Method Article
A Method for Culturing Embryonic C. elegans Cells
In This Article
Summary
We describe here a revised protocol for large-scale culture of embryonic C. elegans cells. Embryonic C. elegans cells cultured in vitro using this method, appear to differentiate and recapitulate the expression of genes in a cell specific manner. Techniques that require direct access to the cells or isolation of specific cell types from the other tissues can be applied on C. elegans cultured cells.
Abstract
C. elegans is a powerful model system, in which genetic and molecular techniques are easily applicable. Until recently though, techniques that require direct access to cells and isolation of specific cell types, could not be applied in C. elegans. This limitation was due to the fact that tissues are confined within a pressurized cuticle which is not easily digested by treatment with enzymes and/or detergents. Based on early pioneer work by Laird Bloom, Christensen and colleagues 1 developed a robust method for culturing C. elegans embryonic cells in large scale. Eggs are isolated from gravid adults by treatment with bleach/NaOH and subsequently treated with chitinase to remove the eggshells. Embryonic cells are then dissociated by manual pipetting and plated onto substrate-covered glass in serum-enriched media. Within 24 hr of isolation cells begin to differentiate by changing morphology and by expressing cell specific markers. C. elegans cells cultured using this method survive for up 2 weeks in vitro and have been used for electrophysiological, immunochemical, and imaging analyses as well as they have been sorted and used for microarray profiling.
Introduction
Caenorhabditis elegans (C. elegans) is a powerful model organism for investigating the molecular bases of cellular function, differentiation, and behavior. While its genome, metabolic and biosynthetic pathways are similar to vertebrates', its genetic and molecular tractability are far greater 2. Among its advantages are its size and simple anatomy, its rapid life cycle (3 days at 25 °C), short life-span (2 weeks) and large number of offspring (>200). Due to its hermaphroditic nature and short life cycle, molecular and genetic manipulations are straightforward in C. elegans, including the generation of transgenic animals 3,4 and the application of gene knock-down techniques such as RNA interference 5. C. elegans body and eggshell are transparent. Therefore cells can be easily visualized in both the adult and the embryo using standard microscopy. In the last 40 years, the C. elegans community has created invaluable resources for C. elegans research including a large collection of mutants, knockouts and transgenics, a detailed description of anatomy and development 6,7, including the full reconstruction of the nervous system 8, and a completely sequenced genome which is well annotated and available to the whole community (www.wormbase.com).
Despite the numerous advantages, some experimental approaches have been challenging in C. elegans. These include the ones that require accessibility to the plasma membrane of the cells and isolation of tissues or cell types. Indeed C . elegans tissues are confined within its pressurized hydrostatic skeleton, which is not easily digested by enzymatic treatment or detergents. At the end of 1990s Miriam Goodman and Janet Richmond pioneered methods for electrophysiological recordings of C. elegans neurons and muscle cells in situ 9,10. While these methods gave us important insights into neuronal and muscle function in vivo, they are challenging and low throughput. Alternative methods to study cell function in vivo had been developed, mostly notably in vivo calcium imaging using genetically encoded calcium sensors such as GCamP and cameleon 11-13. These methods though, do not allow the use of pharmacological tools because they are applied on intact living animals.
The first attempt at culturing C. elegans cells in vitro in large scale was made by Laird Bloom during the preparation of his PhD thesis 14. Unfortunately, difficulties encountered with poor adhesion of the cells to the substrate, poor cell differentiation and survival prevented the establishment of this early protocol as a robust cell culture method. In 1995 Edgar and colleagues published a procedure to investigate cell division and morphogenesis by isolation and culture of a single C . elegans embryos 15. Embryonic cells obtained by digestion of the eggshells with a combination of enzymatic treatment and manual dissociation, continued to proliferate, producing up to ~500 cells 15. Subsequently, Leung and coworkers cultured a small numbers of blastomeres to study intestinal morphogenesis. They showed that one in vitro isolated E blastomere produced polarized intestinal cells that created a structure analogous to the intestinal lumen by interacting with each other through apical adherens junctions 16. Buechner and colleagues also reported a similar method for culturing C . elegans embryonic cells in vitro 17.
Based on this early work, Christensen and colleagues developed a robust protocol for culturing embryonic C . elegans cells in vitro 1. They showed that isolated C . elegans cells can differentiate into various cell types and maintain the features that they possess in vivo, including the expression of cell-specific markers. Several techniques that are challenging in vivo, can be applied on isolated C. elegans embryonic cells. These include electrophysiological 1,18 19, imaging, and immunochemical techniques 20,21, as well as isolation of specific cell types by Fluorescent-Activated Cell Sorting (FACS) for the construction of cell-specific cDNA libraries 22,23. Gene knockdown techniques such as RNA interference (RNAi) can be applied on cultured C. elegans cells 1 and a novel metabolic labeling method using Azido-sugar as a tool for glycoprotein discovery has been recently developed for in vitro cultured C. elegans cells 24.
In conclusion, the cell culture method expands the array of techniques that can be applied to the C. elegans model in an effort to decipher gene function in the context of a living organism. We describe here the protocol for culturing C. elegans embryonic cells in vitro, which is largely based on the protocol first described by Christiansen and colleagues 1.
Protocol
Asterisks (*) indicate new or modified steps as compared to Christensen et al.1
1. Material Setup
- The cell culture procedure requires large quantities of eggs isolated from gravid adults. Grow C. elegans on 8P agar plates seeded with NA22 (available through the C. elegans Genetic Consortium - CGC) bacteria to isolate large quantities of eggs. In these plates the amount of peptone used is 8 times the amount that is normally used for NGM plates. The higher peptone concentration sustains the growth of NA22 bacteria more efficiently, which contrary to OP50-, grow in thick layers.
8P plates recipe:
Dissolve 3 g NaCl, 20 g Bacto-Peptone, 25 g agar in 1 L of sterile distilled water and autoclave for 30 min. Let the medium cool at 55 °C and then add sterile-filtered 1 ml of cholesterol (5 mg/ml in EtOH), 1 ml of 1 MgCl2, 1 ml of MgSO4 and 25 ml of KP buffer (stock of 500 ml: 5 g K2HPO4, 30 g KH2PO4, pH 6.00). Pour liquid agar medium into 10 cm Petri dishes (25 ml/plate).
- The next day spread the whole surface of each enriched peptone agar plate with 1 ml of NA22 E. coli cultured overnight in 2XYT media (16 g Tryptone, 10 g Yeast extract, 5 g NaCl in 1 L of sterile water, pH 7.0) at 37 °C. These bacteria constitute an abundant food source that will form a thick layer supporting the growth of large quantities of gravid adults. Leave seeded plates overnight at room temperature to allow growth of the bacteria. The process can be accelerated by incubation at 37 °C for 4-5 hr.
- Transfer starved animals to seeded 8P plates. Wash animals off a starved NGM plate using 5-6 ml of M9 buffer or water and add 1-2 ml of this suspension to each 8P plate.
- Allow growth and multiplication of the animals until the plates are populated at confluence by gravid adults.
- Isolate the eggs required for the preparation of C. elegans embryonic cells, from gravid adults using 5-6 ml of lysis solution.
Lysis solution recipe
5 ml of Fresh Bleach, 1.25 ml of 10 N NaOH and 18.5 ml of sterile H2O. This mixture must be prepared fresh prior to each use.
- Wash the eggs isolated from gravid adults using egg buffer:
Egg buffer recipe
118 mM NaCl, 48 mM KCl, 2 mM CaCl2, 2 mM MgCl2, 25 mM Hepes, pH 7.3, osmolarity 340 mOsm.
- Remove the eggshell that surrounds the eggs by treatment with chitinase. Chitinase is an enzyme with highest activity at acidic pH 25. Dissolve chitinase (Sigma, catalog no. C6137) in egg buffer pH 6.5 at a final concentration of 2 mg/ml. Store the chitinase stock solution at -20 °C in 1 ml aliquots in sterile 15 ml conical tubes. Aliquots can be stored at -20 °C up to a few months.
- Grow C. elegans cultured cells on autoclaved coverslips (12 mm diameter) covered with peanut lectin. Dissolve peanut lectin in sterile water (0.5 mg/ml). Peanut lectin solution should not be filtered or autoclaved. It also does not need to be treated with UV light. Store 2 ml aliquots at -20 °C for up to 6 months.
- Complete cell culture medium (500 ml) contains 500 ml L-15 culture medium from Gibco, 50 ml Fetal Bovine Serum (heat inactivated), 7.7 g sucrose (45 mOsm), 5 ml of 100 U/ml Penicillin and of 100 μg/ml Streptomycin (2%). The complete medium is then filtered using a 0.20 μm pore filter.
Note that the egg buffer and the culture medium have osmolarity of 340 and 345 mOsm respectively. Indeed, contrary to mammalian cells, C. elegans cells have a relatively high osmolarity that needs to be taken into count when preparing solutions that will come in direct contact with the plasma membrane of the cells. The recipes of these reagents were adjusted to reach the desired osmolarity, which was measured using an osmometer 1. It is not necessary to use an osmometer if these recipes are followed exactly and care is used in preparing these reagents. However, one should use an osmoter if other solutions need to be prepared, whose recipes are not reported here or in any of the publications that use C. elegans cultured cells.
2. Egg Isolation
- It is recommended to begin with at least four 8P plates to collect enough eggs for 12 wells of cultured cells (in 24-well plates).
- Before starting with the procedure thaw one tube of peanut lectin stock solution. Place autoclaved coverslips at the bottom of the wells in a 24-well plate and add 200 μl of peanut lectin to the coverslips. Incubate for 1 hr or until the cells are ready to be plated. Remove completely the peanut lectin and wash the wells once with 1 ml of sterile autoclaved water. Complete removal of the peanut lectin from the coverslips is essential for avoiding cell clumping.
- Wash the gravid adults off the agar plates using sterile autoclaved water. Collect the suspension into two sterile conical 50 ml tube. Leave the tubes on ice for up to 5 min to allow precipitation of the worms at the bottom of the tubes (*). Remove the water with a transfer plastic pipette and replace it with fresh sterile autoclaved water. Pellet worms by table-top centrifugation at 200 x g (~1,200 rpm) for 10 min (*). Repeat this last step at least 3.
- Transfer worms into a sterile 15 ml conical tube and pellet them at 200 x g (~1,200 rpm) for 10 min (*). Do not use higher centrifugation speed to avoid collecting the bacteria at the bottom of the tube as well. Sometimes after centrifugations the worms are not completely pelleted. After each wash and before removal the supernatant, place the tubes for 5 min on ice. In chilled water worms precipitate to the bottom of the tube.
- Remove the water and add 5-6 ml of lysis solution (see Material set up). Rock the suspension gently for 5-10 min and then begin monitoring worm lysis under the stereomicroscope every 2-3 min. A drop of suspension can be also placed on a coverslip for easier inspection. The incubation time varies depending on the freshness of the bleach; buy small bottles of bleach and open a new bottle every month.
- When ~70-80% of worms are lysed (10 min from the beginning of the incubation), stop the lysis reaction by adding 9 ml of egg buffer pH 7.3 (see Material set up). Centrifuge the suspension at 200 x g (~1,200 rpm)for 10 min. From this point on have a Bunsen burner on, on the bench to prevent re-contamination of the eggs (*).
- Carefully remove the supernatant using a sterile plastic transfer pipette and wash the pellet 3-4x with egg buffer until the solution is clear. Make sure to mix the pellet well in the egg buffer during each wash.
- Eggs are separated from the animal carcasses using 30% sucrose solution. Resuspend the pellet in 2 ml of sterile egg buffer and add 2 ml of 60% sucrose solution (stock in sterile egg buffer). Mix well and centrifuge for 20 min at 200 x g (~1,200 rpm) (*).
- Carefully remove the tubes from the centrifuge. The eggs are floating at the top of the solution. Using a P1000 pipettor and sterile tips, transfer all eggs into a fresh sterile 15 ml conical tube.
- Add 10 ml of sterile egg buffer to the tube and centrifuge at 200 x g (~1,200 rpm) for 10 min. Repeat the wash 3 x. Make sure the eggs are completely resuspended in the egg buffer during each wash.
3. Embryonic Cells Dissociation
Conduct the next steps of the procedure under sterile conditions using a laminar flow hood. While animals are gown on bacteria plates, the washes and the treatment with the lysis solution containing bleach should eliminate most if not all the bacteria. Thus using a laminar hood at this point of the procedure prevents new contamination of the egg suspension.
- Resuspend pelleted eggs in 1 ml of 2 mg/ml chitinase (stock in egg buffer pH 6.5 (*)) and transfer them to a new sterile 15 ml conical tube. Rock the tube for 10-30 min at room temperature. The exact incubation time changes according to the freshness of the enzyme and the temperature of the room and should therefore be determined for each preparation. It is recommended to start monitoring the eggs under an inverted cell-culture microscope after 10 min of incubation. Note: in our experience, low pH increases the chitinase enzymatic activity. For this reason we use egg buffer at pH 6.5 to dissolve chitinase (recipe reported above, where pH is adjusted to 6.5 using NaOH).
- When ~ 80% of the eggshells are digested by the chitinase treatment (Figures 1 A-B), pellet the eggs by centrifugation at 900 x g (~2,500 rpm) for 3 min (*). Using a P1000 pipettor and sterile tips, remove carefully the supernatant and add 3 ml of L-15 medium (*).
- Transfer the eggs into a 6 cm diameter plate and gently dissociate the cells using a 10 ml sterile syringe equipped with a 18 G needle. Monitor the degree of dissociation by placing a drop of suspension into a fresh plastic Petri dish and by viewing under the microscope. Do not aspirate air into the syringe during this procedure to avoid damaging cells. Continue the dissociation until ~ 80% of the cells are dissociated.
- Filter the suspension using a sterile 5 μm Millipore filter. Cell suspensions must be filtered in order to remove cell clumps, undigested eggs and hatched larvae. Filter additional 4-5 ml of fresh L-15 media through the filter to recover all the cells. Do not use excessive force during the filtration step to avoid damaging the filter and/or the cells.
4. Culturing Cells
- Pellet the dissociated cells by centrifugation at 900 x g (~2,500 rpm) for 3 min (*). Using a P1000 pipettor and sterile tips carefully remove all the supernatant. Resuspend the pelleted cells in complete L-15 medium and plate 1 ml/well. The amount of the medium added depends on the number of 8P plates used, the confluence of the worms on the plates, and the type of experiments that will be performed on the cells. The cell density can be determined using a hemocytometer. For patch-clamp recordings plating density of ~ 230,000 cells/cm2 is optimal.
- Keep the 24 wells plate in a plastic Tupperware container containing wet paper towels to avoid evaporation of culture medium. Store the container in a humidified incubator at 20 °C and ambient air.
- The cells are usually ready for the experiments within 24 hr when the morphological differentiation and expression of GFP markers are complete. Cells can be kept in culture for up to 2 weeks but they are usually most healthy up to 7-9 days after plating. The medium needs to be replaced once a day to maintain healthy cells.
Results
C. elegans cultured cells differentiate and express cell specific markers
Christensen and colleagues using trypan blue staining demonstrated that >99% of embryonic C. elegans cells survive the isolation procedure. At day 9 and 22 after plating, 85% and 65%, respectively are still alive 1. Isolated embryonic C. elegans cells must adhere to a substrate in order to differentiate. Cells that fail to adhere form clumps and it is not clear whether they survive. Differentiation of the cells begins 2-3 hr after plating and continues for ~ 24 hr. Within 24 hr, cells take on specific morphologies. Thus, neurons send out processes 1,18-20, muscle cells form finger-like elongated structures similar to the ones seen in vivo 1 and canal cells form a lumen 17. The morphological features in vitro are remarkably similar to the ones in vivo. For example, in vitro cultured ALM and PLM touch neurons (identified by expression of Pmec-4::gfp) develop only two neuronal processes with one longer than other, as they do in vivo 1,20 (Figure 2A). This suggests that at least some of the molecular mechanisms that drive differentiation of C. elegans cells are cell-autonomous and thus can be recapitulated in vitro.
In the 1990's, Chalfie pioneered the use of Green Fluorescent Protein (GFP) in C. elegans to label cell types in which a gene of interest is expressed 26. Work done so far on cultured C. elegans embryonic cells supports that expression of GFP in specific cell types can be recapitulated in vitro 1,18-20,27 (Figure 2A). Moreover the ratio of cells that express GFP in vitro is similar to the ratio found in vivo. For example, unc-4 homeodomain reporter gene is expressed in 13 motor neurons in mature embryo (13 of 550 cells- 2.3%) 28,29. Fox and colleagues, counted unc-4::GFP cells in culture and found that they are 2% of the total number of cells 23. Similarly, UNC-119, a protein required for G protein trafficking 30, is expressed in most C . elegans neurons and some muscle cells (76% of the cells in the L1 larva 31). Christensen and colleagues reported that in culture unc-119::GFP expression was observed in 74-76% of the cells resembling neurons and muscle cells 1. Finally, MEC-4, a mechanosensitive Na+/Ca2+ channel subunit needed for gentle touch 32 is expressed in 6 touch neurons in the adult C. elegans. Of these six touch neurons, four (ALMs and PLMs) are embryonically derived. Thus 4 of 550 (0.7%) embryonic cells should express GFP under the control of the mec-4 promoter. Indeed, neurons in culture that express Pmec-4::GFP constitute ~0.5% of the total number of cells 18,20.
Protein markers and posttranslational modifications that are cell-specific can be also observed in vitro. For example, alpha tubulin is acetylated only in touch neurons in C. elegans 33. In vitro, processes of touch neurons stain with an antibody raised against acetylated-alpha-tubulin 20 (Figure 2B). Cultured touch neurons also express the toxic mutant channel MEC-4(d), which causes death of the touch neurons in vivo 34. Indeed, GFP expressing neurons prepared from a mec-4(d); pmec-4::GFP strain are initially present in culture but then degenerate 20. Importantly, touch neurons prepared from mec-4(d); pmec-4::GFP behave in vitro similarly to how they behave in vivo. For example, they can be rescued from death by treatment with amiloride and dantrolene, that spares mec-4(d) touch neurons from degeneration in vivo 20,35. To conclude, not only C. elegans embryonic cells morphologically resemble cells in vivo, but they also express cell specific markers and are present in the same proportion they are present in vivo. Taken together, these data support that cultured C. elegans cells constitute an overall good system to study biological processes both at the cellular and molecular levels.
Patch-clamp electrophysiological analysis of cultured C. elegans cells
The patch-clamp technique, which was introduced by Sakmann and Neher in the Seventies for the study of ion channels activity from cells in isolation or in tissues can be applied to cultured C. elegans cells 36. Christensen and colleagues were the first to study ion channels in cultured C. elegans cells using patch-clamp 1. Since then, K+, anionic, and cationic non-selective channels (Figures 2 C-E), as well as dopamine transporters-dependent channels have been described in cultured C. elegans 1,18,19,37. These studies advanced our understanding of the role of ionic conductances in the function of C. elegans neurons. There are a few modifications that need to be considered when using the patch clamp technique to record ion channel activity from cultured C. elegans cells. These modifications are mostly relevant to whole cell recordings. First, C. elegans cells are small, for example, neurons have a diameter of 1-2 μm. Thus, glass pipettes need to have a small tip (0.5 μm). Small pipette tips increase the input resistance, resulting in stimulation and recording errors. To alleviate this problem, pipettes can be constructed to have a small tip but a wide cone. Goodman and Lockery developed a method employing a fire polisher equipped with a high-pressure air pump for molding pipettes to this shape 38. Second, due to the small size of the cells, gaining whole cell access using suction usually results in damage to the cell. Much higher success is usually achieved by applying a high voltage impulse (electrical zapping) to the patch of membrane under the tip of the pipette. The other configurations of the patch clamp technique (cell attached, inside-out and outside-out) are straightforward to apply to C. elegans cultured cells, with the only modification that the tip of the pipette should be small to take into account the small size of C. elegans cells. The perforated patch technique is notoriously more laborious and time consuming than other patch clamp techniques. However, it has been successfully applied on C. elegans cells in situ 39, 40 and thus should be applicable in culture as well.
Calcium imaging techniques applied to C. elegans cultured cells
The functional role of voltage-gated Ca2+ channels (VGCC) in neurons and glia has been investigated in cultured C. elegans cells by calcium imaging 20, 21, 41. The application of calcium imaging in culture has allowed the use of solutions containing high concentrations of KCl to induce cell depolarization and of calcium channel blockers to discern the contribution of different types of channels to calcium influx. For example, the contribution of Na+/Ca2+ permeable MEC-4(d) channel to the intracellular calcium concentration was determined by calcium imaging using cameleon YC2.12 in the presence and absence of the DEG/ENaC channel blocker amiloride. Those experiments demonstrated that an amiloride-sensitive calcium influx is present in touch neurons expressing MEC-4(d) but not in wild type touch neurons. Using ionophore to permeabilize the membrane of touch neurons and to calibrate cameleon (Figures 3A and B), Bianchi and colleagues also determined that the average free calcium concentration in touch neuron is 200 nM 20 (Figures 3C and D). Similarly, Frokjaer-Jensen and colleagues investigated the role of VGCCs EGL-19 and UNC-36 in regulating depolarization-induced calcium influx in C. elegans cultured mechanosensory neurons expressing cameleon 21. They found that depolarization of touch neurons induced by extracellular K+ in a range between 20 mM and 100 mM, resulted in rise of intracellular calcium revealed by YFP/CFP ratio change between 17% and 70%. Moreover, calcium transients were reduced by egl-19 and unc-36 loss-function mutations and were not detected in the absence of extracellular Ca2+ suggesting that VGCCs play a key role in excitability of C. elegans touch neurons 21.
Stout and Parpura examined the role of voltage-gated Ca2+ channels EGL-19, CCA-1 and UNC-2 in CEPsh sheath glia of the cephalic sensilla in contributing to calcium influx 41. Intracellular calcium dynamics were analyzed in CEPsh glial cells cultured from transgenic animals co-expressing the red fluorescent marker mCherry and the green fluorescent cytosolic Ca2+ indicator GcaMP2.0. In the majority of the CEPsh glial cells, membrane depolarization induced by high extracellular concentrations of KCl induced increase of intracellular Ca2+ as reported by rise in GcaMP2.0 fluorescence. Moreover, treatment with calcium channels blockers Cd2+ and NemA (nemadipine-A) significantly reduced the calcium transients. These data support that voltage dependent rise in intracellular calcium in CEPsh glial cells depends at least in part on voltage-gated calcium channels. The authors, further analyzed intracellular calcium changes in L-type egl-19rf (reduction of function), T-type cca-1 and N, P/Q, R-type channel unc-2 knockout animals and concluded that all three types of Ca2+ channels contribute significantly to intracellular calcium dynamics in these glial cells. Thus, since immature mammalian macroglia respond to depolarization via VGCC-dependent changes in intracellular Ca2+ concentrations, the authors conclude that C. elegans CEPsh glial cells may functionally resemble mammalian macroglia, astrocytes, oligodendrocytes and oligodendrocyte precursor cells.
Other techniques applicable to cultured C. elegans embryonic cells
Fluorescence-Activated Cell Sorting (FACS) has been successfully applied to C. elegans cultured cells to enrich cultures with specific cell types and/or to isolate cells for microarray analysis 23,4243. The major modification that needed to be introduced to the cell culture method was the type of substrate used. Strange and colleagues tested different substrates including collagen IV, poly-L-lysine, fibronectin and laminin and established that poly-L-lysine is the best substrate 37. Poly-L-lysine promotes cell differentiation just as well as peanut lectin, but contrary to peanut lectin allows detachment of the cells from the substrate without damage 36. Several different types of C. elegans cells have been sorted by FACS, including thermosensory, olfactory, motor and mechanosensory neurons, and glia 23,42--45. Cell labeling has been accomplished by expression of GFP reporters and overall, the sorting efficiency is high with isolation of up to 90% of GFP-labeled cells 23.
Gene silencing by RNA interference (RNAi) can be achieved in culture as well. Christensen and colleagues showed that incubation of GFP-expressing cultured C. elegans neurons with dsRNA against GFP resulted in significantly reduced level of GFP expression with a maximal effect 4 days after addition of double-stranded RNA to the culture 1 . Similarly, Shih and colleagues used RNAi in culture to demonstrate that the C. elegans SID-1 is needed for mediating the effect of dsRNA and it is likely needed in vivo for mediating systemic diffusion of RNAi 46. In conclusion, RNAi which is one of the most used and effective methods for gene silencing in C. elegans can be applied in culture. This method could be used for silencing genes whose knockout is lethal or interferes with C. elegans normal development.

Figure 1. C. elegans embryos before and after chitinase treatment. (A) Photograph of eggs prior to exposure to chitinase. The arrow points to the transparent and intact eggshell surrounding an embryo. (B) Eggs treated with chitinase for 10 min. The eggshells have been digested and are no longer visible around the embryos. The arrows point to three-fold embryos released from the eggshell. The blue box surrounds a group of cells that are still attached to each other.
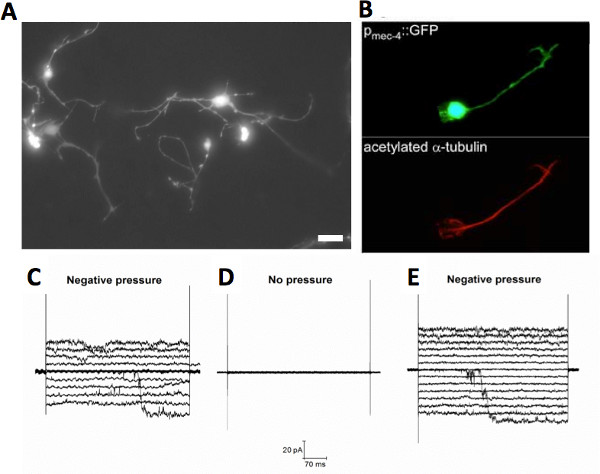
Figure 2. Cultured C. elegans touch neurons. (A) Fluorescent micrograph of cultured C. elegans touch neurons expressing GFP under the touch specific promoter mec-4 (Pmec-4::GFP), scale bar 5 μm. (B) Fluorescent micrographs of a touch neuron expressing Pmec-4::GFP (upper panel), which was stained with a monoclonal antibody against acetylated alpha tubulin (Sigma), a posttranslational modification of alpha tubulin that occurs in touch neurons only 33. Adapted from 20. (C-E) Example of mechanosensitive channels recorded in wild type cultured touch neurons in the cell-attached configuration of the patch clamp technique. The channel has a conductance of ~ 100 pS and it is present in touch neurons isolated from mec-4 knockout animals (mec-4(u253)) as well. These data suggest this channel is not the MEC channel 20. Click here to view larger figure.

Figure 3. Estimation of the free intracellular calcium concentration in cultured touch neurons using cameleon. (A) Pseudocolor images of cultured touch neurons expressing cameleon YC2.12 in 0 mM Ca2+ (+EGTA, left) and in 10 mM Ca2+(right). The pseudocolor scale is shown on the right. (B) Representative cameleon fluorescent changes associated with changes in intracellular calcium concentration in a permeabilized touch neuron. Prior to recording, cells were incubated for 30 min in a solution containing the calcium ionophore Br-A23187 (10 μM) and a defined concentration of free calcium (250 nM in this case). Rotenone (10 mM) and 2-deoxy-D-glucose (1.8mM) were also added to the solution to block active pumps. The neuron was then perfused with a solution containing 0 mM (+5 mM EGTA) Ca2+ and 10 mM Ca2+ to determine the minimum and maximal fluorescence ratio changes. (C) Cameleon calibration curve showing the results obtained from 4-7 cells whose fluorescence changes were evaluated in 11 different free calcium solutions. Each ratio change was normalized to the maximal ratio change for the individual cell. (D) Estimation of the concentration of free calcium in cultured neurons. Cultured touch neurons were imaged for 3 min in a standard extracellular saline solution. Minimal and maximal cameleon ratio changes were then established as described in panel (B). The resting cameleon ratio in cultured touch neurons was 22%±7 (n = 2, 18 cells) of maximal ratio change. Based on the calibration curve shown in (C) this ratio corresponds to a free calcium concentration of ~ 200 nM. Adapted from 20. Click here to view larger figure.
Discussion
C. elegans is a powerful model organism for deciphering the genetic pathways involved in development, behavior and ageing. Its convenience stems primarily from the ease with which it can be genetically manipulated and from its short life cycle. Despite its convenience, C. elegans has its limitations. C. elegans cells are tiny and confined within a pressurized cuticle that limits the application of methods that require direct access to the cells, such as electrophysiological and pharmacological techniques, or isolation of specific cell types, such as the gene-expression profile by microarray analysis. The C. elegans cell culture method has been a solution to many of the limitations of this model organism.
In this manuscript we describe an updated method for isolating and culturing embryonic C. elegans cells in large scale based on early work by Christensen and colleagues 1. We also report published representative results obtained using a variety of techniques including immunocytochemistry, electrophysiology and calcium imaging that have advanced our understanding of the physiological processes and genetic requirements underlying cell function in C. elegans. Work done so far on cultured C. elegans cells supports that they differentiate in vitro as they do in vivo recapitulating the expression of cell specific markers 1,18-20,22,23,27,44. While not all cell types have been characterized in culture, this body of work suggests that the cell culture method allows the study of C. elegans cells in isolation without compromising their identity.
The protocol is simple and easily applicable in any laboratory. Things that need to be kept in mind for successfully culturing C. elegans embryonic cells are the following: 1) Animals must be synchronized so that the majority of them will be gravid adults at the time of the cell culture procedure. Starting the worm culture from a starved plate is a good option. 2) The bacteria used to grow C. elegans need to be eliminated prior to worm lysis to avoid contamination of the cell culture. It is recommended that large volumes of sterile water are used for the washes and that animals are centrifuged at the suggested (not at higher) speed to avoid precipitation of the bacteria as well. Indeed, while treatment with the lysis buffer containing bleach should eliminate bacteria, starting from a relatively bacteria-free animal suspension is recommended. 3) Animals should be treated with bleach/NaOH until ~80% of them have released their eggs. Shorter and longer incubation times reduce the efficiency of egg recovery and damage the eggs respectively. 4) Eggshell digestion by chitinase should be monitored closely. Manual dissociation should be started when ~80% of the eggshells have been digested. Longer and shorter incubation times both result in cell damage, due to direct damage of the plasma membrane by the enzyme and damage caused by prolonged and harsher manual dissociation in the attempt to isolate the cells, respectively. 5) Cell filtration through the 5 μm filter should not be forced. If the filter gets clogged, it should be changed with a fresh one to avoid cell damage.
Despite the fact that cultured embryonic cells have revolutionized the field in many ways, they are not the answer for every scientific question and still have limitations. For example, the genes expressed in culture are mostly embryonic since cells are isolated from embryos. Subcellular compartments may not develop correctly in culture. For example, the development and maintenance of the cilia of certain amphid sensory neurons such as AWA and AWC depend on the interaction with amphid glia, which is lost in culture 44. Cells lose their natural neighbors and do not make physiologically relevant cell-to-cell contacts. For example, it is not known whether cells form tight junctions, electrical or chemical synapses and if they do, these are not likely to be with their physiological partners. Furthermore, cells are plated on a single substrate, peanut lectin. The extracellular matrix in vivo is likely to be a complex and difficult to reproduce mixture of many molecules including peanut lectin, laminin, collagen, and fibronectin. Moreover, specialized extracellular matrix maybe needed for certain types of cell functions. For example, the mechanosensitive channel expressed in body touch neurons and formed by MEC-4 and MEC-10 subunits cannot be gated in culture 18, but can be readily gated in vivo 47. This most likely stems from the fact that extracellular collagen MEC-5 normally produced by neighboring seam cells in vivo, is absent in culture48. MEC-5, together with extracellular matrix proteins MEC-1 (a 1999 aa long protein with a Kunitz-type domain and two EGF domains) and MEC-9 (a 834 aa long protein which contains several Kunitz-type domains, EGF repeats and a glutamic acid-rich domain) are needed for touch sensation and are thought to bind to the extracellular domains of the MEC channel complex and to exert gating tension on the complex during mechanical stimulation.
To conclude, C. elegans embryonic cells can be cultured in vitro and they appear to differentiate recapitulating the expression of cell specific genes. The protocol for isolation and culture of the cells is straightforward and can be applied in any laboratory with minimal experience in cell culture techniques. Bearing in mind the limitations of this technique, the cell culture method can be and has proven to be a powerful technique when direct cells access and/or isolation of specific cell types are needed. A protocol for isolating cells from larvae has been also recently developed (not described here)49. Widespread interest in developing complementary methods is making C. elegans an even more powerful system for ultimately understanding the link between gene function and behavior, development or ageing.
Materials
| Name | Company | Catalog Number | Comments |
| REAGENTS | |||
| Bacto Peptone | VWR International Inc. | 90000-382 | |
| Difco Agar Granulated | VWR International Inc. | 90000-784 | |
| Bacto Tryptone | VWR International Inc. | 90000-284 | |
| Bacto Yeast Extract | VWR International Inc. | 90000-724 | |
| Leibovitz's L-15 Medium (1x) Liquid | Invitrogen | 11415-064 | |
| Fetal Bovine Serum | Invitrogen | 16140-063 | |
| Penicillin-streptomycin | Sigma | P4333-100ML | |
| Chitinase from Streptomyces Griseus | Sigma | C6137-25UN | |
| NA22 Escherichia coli | Caenorhabditis Genetics Center | ||
| Peanut Lectin | Sigma | L0881-10MG | |
| Sucrose | Sigma | 57903-1KG | |
| D-(+)Glucose | Sigma | 67528-1KG | |
| Ethylene glycol-bis (2-amin–thylether), N,N,N',N'- tetraacidic acid (EGTA) | Sigma | E0396-25G | |
| Hepes | Sigma | H3375-500G | |
| Cholesterol | |||
| NaCl | Sigma | 57653-1KG | |
| KCl | Sigma | P9333-500G | |
| CaCl2 | Sigma | C1016-500G | |
| MgCl2 | Sigma | M8266-100G | |
| MgSO4 | Sigma | M2643-500 g | |
| K2HPO4 | Sigma | P2222-500G | |
| KH2PO4 | Sigma | P9791-500G | |
| NaOH | Sigma | S8045-500G | |
| KOH | Sigma | P1767-500G | |
| Ethanol | |||
| Autoclaved distilled H2O | |||
| Bleach | |||
| EQUIPMENT | |||
| 101-1000 μl Blue Graduated Pipet Tips | USA Scentific | 1111-2821 | |
| 10 ml Sterilized Pipet Individually Wrapped | USA Scentific | 1071-0810 | |
| Ergonomic Variable Volume (100-1000 μl) Pipettor with tip ejector | VWR International Inc. | 89079-974 | |
| Portable Pipet Aid, Drummond | VWR International Inc. | 53498-103 | |
| Transfer Plastic Pipet Sterile | VWR International Inc. | 14670-114 | |
| 15 ml Conical Tube | USA Scentific | 1475-1611 | |
| 50 ml Conical Tube | USA Scentific | 1500-1811 | |
| Sterile 18 gauge Needles | Becton, Dickinson and Co. | 305196 | |
| Sterile 10 ml Syringes | Becton, Dickinson and Co. | 305482 | |
| Plastic Syringe Filters Corning 0,20 μm pore size | Corning | 431224 | |
| Acrodic 25 mm Syringe filter w/5 μm versapor Membrane | VWR International Inc. | 28144-095 | |
| 60x15 mm Petri Dish Sterile | VWR International Inc. | 82050-548 | |
| 100x15 mm Petri Dish Sterile | VWR International Inc. | 82050-912 | |
| 12 mm Diameter Glass Coverslips | VWR International Inc. | 48300-560 | |
| Clear Cell Culture Plates 24 Well Flat Bottom w/lid | Thomas scientific | 6902A09 | |
| Dumont #5- Fine Forceps | Fine Science Tools | 11254-20 | |
| Centrifuge 5702 | Eppendorf | 022629883 | |
| Laminar Flow Hood | |||
| Inverted Microscope with x10 objective | |||
| Ambient air humidified Incubator | |||
References
- Christensen, M., et al. A primary culture system for functional analysis of C. elegans neurons and muscle cells. Neuron. 33, 503-514 (2002).
- Riddle, D. L., Blumenthal, T., Meyer, B. J., Priess, J. R., Riddle, D. L., Blumenthal, T., Meyer, B. J., Priess, J. R. . C. elegans II. , (1997).
- Stinchcomb, D. T., Shaw, J. E., Carr, S. H., Hirsh, D. Extrachromosomal DNA transformation of Caenorhabditis elegans. Molecular and cellular biology. 5, 3484-3496 (1985).
- Mello, C. C., Kramer, J. M., Stinchcomb, D., Ambros, V. Efficient gene transfer in C. elegans: extrachromosomal maintenance and integration of transforming sequences. The EMBO journal. 10, 3959-3970 (1991).
- Fire, A., et al. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 391, 806-811 (1998).
- Sulston, J. E., White, J. G. Regulation and cell autonomy during postembryonic development of Caenorhabditis elegans. Developmental biology. 78, 577-597 (1980).
- Chalfie, M. Caenorhabditis elegans development. Current opinion in cell biology. 1, 1122-1126 (1989).
- White, J. G., Southgate, E., Thomson, J. N., Brenner, S. The structure of the nervous system of the nematode Caenorhabditis elegans. Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 314, 1-340 (1986).
- Goodman, M. B., Hall, D. H., Avery, L., Lockery, S. R. Active currents regulate sensitivity and dynamic range in C. elegans neurons. Neuron. 20, 763-772 (1998).
- Richmond, J. E., Jorgensen, E. M. One GABA and two acetylcholine receptors function at the C. elegans neuromuscular junction. Nature. 2, 791-797 (1038).
- Miyawaki, A., Griesbeck, O., Heim, R., Tsien, R. Y. Dynamic and quantitative Ca2+ measurements using improved cameleons. Proceedings of the National Academy of Sciences of the United States of America. 96, 2135-2140 (1999).
- Kerr, R., et al. Optical imaging of calcium transients in neurons and pharyngeal muscle of C. elegans. Neuron. 26, 583-594 (2000).
- Nakai, J., Ohkura, M., Imoto, K. A high signal-to-noise Ca(2+) probe composed of a single green fluorescent protein. Nature. 19, 137-141 (2001).
- Bloom, L. . Genetic and molecular analysis of genes required for axon outgrowth in Caenorhabditis elegans. , (1993).
- Edgar, L. G. Blastomere culture and analysis. Methods in cell biology. 48, 303-321 (1995).
- Leung, B., Hermann, G. J., Priess, J. R. Organogenesis of the Caenorhabditis elegans intestine. Developmental biology. 216, 114-134 (1999).
- Buechner, M., Hall, D. H., Bhatt, H., Hedgecock, E. M. Cystic canal mutants in Caenorhabditis elegans are defective in the apical membrane domain of the renal (excretory) cell. Developmental biology. 214, 227-241 (1999).
- Suzuki, H., et al. In vivo imaging of C. elegans mechanosensory neurons demonstrates a specific role for the MEC-4 channel in the process of gentle touch sensation. Neuron. 39, 1005-1017 (2003).
- Carvelli, L., McDonald, P. W., Blakely, R. D., Defelice, L. J. Dopamine transporters depolarize neurons by a channel mechanism. Proceedings of the National Academy of Sciences of the United States of America. 101, 16046-16051 (2004).
- Bianchi, L., et al. The neurotoxic MEC-4(d) DEG/ENaC sodium channel conducts calcium: implications for necrosis initiation. Nature. 7, 1337-1344 (2004).
- Frokjaer-Jensen, C., et al. Effects of voltage-gated calcium channel subunit genes on calcium influx in cultured C. elegans mechanosensory neurons. Journal of neurobiology. 66, 1125-1139 (2006).
- Von Stetina, S. E., et al. Cell-specific microarray profiling experiments reveal a comprehensive picture of gene expression in the C. elegans nervous system. Genome biology. 8, R135 (2007).
- Fox, R. M., et al. A gene expression fingerprint of C. elegans embryonic motor neurons. BMC genomics. 6, 42 (2005).
- Burnham-Marusich, A. R., et al. Metabolic Labeling of Caenorhabditis elegans Primary Embryonic Cells with Azido-Sugars as a Tool for Glycoprotein Discovery. PloS one. 7, e49020 (2012).
- Kim, K. J., Yang, Y. J., Kim, J. G. Purification and characterization of chitinase from Streptomyces sp. M-20. Journal of biochemistry and molecular biology. 36, 185-189 (2003).
- Chalfie, M., Wolinsky, E. The identification and suppression of inherited neurodegeneration in Caenorhabditis elegans. Nature. 345, 410-416 (1990).
- Parpura, V. Voltage-gated calcium channel types in cultured C. elegans CEPsh glial cells. Cell calcium. 50, 98-108 (2011).
- Miller, D. M., Niemeyer, C. J. Expression of the unc-4 homeoprotein in Caenorhabditis elegans motor neurons specifies presynaptic input. Development. 121, 2877-2886 (1995).
- Lickteig, K. M., et al. Regulation of neurotransmitter vesicles by the homeodomain protein UNC-4 and its transcriptional corepressor UNC-37/groucho in Caenorhabditis elegans cholinergic motor neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 21, 2001-2014 (2001).
- Zhang, H., et al. UNC119 is required for G protein trafficking in sensory neurons. Nature. 14 (7), (2011).
- Maduro, M., Pilgrim, D. Identification and cloning of unc-119, a gene expressed in the Caenorhabditis elegans nervous system. Genetics. 141, 977-988 (1995).
- Chalfie, M., Sulston, J. Developmental genetics of the mechanosensory neurons of Caenorhabditis elegans. Developmental biology. 82, 358-370 (1981).
- Fukushige, T., et al. MEC-12, an alpha-tubulin required for touch sensitivity in C. elegans. Journal of cell science. 112 (Pt. 3), 395-403 (1999).
- Driscoll, M., Chalfie, M. The mec-4 gene is a member of a family of Caenorhabditis elegans genes that can mutate to induce neuronal degeneration. Nature. 349, 588-593 (1991).
- Xu, K., Tavernarakis, N., Driscoll, M. Necrotic cell death in C. elegans requires the function of calreticulin and regulators of Ca(2+) release from the endoplasmic reticulum. Neuron. 31, 957-971 (2001).
- Sakmann, B., Neher, E. Patch clamp techniques for studying ionic channels in excitable membranes. Annual review of physiology. 46, 455-472 (1984).
- Strange, K., Christensen, M., Morrison, R. Primary culture of Caenorhabditis elegans developing embryo cells for electrophysiological, cell biological and molecular studies. Nature protocols. 2, 1003-1012 (2007).
- Goodman, M. B., Lockery, S. R. Pressure polishing: a method for re-shaping patch pipettes during fire polishing. Journal of neuroscience. 100, 13-15 (2000).
- Nickell, W. T., Pun, R. Y., Bargmann, C. I., Kleene, S. J. Single ionic channels of two Caenorhabditis elegans chemosensory neurons in native membrane. The Journal of membrane biology. 189, 55-66 (2002).
- Ward, A., Liu, J., Feng, Z., Xu, X. Z. Light-sensitive neurons and channels mediate phototaxis in C. elegans. Nature neuroscience. 11, 916-922 (2008).
- Parpura, V. Cell culturing of Caenorhabditis elegans glial cells for the assessment of cytosolic Ca(2)(+) dynamics. Methods Mol. Biol. 814 (2), 153-174 (2012).
- Zhang, Y., et al. Identification of genes expressed in C. elegans touch receptor neurons. Nature. 418, 331-335 (2002).
- Cinar, H., Keles, S., Jin, Y. Expression profiling of GABAergic motor neurons in Caenorhabditis elegans. Current biology : CB. 15, 340-346 (2005).
- Bacaj, T., Tevlin, M., Lu, Y., Shaham, S. Glia are essential for sensory organ function in C. elegans. Science. 322, 744-747 (2008).
- Colosimo, M. E., et al. Identification of thermosensory and olfactory neuron-specific genes via expression profiling of single neuron types. Current biology : CB. 14, 2245-2251 (1016).
- Shih, J. D., Fitzgerald, M. C., Sutherlin, M., Hunter, C. P. The SID-1 double-stranded RNA transporter is not selective for dsRNA length. RNA. 15, 384-390 (2009).
- O'Hagan, R., Chalfie, M., Goodman, M. B. The MEC-4 DEG/ENaC channel of Caenorhabditis elegans touch receptor neurons transduces mechanical signals. Nature. 8, 43-50 (2005).
- Du, H., Gu, G., William, C. M., Chalfie, M. Extracellular proteins needed for C. elegans mechanosensation. Neuron. 16, 183-194 (1996).
- Zhang, S., Banerjee, D., Kuhn, J. R. Isolation and culture of larval cells from C. elegans. PloS one. 6, e19505 (2011).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved