Method Article
Rapid Whole-Mount High-Resolution Imaging of Small Animal Vasculature for Quantitative Studies
In This Article
Summary
This protocol introduces a rapid method for quantitative whole-mount three-dimensional vascular imaging using light-sheet fluorescence microscopy. The efficacy of the method is demonstrated using the pharyngeal arch artery system of the chick embryo model, with hemodynamic forces quantified via computational fluid dynamics.
Abstract
In small animal models of cardiovascular development and diseases, subject-specific computational simulations of blood flow enable quantitative assessments of hemodynamic metrics that are difficult to measure experimentally. Computational fluid dynamic simulations shed light on the critical roles of mechanics in cardiovascular function and disease progression. Acquiring high-quality volumetric images of the vessels of interest is central to the accuracy and reproducibility of morphological measurement and flow quantitation results. This study proposes a rapid, cost-effective, and accessible method for whole-mount high-resolution imaging of small animal vasculature using light-sheet fluorescence microscopy. The modified iDISCO+ (immunolabeling-enabled three-dimensional imaging of solvent-cleared organs) light-sheet sample preparation protocol involves (1) labeling vasculature with a fluorescent agent, (2) preserving the sample, and (3) rendering the sample transparent. Unlike classical iDISCO+, which uses immunohistochemical staining, the authors label vascular endothelium with FITC-tagged poly-L-lysine, an affordable non-specific fluorescent dye that is highly resistant to photo-bleaching, in a process termed "endo-painting." The rapid labeling reduces sample preparation time from approximately four weeks to less than 3 days. Furthermore, the use of minimally hazardous solvent ethyl cinnamate (ECi) as the clearing agent and imaging solution makes the samples safer to handle and compliant with a wider range of imaging facilities. The proposed protocol is applied to obtain highly resolved light-sheet fluorescence microscopy image stacks of the cardiovascular system in chick embryos ranging from day 3 (HH18) to day 8 (HH34). This study further demonstrates the suitability of this method for vascular quantitation through 3D reconstruction and computational hemodynamic modeling of a day 5 (HH 26) chick embryo.
Introduction
Volumetric imaging is necessary for accurate studies of cardiovascular physiology and disease. Quantitative imaging produces high-resolution image stacks with intact volumetric dimensions. Samples must both be preserved to maintain their in-vivo morphology and lumen volume as well as imaged in a uniform high resolution capacity. From high-resolution imaging stacks, the user can generate high-fidelity three-dimensional vascular renderings that allow for a complete display of vessel shapes, structure, and connectivity1.
Cardiovascular structures possess complex three-dimensional anatomical features that cannot be accurately captured when examining them through a two-dimensional, disjointed lens. Stereoscope widefield morphological imaging and histological sections are inadequate in capturing complex three-dimensional variations1,2,3. Micro and nano-computed tomography images are the gold standard for quantitative small animal volumetric imaging1,4, but are not widely accessible or adopted among the biological community. Recent innovations in tissue clearing and whole organ/small animal microscopy have allowed for quantitative applications of whole mount clearing and vascular labeling techniques5,6,7. Tissue-clearing works to homogenize the scattering of light in tissue samples, thereby reducing delays in light propagation through the medium by lowering the chance of light scattering or absorption. High transparency requires stringent tissue processing that may affect the antigenicity or brightness of fluorescence signal labeling8. Light-sheet microscopy has emerged as a fast, powerful imaging tool widely adopted by biologists9, offering a gain of speed several orders of magnitude over scanning microscopes and the capacity to image samples over 1 cm in size. Through light-sheet fluorescence microscopy (LSFM), a laser illuminates a sample cross-section with increased speed and depth compared to confocal microscopy; for this reason, the method requires high sample transparency.
Here, the authors adapt recent iDISCO+ clearing methods, combining them with endo-painting10 in the chick embryo animal model to showcase the method's efficacy from early to late cardiovascular development. iDISCO (immunolabeling-enabled three-dimensional imaging of solvent-cleared organs) is an organic solvent-based clearing method, which, unlike aqueous clearing-based methods, is not subject to imaging artifacts caused by solvent evaporation. iDISCO differs from iDISCO+ in that the tetrahydrofuran dehydration step of the former (iDISCO) is replaced by a milder Methanol dehydration followed by a lipid extraction step (iDISCO+). Advantages of the iDISCO+ clearing method include immunolabeling of large adult samples and embryos, low tissue shrinkage, and high transparency8,11. Importantly, iDISCO+ allows for the generation of high-resolution image stacks, expanding upon traditional biology immunolabeling techniques to gain information over large organ samples or an entire embryo rather than being limited to the sampling of small regions that lack information on the whole tissue-level organization, as with traditional histology9. Disadvantages of iDISCO+ include the fact that genetically encoded fluorescent proteins are not preserved11. The tissue labeling method of endo-painting was first introduced as a high-throughput screening for cardiovascular defects using HH31-HH36 chick embryo hearts which were perfused with 0.5 mg/ml of FITC-poly-L-lysine in the left ventricular apex. The dye was allowed to bind for 4 min before fixation and storage10.
The present study found that the same FITC-poly-L-lysine concentration could be used for a broader range of embryos (HH18 - HH34) but found the ideal fixation time to vary (from 5-10 min) to ensure brightly labeled vessels. Users of the present endo-DISCO technique may want to adjust dye concentration (decreasing by 0.1 mg/mL at a time) should the solution prove too viscous to label all desired vessels, but are encouraged to first adjust the fixation time and optimize muscular contraction of the left ventricle before adjusting dye concentration. The authors attempted endo-painting with a concentration of 0.1 mg/mL and found that while the dye more easily spread through small vessels, it was more easily washed away upon PFA perfusion. The authors show that the high-resolution imaging stacks generated through the present technique are of sufficient quality for computational hemodynamic modeling. Blood flow paths and corresponding hemodynamic forces, including pressure and wall shear stress distributions, occur in complex localized patterns that can only be resolved through computational flow simulations1,12. These biomechanical forces affect the behavior of adjacent cardiovascular tissues and trigger vascular adaptation, growth, and remodeling13. Understanding local hemodynamic force values sheds critical light on the mechanistic regulators of cardiovascular function and disease initiation or progression2.
Protocol
The Office of Laboratory Animal Welfare interprets Public Health Service policy as applying to the chick model as a "vertebrate animal" only after hatching. These embryos are similarly exempt from Institutional Animal Care and Use Committee (IACUC) jurisdiction. The relevant National Institutes of Health frequently asked questions can be accessed at: http://grants.nih.gov/grants/olaw/faqs.htm#ApplicabilityofthePHSPolicy.
1. Embryo collection, labeling and fixation
- Fashion pulled 0.75 inner diameter glass capillary rods into cut microneedles using a microforge1,12.
NOTE: The size of the tip required depends on the animal age/flow rate desired. The microneedle can alternatively be cut using microdissection forceps or scissors with greatly reduced precision. - Warm Tyrode's solution to approximately 38°C.
- Dissolve FITC poly-L-lysine solid in Tyrode's solution to prepare a 0.5 mg/mL stock solution.
NOTE: This concentration is optimized for labeling chick embryo arteries. The optimal concentration for other tissue types may vary. The extra stock solution should be stored at -20 °C. - Dissect out the avian embryo (ranging from HH18 (day 3) to HH34 (day 8) for the present chick cardiac study) from the egg yolk by cutting around the embryo with curved tip scissors and using a transfer pipette or a spatula to transfer the embryo to a 35 mm Petri dish filled with warm Tyrode's solution.
- Carefully remove the chorionic and allantoic membranes enveloping the embryo and the pericardium membrane around the heart with fine-tip forceps by making tiny (~0.1 mm) incisions in the membranes and pulling them away from the embryo.
- Transfer the embryo to a new, clean Petri dish filled with warm Tyrode's solution to keep the heart beating.
- Fill a 5 mL plastic syringe with warm Tyrode's solution. Trim off the wide end of a 0-20 μL plastic pipette tip and attach it to the flat tip of the syringe. The syringe has now been extended to the fine pipette tip diameter.
- Construct an "injection line," by attaching a segment of 0.03-inch inner diameter silicone tubing to the newly secured pipette tip-syringe apparatus (step 1.7). Attach a glass capillary microneedle to the opposite end of the tubing.
- Mount the microneedle onto the microinjection holder attached to a micromanipulator. Purge the silicone tubing and microneedle of air bubbles.
- Using the micromanipulator, insert the needle into the apex of the heart and perfuse the embryo1,12by slowly injecting Tyrode's solution into the heart. Continue perfusing the embryo until it is largely cleared of blood.
- Use the micromanipulator to pull the needle and injection line back from its insertion site and orient the needle so that it will not be disturbed during the preparation of the next injection line. Remove the silicon tubing from the needle and syringe.
NOTE: Some users may find it optimal to keep the needle attached to the heart, in which case the user should remove the silicon tubing between the needle and syringe, while keeping the needle in place. - Perform endo-painting to label the vascular endothelium with green fluorescence.
- Using a micropipette, load 20-40 μL of FITC poly-L-lysine stock solution into the silicone tubing, careful not to create air bubbles ahead of the FITC liquid barrier.
- Reattach the syringe filled with Tyrode's solution and use the micromanipulator to slowly inject into the apex of the heart, reinserting the needle if necessary. Remove the needle after the FITC poly-L-lysine has been diffused and before any air bubbles enter the heart.
- Let FITC poly-L-lysine sit in the embryo for 5-10 min.
NOTE: (optional quality check) To verify fluorescence, take a picture with a fluorescent stereo or macroscope. The picture may be used to help determine a dehydration factor after embryo clearing is completed.
- Fill a 5 mL syringe with 4% paraformaldehyde (PFA) and attach it to the silicon injection tubing for a third injection step.
- Perfuse the embryo1,12 with 4% PFA until all cardiovascular structures are at full volume capacity and the tissue starts to become more opaque.
- Gently load the embryo into a 2-5 mL vial filled with enough 4% PFA to cover the sample. Take care not to deform the embryo excessively.
NOTE: Choose a large enough vial for the sample of interest to avoid crushing or deforming the sample. - Incubate the embryo overnight at 4 °C with a shaker.
2. Embryo dehydration and clearing
- Carefully pipette out the 4% PFA.
NOTE: From this point on, avoid direct contact with the embryo to prevent tissue damage or deformation. - To wash the embryo, incubate the embryo in fresh phosphate buffer solution (PBS) at room temperature for 30 min while gently shaking. Repeat two additional times for a total of 3 washes.
- Begin dehydrating the embryo in a fume hood. Carefully pipette out the PBS and incubate the embryo in a graded series of 5 methanol incubations at room temperature for 1 h each: 20%, 40%, 60%, 80%, and 100% methanol concentration.
- Leave the embryo in fresh 100% methanol at room temperature overnight.
NOTE: (Optional stopping point). Embryos can be stored at -20 °C in 100% methanol for later use for up to 6 months. - In the fume hood, begin the lipid removal procedure. Incubate embryo in a 2:1 (volume to volume) DCM: methanol solution at room temperature for 3 h, gently shaking.
CAUTION: DCM is toxic and must be handled in the fumehood. Take extra precautions when working with DCM. Double gloves may help provide an extra barrier. - Take Ethyl cinnamate (ECi) out of 4 °C and leave it at room temperature to thaw.
- Wash embryos in fresh 100% DCM at room temperature for 15 min, shaking. Repeat 100% DCM wash to achieve a total of 2 washes.
- Pipette out DCM and incubate the embryo in 100% ECi. Leave the embryo in ECi until it clears in approximately 1 h. Gently shake the tube if necessary.
- (Optional verification step) Examine the quality of clearing and vessel staining with a fluorescent stereo or macroscope. Take a picture of the embryo to determine the stage/age-specific dehydration scaling factor.
- Store the cleared embryo at 4 °C for up to 6 months until ready to image, keeping the embryo protected from light.
3. Acquisition of data
- To image embryos, use a light-sheet fluorescent microscope. Affix the head of an optically cleared embryo to a glass capillary using superglue gel.
- Mount the glass capillary to the sample holder, fill the imaging chamber with ECi, and lower the embryo in the imaging chamber.
- Adjust the parameters and perform imaging.
4. Quantitative application: 3D reconstruction and computational fluid dynamic modeling
NOTE: In these steps, light-sheet generated high-resolution image stacks are loaded in the open-source software SimVascular14 for 3D anatomical reconstruction and computational fluid dynamic modeling. Detailed tutorials exist on the SimVascular website (see Table of Materials). Reconstruction consists of creating pathlines in the vessels of interest, creating 2D segmentations along the pathlines, and combining lofted segmentations into a 3D solid model. Computational modeling consists of preparing a meshed geometry, defining boundary conditions, and running simulations.
- Create a new SVProject under File and upload a high-resolution light-sheet imaging stack by right-clicking on Images and then clicking on add/replace image.
- Follow the software tutorial steps to reconstruct vascular anatomies in silico.
- Create a pathline by right-clicking on Paths and selecting create path. Place path markers along a vessel of interest. Repeat for each vessel of interest.
- For each pathline created, trace 2D vessel cross sections by right-clicking on Segmentations and selecting the Create Contour Group. Select the vessel path and double-click on the vessel name under Segmentation to begin manual segmentation.
- Once all vessels have been segmented, create a model by right-clicking on Models and then selecting create model. Select PolyData type, enter a model name, click on Create Solid Model, and select all the segmentations that should be part of the model.
- Mesh the geometry by running the Tetgen mesher built into SimVascular SVMesher. Define a max edge size and perform both surface and volume meshing.
NOTE: Choose a mesh size that can resolve fine geometric details and localized hemodynamic variabilities in the vessel. Start with the Estimate a global mesh size button. A mesh convergence study may be necessary when finalizing simulations. - Set up a computational blood flow simulation using the SimVascular Solver. Create simulation files by right-clicking on Simulations and selecting Create Simulation Job. Adjust basic parameters and select inlet and outlet BCs for specific boundary conditions.
- Adjust Solver parameters, navigate to the Create Files and Run simulation tab, select mesh file, click on Create Data Files for Simulation.
- Launch the simulation using a high-performance computing workstation or supercomputer.
Results
The rapid whole-mount high-resolution imaging protocol presented here (Figure 1, Table 1) produces clearly outlined vessel lumens as shown in Figure 2, Figure 3, and Figure 4, where the chick embryo vasculature endothelium is GFP fluorescent and therefore outlined in green across embryo stages from early to mature heart development (Figure 4). It is important to find the right combination of poly-L-lysine concentration, dye fixation time, and use of ventricular contractions (when applicable) if the sample vasculature does not appear clearly labeled (see Figure 2 for how embryos should look under a fluorescent stereoscope or macroscope before LSFM imaging.) Maintaining a beating heart through warm Tyrode's solution and fast dissection of the embryo away from the yolk sack aids in the diffusion of the solution throughout the sample/small animal model. The viscosity of the poly-L-lysine can be controlled through the concentration of the stock solution. A less viscous solution may aid in fluorescence penetrance throughout the sample, though the robustness of the labeling should be verified post-PFA perfusion. The user may wish to compensate for a lower concentration of poly-L-lysine stock solution by increasing the injected volume and allowing increased incubation time before fixing the embryo with PFA.
Figure 5 demonstrates the suitability of the presented method for 3D anatomical reconstruction and computational modeling. Wall shear stress values are in line with the authors' previous studies based on nano-computed tomography reconstructions1.
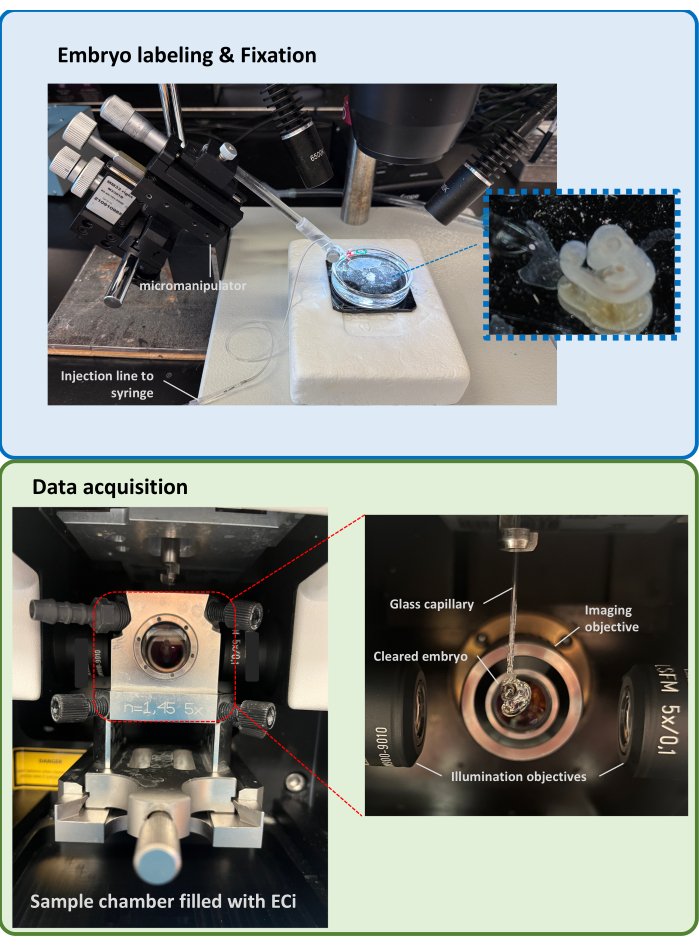
Figure 1: Embryo labeling and fixation set-up (top) and light-sheet microscopy data acquisition set-up (bottom). Please click here to view a larger version of this figure.

Figure 2: Embryos before and after clearing procedures. Representative brightfield and GFP channel illuminated embryos for samples that have undergone steps 1 (before clearing) and 2 (after clearing) of the protocol as seen with a fluorescent macroscope. Note how the whole embryo glows in the GFP channel before clearing, particularly in the heart for which exterior membranes have been removed. All desired vasculature is clearly labeled in the GFP-cleared embryo. Scale bars = 1 mm. Please click here to view a larger version of this figure.
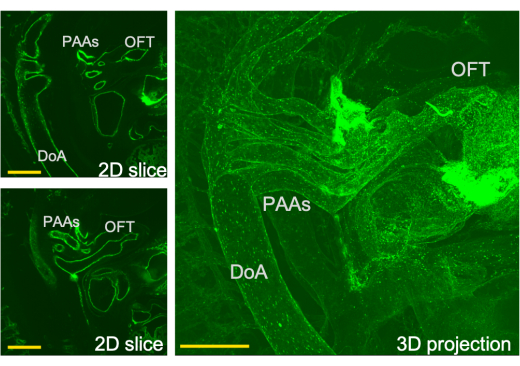
Figure 3: Three-dimensional view of the cardiac outflow system and corresponding slices through this view as obtained by LSFM. PAA- pharyngeal arch artery, OFT-outflow tract, DoA-dorsal aorta. Scale bars = 0.5 mm. Please click here to view a larger version of this figure.
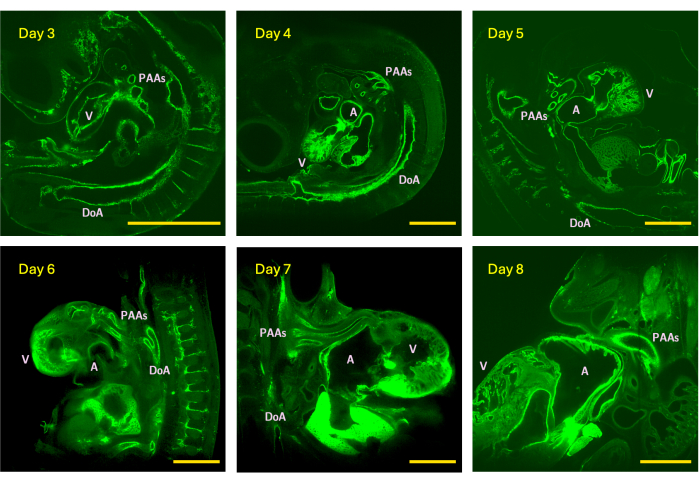
Figure 4: Example z-slices from LSFM image stacks acquired from day 3 (stage HH18), 4 (HH24), 5 (HH26), 6 (HH29), 7 (HH31), and 8 (HH34) chick embryos. Image size represents the maximal (without tiling) field-of-view of the scope equipped with a 5x detection objective. V - ventricle; A - atrium; DoA - dorsal aorta; PAAs - pharyngeal arch arteries. Scale bars = 1 mm. Please click here to view a larger version of this figure.
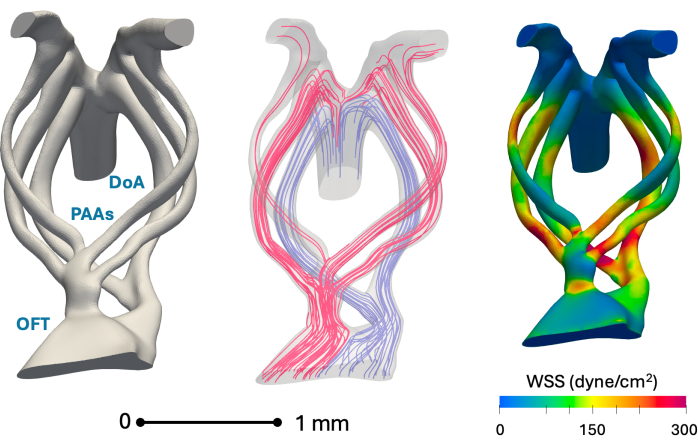
Figure 5: Representative in silico anatomical reconstructions of the day 5 (stage HH26) aortic arch and key hemodynamic simulation results. Left: A reconstructed vascular anatomy model showing the outflow tract (OFT), pharyngeal arch arteries (PAAs), and dorsal aorta (DoA). Middle: Blood flow streamlines at peak systole obtained using blood flow simulation. Red streamlines largely serve cranial regions, while blue serves caudal regions. Right: Peak systolic wall shear stress (WSS) distribution over the vessel wall obtained using blood flow simulation Please click here to view a larger version of this figure.
| Days | Steps | Time | Temperature |
| Day 1 | Embryo harvest | 5-10 min/embryo | RT (benchtop) |
| FITC Endo-paint | 10-30 min/embryo | RT (benchtop) | |
| PFA perfusion fix | Perfusion: 5-15 min/embryo | Perfusion: RT (benchtop) Incubation: 4 °C | |
| Incubation: O/N | |||
| Day 2 | PBS wash | 90 min | RT (benchtop) |
| Dehydration | 5 h + O/N | RT (fumehood) | |
| Day 3 | Lipid removal | 3.5 h | RT (fumehood) |
| Clearing | ≥1 h | RT (benchtop) |
Table 1: Sample preparation overview for rapid quantitative light-sheet imaging.
Discussion
The ability to study biology in 3D is critical to an accurate understanding of morphological complexity, inner organ structure, and vascular connections. Accurate and reliable 3D vascular images are also central to subject-specific computational hemodynamic simulations, which are often the only reliable means of quantifying key hemodynamic parameters such as wall shear stress and pressure distribution. Here, the authors introduce a rapid and accessible sample preparation method for high-resolution 3D vascular imaging in small animals using LSFM. The method reliably produces high-resolution image stacks of HH18 (day 3) to HH34 (day 8) chick embryos, which represent a critical period of cardiac development. From HH24 (day 4) to HH34 (day 8), total embryo size increases from ~30-550 mm3, a 2-fold average growth increase per 24-h period, with heart myocardial size increasing from ~1 mm3 (day 4) to ~17 mm3 (day 8)15. Due to rapid embryo and cardiovascular growth, images for the current study were obtained with an in-plane resolution that varied from 0.61 µm at HH18 (day 3) to 2.28 µm at HH34 (day 8) with a z-step range of 1.92-2.4 µm when focusing on the pharyngeal arch artery system and from 1.22 µm (HH18) to 2.53 µm (HH34) with a z-step range of 1.94-3.5 µm when capturing the whole embryo (Figure 4). The high-resolution image stacks generated are suitable for in silico vascular anatomical reconstruction and subject-specific hemodynamic simulations as demonstrated through HH26 (day 5) models, which were imaged at an in-plane resolution of 1.30 µm and z-step size of 1.92 µm16. As with other whole-mount techniques, the proposed method may be used across animal models, particularly when performing whole-organ imaging5,6,7. The iDISCO+ technique has been applied to neonate and adult mouse hearts5.
The major advantage of the proposed method lies in its use of FITC-tagged poly-L-lysine for fluorescent labeling and ECi for clearing. Poly-L-lysine is positively charged under physiological pH, so it binds non-specifically to vascular endothelium while it is perfused through the sample. The process rapidly and reliably attaches FITC to vessel walls, labeling them with a bright, stable fluorescence that is highly resistant to photobleaching. Traditionally, whole-mount volumetric microscopic imaging, including LSFM, requires samples to be labeled with immunohistochemical staining. This procedure demands the use of expensive antibodies and extends sample preparation time to up to 4 weeks1,8,9. Early embryos do not express traditional vascular markers such as elastin or vascular smooth muscle cells and are, therefore, harder to target with specific antibodies. Furthermore, cleared specimens are often imaged while submerged in the clearing solution. Classical iDISCO+ clearing procedure requires the hazardous compound dibenzyl ether, which is often not allowed in imaging facilities8. ECi is a minimally hazardous compound that is safe to handle outside of laboratory settings and is less likely to damage optical equipment. If desired, the proposed technique may be combined or multiplexed with immunostaining, provided that the chosen spectral emission properties allow for co-expression experiments. Multiplexing may increase sample labeling and preparation time.
The proposed protocol has some limitations. The procedure is only applicable to sacrificed embryos and excised tissue, precluding the possibility of longitudinal studies. FITC poly-L-lysine endo-painting, while rapid and economical, requires a high level of dexterity and precision. Furthermore, as the dye does not readily penetrate through tissue, it will only label vessel walls it can reach, presenting challenges for studying small capillary networks. Such challenges can be managed by keeping the heart beating when administering the poly-L-lysine and adjusting the viscosity/concentration of the injected solution. The non-specificity of the dye makes it difficult to distinguish between vessel types1. Excessive FITC poly-L-lysine that leaks out of the vasculature during the injection process can also bind to non-vascular tissues such as superficial skin and membrane, which may interfere with the illuminating light sheet during imaging and reduce imaging quality. The user should be vigilant when injecting the poly-L-lysine so as to avoid leakage. The current protocol is validated extensively using early-stage chick embryos for imaging major systemic arteries. The concentration, volume, and fixation time of FITC poly-L-lysine injection may need to be further optimized for different use cases.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported by an American Heart Association Career Development Award, Burroughs Wellcome Fund Career Award at the Scientific Interface, Additional Ventures Single Ventricle Research Fund, and the UCSD School of Medicine Microscopy Core (Grant P30 NS047101). The authors thank Dr. Bobby Thompson for his introduction to endo-painting, the UCSD School of Medicine Microscopy Core, and Robert Porter (UCSD) for experimental support.
Materials
| Name | Company | Catalog Number | Comments |
| #5 forceps | Fine Science Tools | 11252-30 | |
| #55-forceps | Fine Science Tools | 11295-51 | |
| 0.03 inch inner diameter silicone tubing | VWR | 32829-182 | |
| 20 μL pipette tips | VWR | 76322-134 | |
| 35 mm Petri dish | VWR | 10799-192 | |
| 5 mL plastic syringe | VWR | BD 309646 | |
| Dichloromethane (DCM) | Sigma-Aldrich | 270997 | Refer to MSDS. Stored in side cabinet under fume hood |
| Ethyl cinnamate (ECi) | Sigma-Aldrich | 112372 | Stored at 4 °C |
| Fine Curved scissors | Fine Science Tools | 14061-09 | |
| FITC-poly-L-lysine | Sigma-Aldrich | P3069 | Store at -20 °C (powder, stock solution), 4ºC (working solution) |
| Fluoresent microscope | EVIDENT SCIENTIFIC | MVX10 | |
| Glass capillary tubes (0.75 mm ID) | Sutter Instrument | FG-GB100-75-10 | |
| Lightsheet microscope | Zeiss | Z.1 system | |
| Methanol | Sigma-Aldrich | M1775 | Refer to MSDS. Stored in flammable cabinet under fume hood |
| Microforge | Narishige International USA, Inc. | MF2 | |
| Micromanipulator | World Percision Instrrument | M3301R | |
| Paraformaldehyde (PFA) 4% | Thermo Scientific | J19943.K2 | Refer to MSDS. Stored at -20 °C (powder), 4 °C (4% working solution) |
| Phosphate buffered saline (PBS) | Cytiva | SH30256.01 | Stored on benchtop |
| SimVascular | open source software www.simvascular.org | ||
| Tyrode’s Solution | Made in-house |
References
- Lindsey, S. E., Butcher, J. T., Vignon-Clementel, I. E. Cohort-based multiscale analysis of hemodynamic-driven growth and remodeling of the embryonic pharyngeal arch arteries. Development. 145 (20), dev162578 (2018).
- Lindsey, S. E., Vignon-Clementel, I. E., Butcher, J. T. Assessing early cardiac outflow tract adaptive responses through combined experimental-computational manipulations. Ann Biomed Eng. 49 (12), 3227-3242 (2021).
- Salman, H. E., et al. Effect of left atrial ligation-driven altered inflow hemodynamics on embryonic heart development: Clues for prenatal progression of hypoplastic left heart syndrome. Biomech Model Mechanobiol. 20 (2), 733-750 (2021).
- Henning, A. L., Jiang, M. X., Yalcin, H. C., Butcher, J. T. Quantitative three-dimensional imaging of live avian embryonic morphogenesis via micro-computed tomography. Dev Dyn. 240 (8), 1949-1957 (2011).
- Anbazhakan, S., et al. Blood flow modeling reveals improved collateral artery performance during the regenerative period in mammalian hearts. Nat Cardiovasc Res. 1 (8), 775-790 (2022).
- Pan, C., et al. Shrinkage-mediated imaging of entire organs and organisms using uDISCO. Nat Methods. 13 (10), 859-867 (2016).
- Renier, N., et al. iDISCO: a simple, rapid method to immunolabel large tissue samples for volume imaging. Cell. 159 (4), 896-910 (2016).
- Vieites-Prado, A., Renier, N. Tissue clearing and 3D imaging in developmental biology. Development. 148 (18), dev199369 (2021).
- Rios Coronado, P. E., Red-Horse, K. Enhancing cardiovascular research with whole-organ imaging. Curr Opin Hematol. 28 (3), 214-220 (2021).
- Miller, C. E., et al. Confocal imaging of the embryonic heart: How deep. Microsc Microanal. 11 (3), 216-223 (2005).
- Renier, N., et al. Mapping of brain activity by automated volume analysis of immediate early genes. Cell. 165 (7), 1789-1802 (2016).
- Lindsey, S. E., et al. Growth and hemodynamics after early embryonic aortic arch occlusion. Biomech Model Mechanobiol. 14 (4), 735-751 (2015).
- Humphrey, J. D. Constrained mixture models of soft tissue growth and remodeling-twenty years after. J Elast. 145 (1), 49-75 (2021).
- Updegrove, A., et al. SimVascular: An open-source pipeline for cardiovascular simulation. Ann Biomed Eng. 45 (3), 525-541 (2017).
- Kim, J. S., Min, J., Recknagel, A. K., Riccio, M., Butcher, J. T. Quantitative three-dimensional analysis of embryonic chick morphogenesis via microcomputed tomography. Anat Rec. 294 (1), 1-10 (2011).
- Zhang, D., Lindsey, S. Evaluation of high-resolution image accuracy for small animal vascular flow quantitation. Bull Am Phys Soc. , (2024).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved