Method Article
Modeling Posthemorrhagic Hydrocephalus of Prematurity in Rats
In This Article
Summary
Posthemorrhagic hydrocephalus of prematurity (PHHP) can be modeled in neonatal rats by combining chorioamnionitis and intraventricular hemorrhage. The combination of these prenatal and postnatal events accurately recapitulates the clinical hallmarks of PHHP, including macrocephaly, ventriculomegaly, and elevated intracranial pressure, through the lifespan.
Abstract
Posthemorrhagic hydrocephalus of prematurity (PHHP) is a serious sequela of severe intraventricular hemorrhage (IVH) in very preterm infants less than 32 weeks gestational age (GA). PHHP is defined by the accumulation of cerebrospinal fluid (CSF) associated with clinical symptoms of elevated intracranial pressure (ICP). Infants with PHHP suffer lifelong shunt dependence, with half requiring repeat surgery in the first year of life and many requiring multiple additional surgeries throughout the lifespan. Prenatal chorioamnionitis predisposes preterm infants to severe IVH and the need for surgical treatment of PHHP trends with neonatal sepsis. These clinical features suggest that systemic inflammation is an integral component of PHHP pathophysiology.
Here, we define an animal model that recapitulates all clinical aspects and essential features of PHHP in rats. The goal of this protocol is to illustrate how in utero chorioamnionitis and postnatal IVH using lysed red blood cells can be combined to yield PHHP. This preclinical approach yields progressive macrocephaly and domed craniums, elevated intracranial pressure, and ventriculomegaly that can be detected via magnetic resonance imaging (MRI) or via microscopy. In addition to sustained disruption in CSF dynamics, rats also have cognitive delay and functional disability into adulthood. Accordingly, this preclinical platform facilitates unique and unparalleled translational studies of PHHP that can incorporate molecular, cellular, biochemical, histologic, imaging, and functional outcome measures. It can also be used for rigorous analysis of the choroid plexus, ependymal motile cilia, and glymphatic system in parallel. Last, it can also be an invaluable preclinical tool for the investigation of novel surgical intervention strategies and non-surgical therapeutic approaches for the treatment of hydrocephalus.
Introduction
Posthemorrhagic hydrocephalus of prematurity (PHHP) remains a substantial public health concern. Defined by symptomatic accumulation of cerebrospinal fluid (CSF) concomitant with elevated intracranial pressure (ICP) secondary to intraventricular hemorrhage (IVH), PHHP is a severe manifestation of encephalopathy of prematurity and a significant contributor to the global burden of prematurity and acquired hydrocephalus1,2. Globally, approximately 400,000 infants each year are born with or acquire the lifelong burden of hydrocephalus3 and many die due to lack of treatment3. PHHP is common in developed countries in very preterm infants (<32 weeks' gestation) with severe IVH, and often affects the sickest of infants who are already suffering from other life-threatening co-morbidities4,5.
The only available treatment for hydrocephalus is surgery6. Surgical procedures yield better longevity when infants are older than 6 months at the time of the first permanent intervention, whether for a ventriculoperitoneal (VP) shunt to divert cerebrospinal fluid (CSF), endoscopic third ventriculostomy (ETV), or ETV with choroid plexus coagulation (ETV-CPC)7. The most common option, VP shunts, often fail within a year and predispose children to a lifetime of complications, repeat surgeries, and hospitalizations at a tremendous cost to the child, the family, and society.8 In particular, the anxiety from a shunt potentially failing at any time is burdensome to families9. Care for children with symptomatic hydrocephalus, including frequent surgeries, is a leading cause of pediatric healthcare expenditures10,11,12,13,14. The annual estimated cost for shunt-related expenditures in children was $2 billion in 200315. While children with shunts comprise only 0.6% of hospital admissions, they generate 3.1% of pediatric hospital charges15. Thus, the discovery of safe, non-surgical therapies for the treatment of PHHP is paramount.
In infants, PHHP develops after IVH over a clinical time course that lasts weeks to months after the initial identification of the brain bleed. A study conducted by the Hydrocephalus Clinical Research Network (HCRN) confirmed that VP shunts remain the best surgical option for neonates with PHHP16. Even for children with PHHP in high-income countries with access to skilled pediatric neurosurgical care, outcomes are far from optimal, with >50% of shunts placed in infants with PHHP requiring surgical revision within the first 2 years8. Despite the clear need to identify safer, more effective treatments for PHHP, research has faced obstacles. Progress has been hampered in part because the preclinical literature on PHHP often fails to appropriately distinguish ventriculomegaly caused by hydrocephalus ex vacuo17,18 from symptomatic hydrocephalus with macrocephaly19,20. Indeed, developmental models of hydrocephalus should include progressive macrocephaly and/or measurements of elevated ICP1.
Merging clinical and preclinical insights has improved study design and propelled our understanding of PHHP2. Studies conducted in diverse centers throughout the globe have shown that IVH is most common in very preterm neonates secondary to chorioamnionitis21,22,23,24,25,26,27,28. In addition to placental infection and inflammation, neonatal sepsis is an additional important risk factor and can play a central role in the progression from IVH to ventriculomegaly to symptomatic PHHP and subsequent surgical intervention29. Preclinical and clinical data support that blood-borne inflammation can cause hydrocephalus20, and systemic inflammation increases secretion of CSF by the choroid plexus30. Further, adults with subarachnoid hemorrhage and IVH who also suffer from sepsis are much more likely to require a shunt31. More recent literature has confirmed that inflammation reduces ependymal motile cilia propulsion of CSF19,20,32 and CSF reabsorption by the glymphatic system33,34,35,36. Overall, systemic inflammation is a key pathophysiological and clinical driver in PHHP1.
Considering these findings, we created an age-appropriate preclinical model of PHHP. This model combines IVH in the immediate and early postnatal period with chorioamnionitis, the principal cause of preterm birth19. This experimental approach begins in utero, with the placental insufficiency, placental inflammation, and intraamniotic inflammation that defines chorioamnionitis7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,
23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,
43,44,45. Specifically, we recapitulate a fetal inflammatory response syndrome, placental neutrophilia, and proinflammatory CNS microenvironment in the preterm period via abdominal laparotomy in pregnant rat dams on embryonic day 18 (E18)37,38,39,40,41,42,43,44,45. Intrauterine injury is induced by temporary bilateral uterine artery occlusion leading to transient systemic hypoxia-ischemia (TSHI) followed by intraamniotic injection of lipopolysaccharide (LPS)37,38,39,40,41,42,43,44,45. Subsequently, to perturb CSF dynamics and catalyze the development of hydrocephalus in the live-born pups, IVH is induced on postnatal day 1. This is accomplished with bilateral intracerebroventricular injection (ICV) of littermate lysed red blood cells (RBCs) into the lateral ventricles19,37,44. Pups are then studied as hydrocephalus develops and throughout their lifespan.
Protocol
The Animal Care and Use Committee (ACUC) at Johns Hopkins University approved all experimental procedures described herein. This protocol utilizes pregnant Sprague-Dawley rat dams and pups of both sexes.
1. Induction of chorioamnionitis on E18
NOTE: The in utero insult portion of this protocol has been previously published in detail, is summarized above, and is the subject of a separate JOVE protocol and video19,37,38,39,40,41,42,43,44,46. Briefly, pregnant female Sprague-Dawley rats undergo abdominal laparotomy on embryonic day 18 (E18) to induce chorioamnionitis, which includes TSHI and intraamniotic LPS administration.
- Anesthesia
- Induce anesthesia in the E18 pregnant rat dam with 2-4% isoflurane.
- Remove the pregnant dam from the induction chamber and place the rat in the supine position on a draped surgical circulating water blanket set at 37 °C.
- Apply ophthalmic ointment to prevent corneal drying. Gently squeeze a paw to confirm the absence of toe pinch reflex. Monitor the anesthetic depth every 15-20 min and increase the isoflurane in case of a positive toe pinch response.
- Administer buprenorphine extended release (0.1 mg/kg SC) at the nape of the neck.
- Surgical preparation and scrub
- Using standard sterile technique, shave the abdomen.
- Scrub the abdomen 3x with alternating betadine and 70% ethanol.
- Drape the animal using sterile surgical drapes.
- Abdominal laparotomy
- Make a 3 cm midline incision on the prepared abdominal skin with a scalpel.
- Use forceps and surgical scissors to hold up the abdominal fascial layer and make an incision of the avascular linea alba of the muscle layer to access the peritoneal cavity.
- Externalize the uterus.
- Isolate and clamp the uterine arteries with aneurysm clips for 60 min. Maintain the temperature and keep the intraabdominal contents moist with sterile saline.
- Remove the clips and inject 100 µL of LPS (4 µg/sac of LPS solution) into each amniotic sac of each fetus. Do not disturb the fetus or the placenta.
- Irrigate the uterine horns and field generously 3x with sterile saline.
- Closing the laparotomy
- Replace the uterine horns in the peritoneal cavity.
- Reapproximate the musculofascial layer edges and close using a running 3-0 suture.
- Reapproximate the skin layer and close the skin using a running 3-0 suture.
- Use a 26 G needle to subcutaneously inject 0.125% bupivacaine around the wound edges.
- For sham controls, perform the laparotomy for the same length of time to control for the duration of anesthesia. Do not clamp the arteries and do not administer any intraamniotic injections. At the conclusion of the procedure, close the laparotomy in two layers (abdominal muscle fascia and skin) using 3-0 suture. In all cases, the pups are born at term (E21/22) and are cared for by the dam.
2. Preparation of lysed red blood cells on P1
- Collection of blood
- Take one male and one female Sprague-Dawley rat pup at postnatal day 1 (P1) from a litter that experienced chorioamnionitis on E18. Rapidly decapitate each donor pup with dedicated surgical scissors.
NOTE: We use 1 male and 1 female pup for blood collection to eliminate a potential sex bias by representing each in the donor cohorts. Additionally, we use a sex-matched pair to guarantee sufficient volume and yield of lysed RBCs to inject their littermates. Typically, each donor pup yields enough lysed RBCs to perform ICV injection on a maximum of 4-5 littermates. - Collect the blood immediately into a 2 mL microcentrifuge tube containing 0.2 mL of sterile saline, taking care to only collect free-flowing blood post decapitation and not scrape or squeeze to produce more blood as this leads to premature hemolysis. Vortex well.
NOTE: The exact amount of blood varies based on the individual donor animal and weight but should be maximum while maintaining the above precautions. - Chop/mince blood clots with small surgical scissors.
- Centrifuge the blood suspension at 500 × g for 10 min at 4 oC, remove the supernatant, and resuspend the pellet in 0.2 mL of sterile saline. Vortex well.
- Chop/mince post vortex residual blood clots with small surgical scissors.
- Repeat steps 2.1.4-2.1.5 twice more for a total of 3x, cleaning surgical scissors with 70% ethanol spray in between each round of lysing.
- Take one male and one female Sprague-Dawley rat pup at postnatal day 1 (P1) from a litter that experienced chorioamnionitis on E18. Rapidly decapitate each donor pup with dedicated surgical scissors.
- Lysis of red blood cells
- After the final centrifugation, add 0.25 mL of sterile saline to the pellet; vortex well.
- Place the suspension on dry ice for 5 min.
- Remove the suspension from dry ice, place it in an incubator set at 37.5 oC for 5 min until completely thawed, and vortex well.
- Repeat the freeze and thaw cycles for a total of 3x (three freezes and three thaws).
- At the conclusion of the last thaw, vortex and perform a quick spin. The RBCs are now lysed and ready to use.
NOTE: The mixture should be an opaque tomato juice-like color and be easily drawn into syringes.
3. Intracerebroventricular injections of lysed red blood cells on P1
- Anesthesia using hypothermia
- Place a small platform on wet ice to cool.
- Place a dry laboratory wipe on top to protect the pup’s skin.
NOTE: This flat cold surface is used for anesthetizing and injecting the pups. - Transfer on pup (aged P1) from the warming pad onto the task wipe atop cold platform to induce anesthesia by hypothermia.
- Confirm the depth of anesthesia by squeezing a paw and confirming the absence of the toe pinch reflex.
- Set an external surgical lamp to its brightest settings.
- With an assistant using their index and middle finger to gently maintain the animal’s head midline, transilluminate the skull to visualize the lateral ventricles through the skull. Identify bregma by visualizing the superior sagittal sinus (midline) through the skin and palpation of the coronal suture with fine forceps as intersecting landmarks.
- ICV injection
- Wipe the head of the anesthetized pup with a cotton swab soaked in 70% ethanol.
- Identify and mark the injection site as 1 mm lateral from the sagittal suture, halfway between lambda and bregma.
- After visualization, use a 0.3 mL, 8 mm long, 31 G insulin syringe with an ultrafine percutaneous needle to inject 20 µL of lysed RBCs into the right lateral ventricle. Insert the needle straight downward using freehand technique to a depth of approximately half the needle length and inject and remove the needle slowly (injection and removal process over approximately 10-15 s).
- Leave the needle in place for several seconds after the injection to prevent egress of the injected lysed RBCs.
- Repeat with the left lateral ventricle and inject 20 µL of lysed RBCs.
- Place the pup on a warming pad set at 37.5 oC to recover from anesthesia.
- Record the sex of the pup and assign a unique animal identifier.
- Return the pup to the home cage only after full recovery on the warming pad and regaining of consciousness such that animal can safely maintain sternal recumbency.
- Monitor all rat pups daily for health and wellness.
4. Confirmation of successful bilateral intraventricular hemorrhage on P2
- Head ultrasound
- To prepare for head ultrasound, remove the P2 pups from their home cage.
- Place ultrasound gel on the ultrasound probe and position the probe over the top of the cranium.
- With extremely light pressure, move the probe to visualize the ventricles. Confirm bilateral hyperechogenicity in the lateral ventricles representing IVH.
5. Confirmation of successful posthemorrhagic hydrocephalus
- Measurement of intraaural distance (IAD), a surrogate for head circumference, to confirm macrocephaly
- To prepare for measurement, acquire a small tape measure appropriate for measuring head circumference, ideally with clearly visualized millimeter designations.
- Have a masked observer remove the pup from their home cage.
- While gently holding the pup, measure the distance from ear to ear (intraaural distance, IAD) and record the value in millimeters.
- Repeat IAD daily from P1 to P15 and graph the values. Serially track IAD and measure again at P21 upon relocating pups to new cages physically separated from the dam (which is the standard time point for pup weaning). Repeat IAD subsequently every 5 days starting at P25 until P60.
- Measurement of opening pressure to confirm elevated intracranial pressure
- Have a masked observer remove the pup from its home cage.
- Anesthetize with 75-100 mg/kg intraperitoneal (IP) ketamine and 5-10 mg/kg IP xylazine in preparation for euthanasia.
- Check the depth of anesthesia by squeezing a paw and confirming the absence of the toe pinch/pedal reflex.
- Insert a small needle (31 G) connected to a manometer into the cervicomedullary junction CSF space.
- Record the opening pressure on the manometer.
- Remove the needle and decapitate the rat with sharp scissors and proceed with tissue collection.
- Ex-vivo magnetic resonance imaging (MRI) for assessment of ventriculomegaly
- Anesthetize with 75-100 mg/kg intraperitoneal (IP) Ketamine and 5-10 mg/kg IP Xylazine in preparation for euthanasia.
- Check the depth of anesthesia by squeezing a paw and confirming the absence of the toe pinch/pedal reflex.
- Perfuse the rats with phosphate-buffered saline (PBS), followed by 4% paraformaldehyde (PFA) until fixed well.
- Remove the brain and drop-fix the brain in 4% PFA
- Embed the brain in 2% agarose in a 50 mL conical tube. Let it stand at room temperature.
- Transfer the brain to the MRI scanner for ex vivo MRI.
- Run 11.7T MRI as follows: T2 Turbo RARE; TE/TR = 30.0/3000 ms; avg = 2; Echo spacing = 10.000 ms; RARE factor = 8; number of slices = 30; slice thickness = 1 mm; image size = 128 x 128; FOV = 28 mm x 28 mm; slice resolution = 0.219 x 0.219 mm2; FA = 90.0°.
NOTE: While MRI imaging provides evidence of successful PHHP modeling, it is not required to scan all brains in a given cohort to verify the PHHP. IAM and ICP measurement is sufficient to verify as described above. Ultimately, the ability of an investigator to perform in vivo or ex vivo MRI will be dependent on a variety of factors such as MRI scanner access, funds, and technical ability. This step is particularly useful for validation when newly incorporating the PHHP model. It is key to note that in the absence of documented signs of increased intracranial pressure, such as elevated opening pressure, isolated findings of ventriculomegaly on MRI imaging does not represent hydrocephalus.
Results
Using this model, hydrocephalus develops in the days and weeks after injection of lysed red blood cells. A representation of a typical experimental design and progression of hydrocephalus is provided in Figure 1. We evaluated 5-6 sham animals and 6-8 PHHP animals per group. As juveniles, rats with PHHP exhibited macrocephaly (Figure 2), elevated intracranial pressure (Figure 3), and ventriculomegaly (Figure 4). The constellation and combination of these findings represent hydrocephalus. These rats also have developmental delay19 and, as adults, survive with persistent cognitive difficulties and elevated ICP. Males perform worse than females in this model19, which replicates the clinical scenario where males are more prone to develop hydrocephalus3,19. Investigators using this experimental platform to study hydrocephalus will be able to confirm the successful completion of the procedure by visualizing progressive macrocephaly and a domed cranium that develops over the course of 5 days following the induction of IVH and maintained throughout the lifespan (Figure 2). Sustained macrocephaly is an essential, clinically important, easily identifiable sign of a successful procedure. By postnatal day 21 (P21), statistically significant increases in IADs (surrogate of head circumference used clinically) and opening pressure are quantifiable (Figure 3). Similarly, ventriculomegaly, and increases in ventricular volume, are observable on MRI and histology compared to sham controls (Figure 4)1,2,19. The quantification of ventricular volume either by structural MRI imaging or through standard histological procedures like cresyl violet or hematoxylin and eosin staining is an excellent complement to more sophisticated diffusion and functional imaging. These data are consistent with the clinical features of PHHP, including macrocephaly, disrupted CSF dynamics leading to ventricular expansion, and elevated ICP.
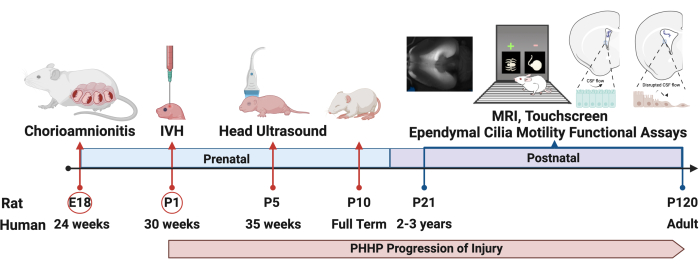
Figure 1: Experimental design. The protocol to induce PHHP starts with the induction of chorioamnionitis in rats at embryonic day 18 (E18) and intraventricular hemorrhage on postnatal day 1 (P1). Hydrocephalus develops and evolves throughout the lifespan and can be evaluated by multiple metrics, including functional assays, through adulthood. Abbreviations: PHHP = posthemorrhagic hydrocephalus of prematurity; IVH = intraventricular hemorrhage. Please click here to view a larger version of this figure.

Figure 2: Macrocephaly typifying PHHP. Rats with PHHP have enlarged, domed craniums, and macrocephaly compared to sham controls. Abbreviation: PHHP = posthemorrhagic hydrocephalus of prematurity. Please click here to view a larger version of this figure.
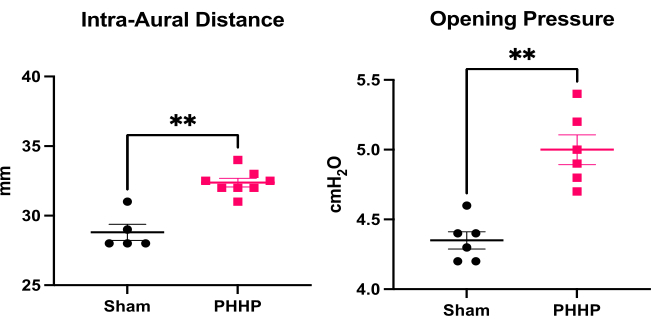
Figure 3: Quantification of macrocephaly and elevated intracranial pressure. Rats with PHHP (n=8) have increased intra-aural distance (a surrogate for head circumference) and increased opening pressure (intracranial pressure, n=6) on postnatal day 21 (P21) compared to sham controls (n=5-6). (t-test **p < 0.01, error bars represent standard error of the mean). Abbreviation: PHHP = posthemorrhagic hydrocephalus of prematurity. Please click here to view a larger version of this figure.
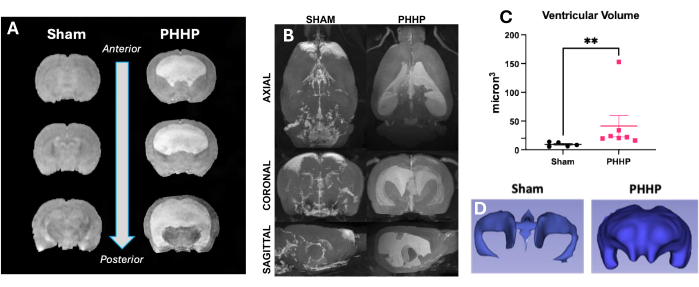
Figure 4: Rats with PHHP have moderate-severe ventriculomegaly. Rats with PHHP have enlarged ventricles and increased ventricular volume compared to sham controls clearly identifiable on magnetic resonance imaging. (A) T2 structural imaging shows ventricular dilation in the coronal plane from anterior to posterior at P21. (B) Ventricular volume increases in PHHP rats compared to sham animals are also visible in axial, coronal, and sagittal planes in adult rats at P60. Ventricular volumes can be quantified using (C) MRI (n=5-7/group) and paired with (D) 3D reconstruction of the ventricular system at any age. (t-test **p < 0.01, error bars represent standard error of the mean). Abbreviation: PHHP = posthemorrhagic hydrocephalus of prematurity. Please click here to view a larger version of this figure.
Discussion
This protocol for the induction of PHHP allows for rigorous, quantifiable, and clinically translatable outcome measures of brain structure and function concomitant with phenotypic hallmarks of hydrocephalus, including chronic elevation of ICP, ventriculomegaly, and macrocephaly, from birth to adulthood4. Biochemical, histological, and functional assays can be used to evaluate the health of the choroid plexus, ependyma, and glymphatic system, as well as gray and white matter19. Additionally, this model can support the integration of functional CSF and live-cell cilia imaging with multimodal neuroimaging and biobehavioral outcomes. It can also be used to combine mechanistic studies using multiparameter flow cytometry and dynamic neural cell assays with histology and immunochemistry to rigorously assess the ventricular microenvironment. Together with digital gait evaluations and touchscreen testing of cognition and executive function2,19, this approach may allow the assessment of cellular, fluid, and neurobehavioral biomarkers not previously utilized in translational studies of PHHP.
For families of children with PHHP, the highest priority after durable shunt function is the improvement of cognitive outcome and the promise of non-surgical treatment strategies47,48,49. The development of pharmacotherapies to address these needs is the first step to transforming the care of children with PHHP2,47,48,49. This preclinical model is amenable to testing drug regimens and emerging pharmaceuticals. It is appropriate for the evaluation of non-surgical interventions and pharmacological treatments designed to modulate CSF dynamics. These drugs can be given through multiple routes of administration in the rats (i.e., intraperitoneal, intravenous, subcutaneous, osmotic minipump) and their hydrocephalus and ventriculomegaly can be tracked, monitored, and quantified throughout the lifespan using imaging and clinical signs. Multiple aspects of brain health can also be assayed including ventricular volume, white matter loss, and functional connectivity. Histology, immunohistochemistry, qPCR, and associated experiments can be performed on tissue collection from specific regions and developmentally distinct endpoints. This platform for study in rats can also facilitate the study of hydrocephalus-associated co-morbid conditions, including cerebral palsy, epilepsy, and chronic pain4.
Mortality in this model is 3-7% and most frequently occurs in the first 48 h post IVH19,44. Occasionally, rat pups fail to gain weight and feed effectively. This failure to thrive can be a direct result of progressive macrocephaly associated with a successful procedure, or cortical/subcortical hemorrhage caused by poor ICV injection technique. In the absence of the necessary precision, cortical hemorrhagic infarction or skull base trauma can be observed. Postnatal care can be disrupted by either the prenatal or postnatal interventions described as the pups are markedly different from sham controls with respect to head shape, body size, and behavior. Proper surgical and injection technique, combined with advanced neuroanatomical proficiency, is crucial to ensure the above representative results are fully realized.
This preclinical platform requires an open abdominal laparotomy to be performed in pregnant rats. This requires advanced surgical skill. In the postnatal period, failure to accurately inject lysed RBCs into the lateral ventricles will not result in IVH nor PHHP and these rats will not grow to demonstrate progressive macrocephaly, elevated opening pressure, or ventriculomegaly. Comfort with freehand injections and the ventricular anatomy of neonatal rodents is essential. Notably, open fontanelles and relatively thin skulls make transillumination possible and facilitate the identification of neuroanatomical landmarks such as bregma and the dorsal aspects of the lateral ventricles necessary for the successful placement of the needle. Unilateral IVH is possible if both lateral ventricles are not accessed, and this may yield transient but not persistent macrocephaly. CSF egress after ventricular access is a suitable indicator of successful injections. Similarly, aspiration of CSF into the needle hub prior to the injection of lysed RBCs ensures the injector is in the proper ventricular space to proceed with injection. While ventriculomegaly on MRI can be present in the absence of hydrocephalus (encephalomalacia), the combination of this finding, along with elevated opening pressure and neurocognitive deficits, represents the successful execution of the PHHP technique.
Analysis of procedural success can be ascertained by comparing the above representative metrics between pups who underwent ICV injection of lysed RBCs and sham pups or in pups who underwent ICV injection versus control pups who experienced chorioamnionitis but not ICV injection of lysed RBCs. Importantly, the sham dams undergo exposure to isoflurane as well as laparotomy and uterine externalization. Unlike in the PHHP model, however, the amniotic sacs and uterus are then returned to the abdominal cavity and the laparotomy completed without uterine artery occlusion or LPS injection. The pups borne out of these sham dams typically do not receive injections of lysed RBCs as it is the specific combination of TSHI+LPS in utero (which the sham fetuses do not experience) followed by IVH that leads to PHHP. Additionally, by having age and sex-matched controls who are not exposed to either TSHI+LPS or IVH, we are able to validate technical success of the model and more directly compare outcomes of PHHP cohorts who received intervention with outcomes of their sham counterparts. This comparison allows for robust efficacy testing of both the model and any therapeutic interventions therein.
Despite the technical proficiency required, this model is advantageous compared to other models of early IVH and hydrocephalus because it is age-appropriate, incorporates systemic inflammation, has evolving PHHP sequelae through adulthood, and provides the opportunity to evaluate translational outcome measures such as sophisticated functional testing and neuroimaging30,50,51,52. It also yields sustained ventriculomegaly and elevated ICP. The use of lysed RBCs increases the translational relevance of this model. Preterm infants that suffer IVH have whole blood released into their ventricles. This blood remains in the ventricular CSF system and degrades over weeks (RBC lysis) leading to a persistent inflammatory response in the ventricles, commonly viewed on routine head ultrasound as ependymal hyper-echogenicity53,54. The use of lysed RBCs corroborates and substantiates prior work evaluating the efficacy of individual blood products/components in creating hydrocephalus and sustained ventriculomegaly55. Unlike packed RBCs, intraventricular injection of lysed RBCs results in significantly enlarged ventricles on MRI 24 h after injection and beyond19,55. Lysed RBC injection has been found to upregulate brain hemoxygenase-1 and ferritin levels in the periventricular space when compared to packed RBC or saline injection55. This is important as the pathophysiology of human IVH is largely due to the breakdown of initial blood and the gradual release of iron degradation products and other blood components concomitant with resultant tissue damage.
Heme-oxygenase 1 is a major enzyme in heme degradation, and ferritin is an iron storage protein; thus, their increased periventricular concentration after lysed RBC injection closely aligns with the human IVH etiology. Lastly, injecting iron only into the ventricular spaces neglects other components of RBCs such as carbonic anhydrase 29. Additionally, iron injection does not maximize the potential severity of the induced IVH, which directly correlates with the likelihood of subsequent hydrocephalus. Like the choice to use lysed RBCs, the rationale behind injecting both ventricles is to increase translation. Clinical literature shows a clear increased association between more severe IVH and subsequent PHHP. Introducing hemorrhages bilaterally in and of itself increases the severity of the IVH as well as the likelihood of extension from the germinal matrix into the ventricular spaces and resultant ventricular dilatation - another characteristic of more severe IVH. Additionally, as described above, bilateral injection also allows for a mode to assess CSF communication. Twenty microliters is the lowest volume to reliably attain sustained hydrocephalus and without significant parenchymal involvement such that rat pup mortality becomes a complicating variable.
In conclusion, the use of an animal model that recapitulates clinical aspects of PHHP, including progressive macrocephaly, elevated intracranial pressure, ventriculomegaly, and cognitive delay into adulthood adds rigor to the field and facilitates unique and unparalleled studies necessary for new therapeutic approaches and improved mechanistic understanding of the complex pathophysiology of this common form of perinatal brain injury.
Disclosures
The authors have no conflicts of interest to disclose.
Acknowledgements
The authors are grateful for the funding provided by the National Institutes of Health (R01HL139492), the Congressionally Directed Medical Research Program (W81XWH1810166, W81XWH1810167, W81XWH2210461, and W81XWH2210462), the Hydrocephalus Association, and the Rudi Schulte Research Institute.
Materials
| Name | Company | Catalog Number | Comments |
| 70% ethanol | Pharmco | 111000200 | Diluted to 70% |
| Betadine surgical scrub | Cardinal Health | NDC-67618-151-17 | |
| Blunt Forceps | Roboz | RS-8100 | |
| Bravmini Plus Cordless Rechargeable Trimmer | Wahl | 41590-0438 | |
| Carbon Steel Surgical blades | Bard-Parker | 371151-11 | |
| centrifuge | Eppendorf | 5424R | |
| Cotton Gauze Sponge | Fisherbrand | 22-362-178 | Small, 6 inch sterile |
| Cotton-tipped Applicators | Fisherbrand | 23-400-114 | 30 G 1 |
| Eye Lubricant | Refresh Lacri Lube | 75929 | |
| Far infrared warming pad | Kent scientific | RT-0501 | |
| Incubator - Genie Temp-Shaker 100 | Scientific Industries | SI-G100 | |
| Insulin Syringes | BD | 328438 | 0.3 cc 3 mm 31 G, ultrafine |
| Isoflurane | Covetrus | 11695067772 | |
| Ketamine hydrochloride injection | Dechra | 17033-101-10 | |
| Kimwipes | Kimtech Science | BXTNI141300 | |
| LPS 011B4 | Sigma | L2630 | |
| microcentrifuge tubes | Thermo Fisher Scientific | 3453 | 2.0 mL |
| Needle | BD | 305122 | 1 mL |
| Needle | BD | 305128 | 25 G 5/8 |
| Needle Holders | Kent Scientific Corp. | INS14109 | 12.5 CM STR |
| OR Towels | Cardinal Health | 287000-008 | |
| Paper measuring tape | Cardinal Health | SKU | |
| Saline Solution, 0.9% | Sigma | S8776 | |
| Scissors | Roboz | RS-6808 | |
| SomnoSuite | Kent Scientific | SS6823B | |
| Sterile Alcohol Prep Pads | Fisherbrand | 06-669-62 | Sterile |
| Surgical gloves | Biogel | 40870 | |
| Surgical Scissors | Roboz | RS-5880 | |
| Surgical Scissors | EST | 14002-16 | |
| Syringe | BD | 309628 | |
| T/Pump (Heat Therapy Pump) | Stryker Medical | TP700 | |
| Vessel Clips | Kent Scientific Corp. | INS14120 | 30 G Pressure |
| Xylazine injection | vet one | NDC 13985-704-10 |
References
- Sevensky, R., Newville, J. C., Tang, H. L., Robinson, S., Jantzie, L. L. Cumulative damage: Cell death in posthemorrhagic hydrocephalus of prematurity. Cells. 10 (8), 1911 (2021).
- Robinson, S., Jantzie, L. L. Pathogenesis of posthemorrhagic hydrocephalus of prematurity: New horizons. Semin Perinatol. 46 (5), 151596 (2022).
- Dewan, M. C., et al. Global hydrocephalus epidemiology and incidence: systematic review and meta-analysis. J Neurosurg. 130 (4), 1065-1079 (2018).
- Robinson, S. Neonatal posthemorrhagic hydrocephalus from prematurity: pathophysiology and current treatment concepts. J Neurosurg Pediatr. 9 (3), 242-258 (2012).
- Gigi, M., Roth, J., Eshel, R., Constantini, S., Bassan, H. Health-related quality of life after post-haemorrhagic hydrocephalus in children born preterm. Dev Med Child Neurol. 61 (3), 343-349 (2019).
- Muir, R. T., Wang, S., Warf, B. C. Global surgery for pediatric hydrocephalus in the developing world: a review of the history, challenges, and future directions. Neurosurg Focus. 41 (5), E11 (2016).
- Kulkarni, A. V., et al. Endoscopic third ventriculostomy and choroid plexus cauterization in infant hydrocephalus: a prospective study by the Hydrocephalus Clinical Research Network. J Neurosurg Pediatr. 21 (3), 214-223 (2018).
- Riva-Cambrin, J., et al. Risk factors for shunt malfunction in pediatric hydrocephalus: a multicenter prospective cohort study. J Neurosurg Pediatr. 17 (4), 382-390 (2016).
- Agajany, N., et al. The impact of neonatal posthemorrhagic hydrocephalus of prematurity on family function at preschool age. Early Hum Dev. 137, 104827 (2019).
- Berry, J. G., et al. Characteristics of hospitalizations for patients who use a structured clinical care program for children with medical complexity. J Pediatr. 159 (2), 284-290 (2011).
- Berry, J. G., et al. Hospital utilization and characteristics of patients experiencing recurrent readmissions within children's hospitals. JAMA. 305 (7), 682-690 (2011).
- Berry, J. G., et al. Trends in resource utilization by children with neurological impairment in the United States inpatient health care system: a repeat cross-sectional study. PLoS Med. 9 (1), e1001158 (2012).
- Drake, J. M., et al. Randomized trial of cerebrospinal fluid shunt valve design in pediatric hydrocephalus. Neurosurgery. 43 (2), 294-303 (1998).
- Simon, T. D., et al. Cerebrospinal fluid shunt infection: Emerging paradigms in pathogenesis that affect `prevention and treatment. J Pediatr. 206, 13-19 (2019).
- Simon, T. D., et al. Hospital care for children with hydrocephalus in the United States: utilization, charges, comorbidities, and deaths. J Neurosurg Pediatr. 1 (2), 131-137 (2008).
- Riva-Cambrin, J., et al. Predictors of success for combined endoscopic third ventriculostomy and choroid plexus cauterization in a North American setting: a Hydrocephalus Clinical Research Network study. J Neurosurg Pediatr. 24 (2), 128-138 (2019).
- Emmert, A. S., et al. Characterization of a novel rat model of X-linked hydrocephalus by CRISPR-mediated mutation in L1cam. J Neurosurg. 132 (3), 945-958 (2019).
- Strahle, J. M., et al. Role of hemoglobin and iron in hydrocephalus after neonatal intraventricular hemorrhage. Neurosurgery. 75 (6), 696-705 (2014).
- Robinson, S., et al. Extended combined neonatal treatment with erythropoietin plus melatonin prevents posthemorrhagic hydrocephalus of prematurity in rats. Front Cell Neurosci. 12, 322 (2018).
- Yung, Y. C., et al. Lysophosphatidic acid signaling may initiate fetal hydrocephalus. Sci Transl Med. 3 (99), 99ra87 (2011).
- Moscuzza, F., et al. Correlation between placental histopathology and fetal/neonatal outcome: chorioamnionitis and funisitis are associated to intraventricular haemorrage and retinopathy of prematurity in preterm newborns. Gynecol Endocrinol. 27 (5), 319-323 (2011).
- Salas, A. A., et al. Histological characteristics of the fetal inflammatory response associated with neurodevelopmental impairment and death in extremely preterm infants. J Pediatr. 163 (3), 652-657 (2013).
- Arayici, S., et al. The effect of histological chorioamnionitis on the short-term outcome of preterm infants ≤32 weeks: a single-center study. J Matern Fetal Neonatal Med. 27 (11), 1129-1133 (2014).
- Shankaran, S., et al. Maternal race, demography, and health care disparities impact risk for intraventricular hemorrhage in preterm neonates. J Pediatr. 164 (5), 1005-1011.e3 (2014).
- Lu, H., Wang, Q., Lu, J., Zhang, Q., Kumar, P. Risk factors for intraventricular hemorrhage in preterm infants born at 34 weeks of gestation or less following preterm premature rupture of membranes. J Stroke Cerebrovasc Dis. 25 (4), 807-812 (2016).
- Stark, M. J., Hodyl, N. A., Belegar, V. K., Andersen, C. C. Intrauterine inflammation, cerebral oxygen consumption and susceptibility to early brain injury in very preterm newborns. Arch Dis Child Fetal Neonatal Ed. 101 (2), F137-F142 (2016).
- Lee, J., et al. A new anti-microbial combination prolongs the latency period, reduces acute histologic chorioamnionitis as well as funisitis, and improves neonatal outcomes in preterm PROM. J Matern Fetal Neonatal Med. 29 (5), 707-720 (2016).
- Edwards, J. M., Edwards, L. E., Swamy, G. K., Grotegut, C. A. Magnesium sulfate for neuroprotection in the setting of chorioamnionitis. J Matern Fetal Neonatal Med. 31 (9), 1156-1160 (2018).
- Alan, N., et al. Reduced ventricular shunt rate in very preterm infants with severe intraventricular hemorrhage: an institutional experience. J Neurosurg Pediatr. 10 (5), 357-364 (2012).
- Karimy, J. K., et al. Inflammation-dependent cerebrospinal fluid hypersecretion by the choroid plexus epithelium in posthemorrhagic hydrocephalus. Nat Med. 23 (8), 997-1003 (2017).
- Wessell, A. P., et al. A sustained systemic inflammatory response syndrome is associated with shunt-dependent hydrocephalus after aneurysmal subarachnoid hemorrhage. J Neurosurg. 130 (6), 1984-1991 (2018).
- Abdi, K., et al. Uncovering inherent cellular plasticity of multiciliated ependyma leading to ventricular wall transformation and hydrocephalus. Nat Commun. 9 (1), 1655 (2018).
- Goulding, D. S., et al. Neonatal hydrocephalus leads to white matter neuroinflammation and injury in the corpus callosum of Ccdc39 hydrocephalic mice. J Neurosurg Pediatr. 25 (5), 476-483 (2020).
- Hussain, R., et al. Potentiating glymphatic drainage minimizes post-traumatic cerebral oedema. Nature. 623 (7989), 992-1000 (2023).
- Cai, Y., et al. The relationship between inflammation, impaired glymphatic system, and neurodegenerative disorders: A vicious cycle. Neurobiol Dis. 192, 106426 (2024).
- Mogensen, F. L., Delle, C., Nedergaard, M. The glymphatic system (en)during inflammation. Int J Mol Sci. 22 (14), 7491 (2021).
- Jantzie, L. L., Winer, J. L., Maxwell, J. R., Chan, L. A., Robinson, S. Modeling encephalopathy of prematurity using prenatal hypoxia-ischemia with intra-amniotic lipopolysaccharide in rats. J Vis Exp. (105), e53196 (2015).
- Kitase, Y., et al. Chorioamnionitis disrupts erythropoietin and melatonin homeostasis through the placental-fetal-brain axis during critical developmental periods. Front Physiol. 14, 1201699 (2023).
- Maxwell, J. R., Denson, J. L., Joste, N. E., Robinson, S., Jantzie, L. L. Combined in utero hypoxia-ischemia and lipopolysaccharide administration in rats induces chorioamnionitis and a fetal inflammatory response syndrome. Placenta. 36 (12), 1378-1384 (2015).
- Ozen, M., et al. Chorioamnionitis precipitates perinatal alterations of heme-oxygenase-1 (HO-1) homeostasis in the developing rat brain. Int J Mol Sci. 22 (11), 5773 (2021).
- Yellowhair, T. R., et al. CXCR2 blockade mitigates neural cell injury following preclinical chorioamnionitis. Front Physiol. 10, 324 (2019).
- Yellowhair, T. R., et al. Chorioamnionitis in rats precipitates extended postnatal inflammatory lymphocyte hyperreactivity. Dev Neurosci. , 1-11 (2019).
- Yellowhair, T. R., et al. Preclinical chorioamnionitis dysregulates CXCL1/CXCR2 signaling throughout the placental-fetal-brain axis. Exp Neurol. 301 (Pt B), 110-119 (2018).
- Jantzie, L. L., et al. Complex pattern of interaction between in utero hypoxia-ischemia and intra-amniotic inflammation disrupts brain development and motor function. J Neuroinflammation. 11, 131 (2014).
- Jantzie, L. L., et al. Repetitive neonatal erythropoietin and melatonin combinatorial treatment provides sustained repair of functional deficits in a rat model of cerebral palsy. Front Neurol. , 233 (2018).
- Maxwell, J. R., et al. Prenatal alcohol exposure and chorioamnionitis results in microstructural brain injury in a preclinical investigation. Ann Pediatr Res. 4 (1), 1031 (2020).
- Karimy, J. K., et al. Outcomes of the 2019 hydrocephalus association workshop, "Driving common pathways: extending insights from posthemorrhagic hydrocephalus". Fluids Barriers CNS. 20 (1), 4 (2023).
- Jakopin, N. E., et al. Establishing ranked priorities for future hydrocephalus research. J Neurosurg. 139 (2), 492-501 (2022).
- Koschnitzky, J. E., et al. Opportunities in posthemorrhagic hydrocephalus research: outcomes of the Hydrocephalus Association Posthemorrhagic Hydrocephalus Workshop. Fluids Barriers CNS. 15 (1), 11 (2018).
- Strahle, J., et al. Mechanisms of hydrocephalus after neonatal and adult intraventricular hemorrhage. Transl Stroke Res. 3 (Suppl 1), 25-38 (2012).
- Segado-Arenas, A., et al. Cognitive impairment and brain and peripheral alterations in a murine model of intraventricular hemorrhage in the preterm newborn. Mol Neurobiol. 55 (6), 4896-4910 (2018).
- Li, Q., et al. Targeting germinal matrix hemorrhage-induced overexpression of sodium-coupled bicarbonate exchanger reduces posthemorrhagic hydrocephalus formation in neonatal rats. J Am Heart Assoc. 7 (3), e007192 (2018).
- Gaisie, G., Roberts, M. S., Bouldin, T. W., Scatliff, J. H. The echogenic ependymal wall in intraventricular hemorrhage: sonographic-pathologic correlation. Pediatr Radiol. 20 (5), 297-300 (1990).
- Mohammad, K., et al. Consensus approach for standardizing the screening and classification of preterm brain injury diagnosed with cranial ultrasound: A Canadian perspective. Front Pediatr. 618236, (2021).
- Gao, C., et al. Role of red blood cell lysis and iron in hydrocephalus after intraventricular hemorrhage. J Cereb Blood Flow Metab. 34 (6), 1070-1075 (2014).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved