effects of two members of the Wnt signaling pathway, Wnt7A and Frizzled-9 (Fzd-9). Concurrent overexpression of Wnt7a and Fzd-9 caused an inhibition of colony formation in CMT167 cells. This shows that expression of Wnt7a ligand and its Frizzled-9 receptor is sufficient to suppress tumor growth in a murine lung carcinoma model.
Introduction
The soft agar colony formation assay is a technique widely used to evaluate cellular transformation in vitro. Historically, another assay, the clonogenic assay, described by Puck et al. in 1956 was used to evaluate the ability of cells to form colonies1. In this technique, cells were dispersed onto a culture plate and grown in the presence of 'feeder' cells or conditioned medium to provide necessary growth factors. The limitation of this technique was that it only provided information regarding colony formation. Normal cells are prevented from anchorage-independent growth, due to a particular type of apoptotic death, called anoikis2. However, transformed cells have the capability to grow and divide without binding to a substrate. To capitalize on this concept, researchers developed the soft agar colony formation assay. The soft agar colony formation assay has since been modified, in more recent years, to address specific needs. One variation involves incorporation of fluorometric dye to allow for high-throughput colony counting. Another variation involves the use of specialized agar solution to allow for retrieval of viable cells after colony formation when protein or DNA samples are needed.
In the traditional soft agar colony formation assay, cells are grown in a layer of soft agar mixed with cell culture medium that rests on another layer of soft agar, also mixed with cell culture medium, but containing a higher concentration of agar. This prevents cells from adhering to the culture plate, yet allows transformed cells to form visible colonies. The rationale behind this technique is that normal cells depend on cell to extracellular matrix contact to be able to grow and divide. Conversely, transformed cells have the ability to grow and divide irrespective of their surrounding environment. Therefore, cells able to form colonies in an anchorage-independent manner were considered to be transformed and carcinogenic. The overall goal of this method is to measure this capability in cells in a semi-quantitative and stringent manner.
The Wnt signaling pathway is critical in embryogenesis and often de-regulated in tumorigenesis3-6. There are multiple pathways associated with Wnt signaling. The canonical pathway involves Wnt signaling and regulation of downstream gene transcription through its effects on the transcriptional coactivator beta-catenin. Wnts also signal through several non-canonical pathways, for example, the planar cell polarity pathway, which regulates elements involved in cytoskeletal structure7, and the Wnt-calcium pathway, which regulates release of calcium from the endoplasmic reticulum8. Wnt ligands exert their activity through binding Frizzled receptors. Although several Wnts have been shown to be upregulated in lung cancer, Wnt7a has been shown to be down-regulated in non-small cell lung cancer through promoter methylation9. Wnt7a binds Fzd9 and acts as a tumor suppressor through a non-canonical pathway. Restoration of Wnt-7a and Fzd-9 inhibits the growth of non-small cell lung cancer cells10. The effects of Wnt7a/Fzd9 are mediated through the activation of ERK-5, which in turn, activates peroxisome proliferator-activated receptor γ (PPARγ)11,12. Here, we show that overexpression of Wnt7a and Fzd9 results in the suppression of anchorage-independent growth of a murine lung carcinoma cell line. Murine CMT167 cells were derived from a lung carcinoma in C57BL/lcrf mice13 and were stably transfected with Wnt7A and Fzd9. Overexpression of Wnt7A and Fzd9 were confirmed by quantitative-PCR (Q-PCR) and the functionality of Wnt7A and Fzd9 overexpression was confirmed through downstream activation of PPARγ.
Protocol
1. Preparation of Materials and Reagents
- Label each well of a tissue culture treated 6-well plate appropriately for each cell line or condition being investigated.
- Prepare 2x cell culture medium by dissolving 1 g of powder medium and 0.2 g of sodium bicarbonate in de-ionized water to a final volume of 50 ml.
- Pass this medium through a 0.2 μm filter to sterilize.
- Add additional components needed for normal culture of the cell line of interest. For example, grow CMT 167 cell line in RPMI 1640 medium supplemented with 10% FBS and 1% penicillin/streptomycin solution. Warm medium to 37 °C in hot water bath prior to use.
- Prepare 1x cell culture medium separately as you would for normal cell culture of the cell line of interest.
- Prepare 1% noble agar by adding 1 g of noble agar to 100 ml of deionized water.
NOTE: Noble agar will not dissolve completely with agitation alone. - Prepare 0.6% noble agar by adding 0.6 g of noble agar to 100 ml of de-ionized water. Both agar solutions can be made in 100 ml glass bottles with closable lids for long-term storage.
- Autoclave the noble agar mixtures to sterilize. These mixtures can be made in advance and stored at 4 °C but should be heated again at the time of the experiment until agar has completely dissolved.
- Prepare nitroblue tetrazolium chloride solution by making a 1 mg/ml stock solution in 1x PBS (8 g NaCl, 0.2 g KCl, 1.44 g Na2HPO4, and 0.24 g KH2PO4 in H2O to final volume of 1,000 ml). This will be used at the end of the experiment to stain the colonies.
2. Plating of Bottom Layer of Agar
- Loosen the cap on the bottle of 1% noble agar and microwave for about 1-2 min. While heating in a microwave, monitor the solution closely to avoid boiling over. Continue heating, while mixing intermittently, until agar is completely dissolved and the solution is clear.
NOTE: Use heat-resistant gloves to handle flask after heating. Failing to do so may cause burn or serious injury. - Place melted agar solution and pre-warmed 2x culture medium in an ice bucket filled with hot tap water (42 °C). Also place a 50 ml conical tube in a tube holder in the ice bucket with hot water. Transfer bucket to cell culture hood for subsequent steps.
- For the bottom layer of agar, you will need 1.5 ml of a mix of agar and medium per well of a 6-well plate.
- To ensure an adequate amount of the mixture, prepare a total of 12 ml for each 6-well plate.
- Start by adding 6 ml of culture medium to the 50 ml conical tube and then 6 ml of the 1% noble agar solution.
- Invert the conical tube several times to mix. Working at a brisk pace will prevent premature hardening of the soft agar.
- Draw up approximately 5.5 ml of mixture into a 5 ml serological pipette.
- Allow the air bubbles to rise to top of pipette column before depositing 1.5 ml of this mixture into each well. Use caution to avoid deposition of any air bubbles into the plate wells.
- Cover the plates and allow agar mixture to solidify at room temperature, in cell culture hood, for 30 min.
3. Plating the Upper Layer of Agar Containing Cells
- Once the lower layer of agar has solidified, begin preparation of the upper layer.
- First, harvest cells by trypsinization and dilute them 1:5 in culture medium (e.g. for 1 ml of trypsin, add 4 ml of medium) into a 15 ml conical tube.
- Count cells and calculate the number of cells needed per well to prepare a final cell suspension at this time. This number will vary depending on cell type. Use 5,000 cells/well as a starting point and adjust as needed. For this cell number, you would prepare a cell suspension of 6,667 cells/ml (i.e. each well will receive 0.75 ml of this suspension and 0.75 ml of agar for a total volume of 1.5 ml; the concentration of cells will also be diluted 1:2 for a final total cell count of 5,000).
- The volume of cell suspension needed per well of a 6-well plate will again be 1.5 ml. Prepare additional cell suspension totaling 12 ml per 6-well plate.
- Melt 0.6% agar solution in a microwave as above and place into ice bucket containing hot water along with a 50 ml conical tube in a tube holder and the final cell suspension from Step 3.3.
- Transfer the ice bucket with melted 0.6% agar to cell culture hood for subsequent steps.
- Mix 0.6% agar and cell suspension in a 1:1 ratio, preparing a total volume of 12 ml per 6-well plate. 1.5 ml will be required per well but extra should be made as above.
- Pipette 6 ml of cell suspension into the 50 ml conical tube.
- Then, add 6 ml of 0.6% agar to the tube.
NOTE: The temperature of this mixture must be kept around 42 °C to avoid premature hardening and to maximize cell survival. - Working quickly, pipette this mixture 2-3x to distribute cells, then draw up 5.5 ml of mixture into a 5 ml serological pipette.
- Allow any air bubbles to rise to top of pipette column before depositing 1.5 ml of this mixture into each well. Use caution to avoid deposition of any air bubbles into the plate wells.
- Allow cell/agar mixture to solidify at room temperature, in cell culture hood, for 30 min before placing into a 37 °C humidified cell culture incubator.
- The time required for adequate colony formation varies for each cell line, typically around 21 days.
- A layer of growth medium should be maintained over the upper layer of agar to prevent desiccation. 100 μl of medium added twice weekly is sufficient for this purpose.
4. Staining the Plates and Counting Colonies
- Stain cells by adding 200 μl of nitroblue tetrazolium chloride solution per well and incubating plates overnight at 37 °C.
- Once colonies are stained, take photographs of wells using an imager and count colonies using image analysis software.
Results
The expression of Wnt7A and Fzd9 in CMT167 cells is effective in tumor suppression as illustrated by our soft agar colony formation assay. Preliminarily, we used Q-PCR to show that Wnt7A and Fzd9 mRNA are expressed in low levels in CMT167 cells. CMT167 cells showed low levels of endogenous Wnt7A and Fzd9 when compared to MLE-12 cells, an SV40-transformed murine lung epithelial cell line (Figure 1). We then transfected CMT167 cells with two retroviral overexpression vectors expressing human constructs of Wnt7A (LNCX-Wnt7A) and Fzd9 (LPCX-Fzd9) to create a stable cell line that expresses both Wnt7A and Fzd9 (CMT LL Wnt7a/Fzd9). We confirmed expression of Wnt7A and Fzd9 by Q-PCR in this cell line (Figure 2). As our previous work has shown, PPARγ is a downstream effector of Wnt7A/Fzd9 signaling. Wnt signaling pathways are diverse. Therefore, proper expression and activation of a particular Wnt and its Fzd receptor must be assessed using a downstream effector that is known to be activated by the Wnt/Fzd pair of interest. For Wnt7A and Fzd9, PPARγ was chosen for this reason. We transfected CMT167 cells overexpressing empty vectors (LNCX/LPCX) or Wnt7A/Fzd9 with a Luciferase reporter construct containing a PPARγ response element. We showed that our CMT167 LL Wnt7A/Fzd9 cell line had almost six-fold increased PPAR-RE activity (Figure 3). Finally, as mentioned previously, to confirm the tumor suppressor activity of Wnt7A and Fzd9 in vitro, we performed a soft agar colony formation assay. CMT167 vector expressing and Wnt7A/Fzd9 expressing stable cell lines were plated onto soft agar plates and allowed to form colonies for two to three weeks. CMT167 LL Wnt7A/Fzd9 cells formed significantly less colonies when compared to vector-transfected cells, illustrating the tumor suppressor function of Wnt7A and Fzd9 (Figure 4). Pictures were taken of all plates, and colonies were counted using an imager and imaging software.

Figure 1: Decreased levels of Wnt7A and Fzd9 mRNA in CMT167 cells compared to MLE-12 cells. Q-PCR analysis was performed to determine mRNA levels of Wnt7a and Fzd9 normalized to GAPDH. Levels in CME167 murine lung carcinoma cells were compared to those in MLE-12 SV40-transformed murine lung epithelial cells, which were normalized to 1.0. CMT167 cells showed lower levels of Wnt7a (A) and Fzd9 (B) mRNA when compared to MLE-12 cells.

Figure 2: Overexpression of Wnt7a/Fzd9 in CMT167 LL Wnt7a/Fzd9 stable cell line. Wnt7A/Fzd9 stable overexpression human constructs were made using an LNCX retroviral vector. Q-PCR was performed for Wnt7A and Fzd9, and mRNA levels were normalized to GAPDH in empty-vector transfected cells and in Wnt7A/Fzd9 overexpressing cells. CMT167 LL Wnt7a/Fzd9 cells expressed high levels of Wnt7a (A) and Fzd9 (B) mRNA.
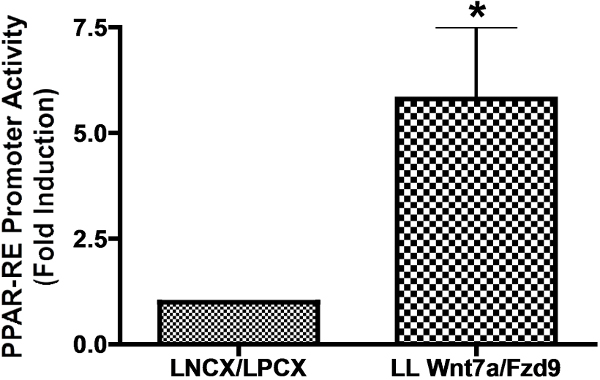
Figure 3: CMT167 Wnt7a/Fzd9 stable overexpressing cell line exhibits increased PPAR-RE promoter activity. CMT167 LL Wnt7a/Fzd9 or empty vector CMT167 LNCX/LPCX stable overexpressing cells were transfected with a PPAR response element promoter luciferase plasmid using Lipofectamine reagent. Promoter activity was quantified compared to vector-transfected cells, where promoter activity was normalized to 1.0. CMT167 LL Wnt7a/Fzd9 stable overexpressing cells showed an approximately six-fold increase in PPAR promoter activity.

Figure 4: Soft Agar Colony Formation Assay of vector-expressing CMT167 LNCX/LPCX cells and CMT167 LL Wnt7a/Fzd9 overexpressing cells. Vector-expressing CMT167 LNCX/LPCX cells and CMT167 LL Wnt7A/Fzd9 overexpressing cells were plated in a soft agar colony formation assay per protocol described above. CMT167 LL Wnt7A/Fzd9 cells showed a marked decrease in colony formation. Representative wells showing colonies for each cell line. Picture of wells are representative of three independent experiments.
Discussion
In vitro confirmation of tumor suppressive function of signaling proteins is difficult. One of the most rigorous assays available to investigate this property is the soft agar colony formation assay. Here, we have illustrated the soft agar colony formation assay using a murine lung carcinoma cell line stably overexpressing Wnt7a and Fzd9 compared to its parental CMT167 cell line.
There are several important points to consider regarding the soft agar assay. The most critical step in this assay is plating of the cells. Cell counts must be accurate, and the agar solution must not be too hot. If no colonies are formed, the cells may have been damaged due to heat stress. In this situation, the assay should be repeated, taking precautions to keep agar temperature as close to 42 °C as possible. Alternatively, a greater number of cells can be plated.
There are a few limitations to the soft agar assay. One such limitation is that it takes two to three weeks for completion. Another is that it does not allow for cell retrieval upon completion. Modifications of this technique employ specialized agar solutions to allow for cells to be harvested for DNA or protein upon assay completion. Alternative methods also utilize fluorometric dye to allow for high throughput assays. Nevertheless, the traditional soft agar colony formation assay remains as one of the most rigorous tests for confirmation of anchorage-independent cell growth.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This study was supported by a Merit Award from the U.S. Department of Veterans Affairs, and an NIH grant R01CA1385282522717 to RW.
Materials
| Name | Company | Catalog Number | Comments |
| Cancer Cell Line of Interest | Sigma-Aldrich | 10032302 | CMT-167 Cells |
| Powdered RPMI 1640 Medium | Gibco | 31800-089 | Used to prepare 2x cell culture medium. |
| Liquid RPMI 1650 Medium | Cellgro | 10-040-CV | Referred to as 1x cell culture medium. |
| Fetal Bovine Serum | HyClone | SH30910.03 | Used to supplement cell culture medium. |
| Penicillin/Streptomycin | CellGro | 30-002-Cl | Used in cell culture medium. |
| Difco Noble Agar | BD Biosciences | 214230 | Used to prepare 1.0% and 0.6% agar. |
| Sodium Bicarbonate | Fisher | BP-328-1 | Used in 2x cell culture medium. |
| Trypsin | Cellgro | 25-050-Cl | |
| Sterile Bottle-Top Filters | Fisher | 09-761-126 | Used to sterile filter 2x medium. |
| Lipofectamine Reagent | Invitrogen | 18324-020 | Used in PPAR-RE luciferase assay. |
| 6-well Plates Tissue-culture Treated | |||
| 37 °C/5% CO2 Incubator | |||
| Chemi-Doc Imager | Bio-Rad | Used to take pictures of colonies. | |
| Quantity One Software | Bio-Rad | Used to count cell colonies. | |
| 15 ml Conical Tubes | |||
| 50 ml Conical Tubes | |||
| 250 ml Erlenmeyer Flasks | |||
| Microwave | |||
| 5 ml Serological Pipettes | |||
| Pipette Aid | |||
| Micropipette | |||
| Hemacytometer w/ cover slip | |||
| Pipette Tips | |||
| Inverted Light Microscope | |||
| Centrifuge | |||
| Heat-Resistant Gloves | |||
| Saran Wrap | |||
| Ice Bucket |
References
- Puck, T. T., Marcus, P. I., Cieciura, S. J. Clonal growth of mammalian cells in vitro; growth characteristics of colonies from single HeLa cells with and without a feeder layer. J Exp Med. 103, 273-283 (1956).
- Taddei, M. L., Giannoni, E., Fiaschi, T., Chiarugi, P. Anoikis: an emerging hallmark in health and diseases. The Journal of pathology. 226, 380-393 (2012).
- Wend, P., Holland, J. D., Ziebold, U., Birchmeier, W. Wnt signaling in stem and cancer stem cells. Semin Cell Dev Biol. 21, 855-863 (2010).
- Reya, T., et al. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature. 423, 409-414 (2003).
- Klaus, A., Birchmeier, W. Wnt signalling and its impact on development and cancer. Nat Rev Cancer. 8, 387-398 (2008).
- Clevers, H. Wnt/beta-catenin signaling in development and disease. Cell. 127, 469-480 (2006).
- Takahashi-Yanaga, F., Kahn, M. Targeting Wnt signaling: can we safely eradicate cancer stem cells. Clin Cancer Res. 16, 3153-3162 (2010).
- De, A. Wnt/Ca2+ signaling pathway: a brief overview. Acta Biochim Biophys Sin (Shanghai). 43, 745-756 (2011).
- Tennis, M. A., Vanscoyk, M. M., Wilson, L. A., Kelley, N., Winn, R. A. Methylation of Wnt7a is modulated by DNMT1 and cigarette smoke condensate in non-small cell lung cancer). PLoS One. 7, (2012).
- Winn, R. A., et al. Restoration of Wnt-7a expression reverses non-small cell lung cancer cellular transformation through frizzled-9-mediated growth inhibition and promotion of cell differentiation. J Biol Chem. 280, 19625-19634 (2005).
- Winn, R. A., et al. Antitumorigenic effect of Wnt 7a and Fzd 9 in non-small cell lung cancer cells is mediated through ERK-5-dependent activation of peroxisome proliferator-activated receptor gamma. J Biol Chem. 281, 26943-26950 (2006).
- Tennis, M. A., et al. Sprouty-4 inhibits transformed cell growth, migration and invasion, and epithelial-mesenchymal transition, and is regulated by Wnt7A through PPARgamma in non-small cell lung cancer. Mol Cancer Res. 8, 833-843 (2010).
- Steele, J. G., Rowlatt, C., Sandall, J. K., Franks, L. M. Cell surface properties of high- and low-metastatic cell lines selected from a spontaneous mouse lung carcinoma. Int J Cancer. 32, 769-779 (1983).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved