Method Article
Acclimation Prior to an Intraperitoneal Insulin Tolerance Test to Mitigate Stress-Induced Hyperglycemia in Conscious Mice
In This Article
Summary
Stress-induced elevations in glucose levels can confound interpretation of data derived from a conscious intraperitoneal insulin tolerance test in mice. In this article, we describe a method to acclimate the mice to handling, injections and blood sampling prior to performing the insulin tolerance test in order to limit stress-induced hyperglycemia.
Abstract
The insulin tolerance test is commonly used in metabolic studies to assess whole body insulin sensitivity in rodents. It is a relatively simple test that involves measurement of blood glucose levels over time following a single intraperitoneal injection of insulin. Given that it is performed in the conscious state and blood is often collected via a tail snip, it has the potential to elicit a stress response from animals due to anxiety associated with handling and blood collection. As such, a stress-induced rise in blood glucose can occur, making it difficult to detect and interpret the primary endpoint measure, namely an insulin-mediated reduction in blood glucose. This has been seen in many mouse strains, and is quite common in diabetic db/db mice, where glucose levels can increase, rather than decrease, after insulin administration. Here, we describe a method of acclimating mice to handling, injections and blood sampling prior to performing the insulin tolerance test. We find that this lowers stress-induced hyperglycemia and results in data that more accurately reflects whole body insulin sensitivity.
Introduction
Metabolic tests in rodents are routinely performed to assess various parameters that regulate glucose homeostasis1. The gold standard for assessing whole-body insulin action in vivo is the hyperinsulinemic-euglycemic clamp2. This test involves administration of insulin to raise circulating insulin levels while glucose is infused to maintain euglycemia. The glucose infusion rate required to maintain euglycemia is indicative of insulin action. While it is a powerful tool in metabolic research, the clamp technique in mice is technically challenging and labor intensive, and thus is not well suited as an initial screening tool in characterizing a metabolic phenotype. For these reasons, the simpler intraperitoneal insulin tolerance test (ITT) is often chosen.
The ITT is performed in the conscious state following a fasting period (typically 4-6 hours). A bolus of insulin is administered intraperitoneally, after which blood glucose is monitored over a timeframe that usually lasts 60 min. Blood glucose levels are expected to fall due to the ability of insulin to facilitate glucose uptake into insulin-sensitive tissues; the degree to which this occurs is indicative of whole-body insulin action. In some cases, it has been shown that glucose levels paradoxically increase, rather than decrease, after insulin administration. This phenomenon is likely attributed to a stress response. Handling, injections and blood sampling can all induce stress3,4,5, resulting in activation of the hypothalamic-pituitary-adrenal axis (HPA) and the autonomic nervous system (ANS)6,7,8. It is well known that both the HPA and ANS contribute to increases in circulating glucose levels9,10,11. The presence of stress-induced hyperglycemia at the beginning of an ITT is problematic, as it interferes with the rate and magnitude of the fall in glucose upon insulin administration12. This can lead to erroneous conclusions regarding the presence of insulin resistance. Thus, to mitigate the confounding impact of stress on glucose levels during an ITT, we have developed a method of acclimating mice to handling, injections and blood sampling prior to performing the ITT.
Protocol
All methods described here have been approved by the VA Puget Sound Health Care System’s Institutional Animal Care and Use Committee.
NOTE: Local requirements for monitoring and/or intervention of animals that experience hypoglycemia may differ from those described here.
1. Fasting (t= -210 min)
- After the dark cycle has ended, transfer mice to a new cage with non-nutritious bedding such as cellulose or paper bedding (not corn-cob bedding, which will affect metabolic endpoints if consumed by mice13). Provide mice with ad libitum access to water throughout the fasting period. Be consistent with the fasting time of day and duration across groups of mice. For the data shown, food was removed between 0700 and 0800 hours, which was 1-2 hours after the dark cycle had ended.
- Move cage(s) to the location where the ITT will be performed. This should be a quiet space where stressors such as temperature, noise, light or movement are minimized.
2. Acclimation (t= -150 to -60 min)
IMPORTANT: Handle mice as gently as possible. Avoid use of a restraint device if possible.
- At -150 min, measure body weight. This will be used for calculation of the volume of insulin that will be administered for the ITT.
- Pick up the mouse gently by the tail and rest on a flat tabletop surface while still gently gripping the tail. Use a 20 G needle (or surgical scissors) to make a small incision in the tip of the tail. A drop of blood should begin to form at the site.
- Set the mouse on a smooth hard surface and restrain the mouse gently by the tail.
- To record blood glucose at this time, place a drop of blood from the tail tip on the test strip of a handheld glucometer. If necessary, very gently massage the tail to obtain a drop of blood.
- Draw up 100 µL of sterile saline into an insulin syringe. Pick up the mouse using gentle scruffing and inject intraperitoneally. Record the time of saline injection.
- If multiple mice are being studied, inject the mice with saline at 1 min intervals to allow time for subsequent steps below.
- At 15 min after saline injection, pick up the mouse gently by the tail. Use gauze to gently dislodge any blood clot on the tail tip.
- Optional: Record blood glucose again at this time as described in step 2.3.
- At 30 min after the saline injection gently pick up the mouse by the tail. If desired, obtain another blood glucose measurement as described in step 2.3.
- Return the mouse to the cage, and repeat for other mice as necessary. Leave mice undisturbed until -90 min.
- At -90 min, repeat steps 2.3 through 2.6, including measurement of blood glucose levels if desired.
- Return the mouse to the cage, repeat for other mice as necessary. Leave mice undisturbed until the ITT (for which the baseline blood glucose measurement is done at -5 min).
3. Insulin Tolerance Test (t= -5 to +60 min)
- Prepare a working solution of regular insulin (CAUTION) in sterile saline, such that the desired dose can be injected at 4 µL/g body weight.
NOTE: Caution should be used when handling insulin as accidental injection can result in hypoglycemia. - Prepare a 25% (v/v) dextrose solution in sterile saline to have on hand in case mice develop hypoglycemia requiring intervention.
- At -5 min, pick up the mouse gently by the tail. Determine the baseline blood glucose level at this time, by placing a drop of blood from the tail tip on the test strip of a handheld glucometer. If necessary, very gently massage the tail to obtain a drop of blood.
- Draw up insulin working solution into an insulin syringe (4 µL/g body weight, for a typical dose range of 0.5-2.0 U/kg). Pick up the mouse using gentle scruffing and inject intraperitoneally. Record time of insulin injection.
- If multiple mice are being studied, inject the mice with insulin at 1 min intervals to allow time for subsequent blood sampling and data recording below.
- At 15 min after insulin injection, pick up the mouse gently by the tail. Use gauze to gently dislodge any blood clot on the tail tip. Determine the blood glucose again at this time as described in step 3.3.
- Return the mouse to the cage and monitor for signs of hypoglycemia (e.g., excessive lethargy). If mice develop symptoms of hypoglycemia, measure blood glucose as described in step 5 and administer dextrose if necessary. A mouse undergoing dextrose intervention would be removed from the ITT protocol at this time.
- Repeat steps 3.5 and 3.6 at 30 min after insulin injection.
- Repeat steps 3.5 and 3.6 at 45 min after insulin injection.
- Repeat steps 3.5 and 3.6 at 60 min after insulin injection.
- Return the mice to their home cage, containing a few food pellets on the floor of the cage to aid in recovery from the ITT. Continue to monitor for 30 min and if mice have not regained normal activity/behavior follow procedure described in step 3.6.
NOTE: Figure 1 summarizes the above acclimation and ITT procedure.
Results
Figure 2A and Figure 3A are representative data (individual and mean data, respectively) showing a paradoxical rise in blood glucose levels in diabetic db/db mice in the 15 minutes following insulin administration, consistent with stress-induced hyperglycemia. Note, no rise in blood glucose was evident in the control non-diabetic littermates (db/+ or +/+) that underwent the same procedure. To determine whether acclimation to the ITT procedure was effective in mitigating this increase in blood glucose, the same mice underwent the acclimation protocol described above. Each major step is designated by vertical lines within the graph (Figure 2B) and blood samples were taken each time the mice were handled. Handling and/or saline injection at -150 min resulted in a rise in blood glucose in 3 of the 4 db/db mice (red circles); the fourth mouse had blood glucose levels >600 mg/dL. As 600 mg/dL is the upper limit of detection for the glucometer used, we were unable to ascertain whether blood glucose rose in the fourth mouse. The subsequent handling at -90 min resulted in increased blood glucose in 2 mice. Blood glucose levels were not affected by handling in control mice (blue triangles), though by t = 0, blood glucose levels were lower than at -60 min (150 ± 6 vs. 175 ± 8 mg/dL; n = 4, p = 0.02). Blood glucose levels during the ITT following acclimation decreased at all time points following insulin administration. Mean data for ITT before and after acclimation are shown in Figure 3. Note that overall glucose levels were lower in acclimated versus non-acclimated diabetic db/db mice (Figure 3A; p < 0.05). Further, the fall in blood glucose levels during the first 15 min after insulin administration was greater in non-diabetic littermate controls (Figure 3B; p = 0.002) following the acclimation protocol.

Figure 1. Schematic depicting the handling, injection and blood sampling procedure during acclimation and the ITT. Please click here to view a larger version of this figure.
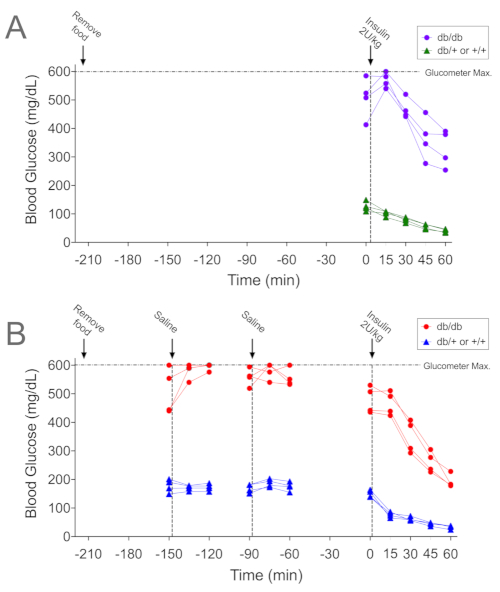
Figure 2. Representative examples of ITTs performed with and without acclimation paradigm. (A) Blood glucose levels in diabetic db/db mice (purple circles) and littermate non-diabetic controls (db/+ or +/+; green triangles) during an ITT (2 U/kg) performed following a 3.5 h (210 minute) fast but no additional handling. (B) Blood glucose levels from the same mice (db/db in red circles, controls in blue triangles) subsequently undergoing the acclimation procedure described here, followed by the ITT (2 U/kg). The time interval between procedures in Panel A versus Panel B was 6 days. Data are shown for individual mice (n=4 per genotype). Please click here to view a larger version of this figure.

Figure 3. Mean blood glucose data for ITT performed before and after acclimation. (A) Mean (± SD) blood glucose levels from the same db/db mice during ITT performed without (purple) or with (red) acclimation procedure; note that the acclimation procedure abolishes the rise in glucose levels between 0 and 15 min. Also, overall glucose levels are lower in acclimated versus non-acclimated diabetic db/db mice (p<0.05). n=4. (B) Mean (± SD) blood glucose levels from the same control db/+ or +/+ mice during ITT performed without (green) or with (blue) acclimation procedure; note that the acclimation procedure results in a steeper decline in glucose levels between 0 and 15 min (p=0.002). n=4. Please click here to view a larger version of this figure.
Discussion
The hyperinsulinemic-euglycemic clamp is considered the gold standard for assessing insulin action in vivo. Modifications to the methodology for performing the clamp have resulted in the technique being done in conscious, unrestrained mice2 that have been previously catheterized using a two-catheter system14 to enable blood sampling via the carotid artery and infusions via the jugular vein. This limits the need to handle or restrain mice during the procedure, thereby reducing stress responses2. It is well recognized that stress can confound various endpoint measures in metabolic studies1,3. Stress can be induced by various factors in addition to handling, including blood sampling, olfactory stimuli, noise, light, and anesthesia3,4,5. Consequent activation of the hypothalamic-pituitary-adrenal axis (HPA) and the autonomic nervous system (ANS) can have profound effects on glucose levels9,10,11.
While measurement of insulin action by clamping mice is the preferred method, it is often not feasible as it is technically challenging and labor intensive. For these reasons, the simpler ITT is more commonly performed in mice. ITTs monitor changes in blood glucose over time (typically 60 min) in response to an intraperitoneal bolus of insulin in mice that are fasted. A small volume of blood is sampled via tail tip at baseline and every 15 min thereafter. Considerations in performing ITTs have been previously discussed, particularly with respect to the period of fasting, the dose of insulin, and presentation of resulting data1,12. Here, we have considered the impact of stress prior to the ITT on the subsequent glucose response to insulin. We came to this from studies in db/db mice, where we consistently observed a paradoxical increase, rather than decrease in blood glucose following administration of insulin. Upon acclimating the mice to handling and injections prior to performing the ITT, we found that we could mitigate the rise in blood glucose. This suggests stress likely plays a role in the apparent hyperglycemia observed at the onset of the ITT. This phenomenon appears to be common in the literature. A PubMed search was performed using the search terms “db” and “insulin tolerance”, yielding 101 results (publication dates 1998 to 2019). Of those, 76 publications had accessible full text content, performed ITTs in db/db mice and displayed ITT data as glucose levels over time (raw values and/or percent of baseline values). Among those 76 studies, blood glucose levels were increased during the ITT in untreated db/db mice in 16 studies (21%). An additional 4 studies showed increases in glucose levels in other groups of mice (e.g., drug-treated db/db or control non-db mice). Finally, an additional 10 studies failed to observe a decrease in glucose levels following insulin administration in db/db mice over the course of the ITT, though this may have been related to the dose of insulin used or a state of marked insulin resistance in the mice. While our
study and literature review focus on db/db mice, it is highly likely that this phenomenon also occurs in other mouse strains. Thus, a greater awareness of the impact of stress is needed when performing ITTs, particularly in terms of how it may affect data interpretation.
To that end, we have developed an acclimation protocol that can be performed prior to initiating the ITT. The critical steps include handling and injecting mice without the use of a restraining device. Special attention should be given to the ambient noise, lighting, temperature and any movement in the room in which the ITT will be performed. It is advised that mice be transferred to this room at the start of the fasting period, so as to minimize exposure to changes in the environment immediately before the ITT begins. It is also important for the investigator to remain calm as this may impact anxiety-related behaviors in mice. In db/db mice, we found that the acclimation procedure abolished the rise in glucose levels between 0 and 15 min during the ITT. Further, in non-diabetic control mice, the acclimation procedure resulted in a steeper decline in glucose levels between 0 and 15 min during the ITT. Importantly, non-diabetic control mice that had not been acclimated did not exhibit a rise in glucose levels at the onset of the ITT, yet upon acclimation they still responded favorably by displaying a greater rate of glucose disposal. The latter suggests that stress during an ITT may not always manifest itself as elevated glucose levels above baseline, however taking steps to mitigate stress may avoid misinterpretation of the data.
The data support the implementation of an acclimation period prior to performing an ITT. However, the method proposed has some limitations. Perhaps the most obvious of these is the need to handle the mice. Specifically, the protocol involves mice being picked up by the tail. A previous study compared glucose levels using this handling method with the “cup” method, which involves being scooped up and being free to roam in the handler’s open gloved hands without direct physical restraint. It was found that the cup method resulted in lower fasting glucose levels and improved glucose tolerance, as well as lower plasma corticosterone concentrations and reduced anxiety-like behaviors15,16. Thus, the method of mice being picked up by the tail may still elicit a stress response, though it is important to recognize that in general, handling positively habituates animals to human contact. Another limitation is the need to sample blood from the tail, which can itself induce stress. In a study comparing catecholamine levels in samples obtained via tail cutting versus artery sampling 17, catecholamines were higher in the former indicating that cut tail sampling can be stressful. However, this is only the case when large blood volumes (~100 μL) are sampled and the tail is massaged or squeezed. Indeed, when minimal volumes of blood (~5 μL) were sampled from the tail, catecholamine levels were not elevated17. Thus, given that the acclimation (and ITT) procedure only requires a small blood volume for glucometer readings, the cut tail method is a useful technique that potentially avoids a stress response. A drawback of the study that limits a more definitive assertion in this regard is the fact that stress hormones were not measured (which would have required a large blood volume).
As evidenced by the data, the acclimation procedure is useful for obtaining more reliable ITT data from both diabetic and non-diabetic mice. It is possible that additional refinements to the procedure will further lessen the impact of stress during an ITT. For example, during the acclimation period, we propose “mock” injections in which mice receive i.p. saline. It would be useful to know whether delivery of the saline is actually necessary, or whether mice can simply be handled and an empty syringe inserted intraperitoneally. Also, the number of times mice are handled, as well as the period between each handling, could be tested. Currently, the protocol indicates that they be handled twice during the acclimation period, with a 60 min interval between the start of each handling period. Additionally, we incorporate a 60 min recovery period from the last handling/injection to the start of the ITT. It remains to be determined whether a shorter or longer recovery period would further mitigate the stress-induced hyperglycemia we observe in db/db mice. Previously, it was reported that in response to stressful stimuli such as handling, noise and i.p. injections, rodents exhibit elevated plasma corticosterone levels for at least 2 hours18, suggesting that perhaps a longer recovery period would be useful. Further, stress responses can differ amongst different strains of mice. For instance, tail handling can trigger seizures in susceptible strains19. In considering all of these variables, we propose that the precise conditions for acclimation prior to an ITT may need to be determined empirically by each investigator, using our protocol as a starting point.
In summary, we have described a method of acclimating mice to handling, injections and blood sampling prior to performing ITTs. We find that this significantly lowers stress-induced hyperglycemia and results in data that more accurately reflects insulin action.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported by the National Institutes of Health grant P30 DK-017047 (University of Washington Diabetes Research Center, Cell Function Analysis Core), and the United States Department of Veterans Affairs, VA Puget Sound Health Care System (Seattle, WA). The contents of this manuscript do not represent the views of the U.S. Department of Veteran Affairs or the United States Government.
Materials
| Name | Company | Catalog Number | Comments |
| Dextrose-50 | Pfizer Injectables | 00409-6648-16 | For use if mouse experiences hypoglycemia. |
| Gauze pads | Fisher Scientific | 22037907 | To dislodge blood clot on the tail tip. |
| Glucometer | Accu-Chek | M001_us | To measure blood glucose. |
| Gram scale | To measure body weight. | ||
| Insulin (Novolin R) | Novo Nordisk | 0169-1833-11 | For injection. |
| Insulin syringes | VWR | BD-329461 | For injections. |
| Minute timer | |||
| Sterile 20 G needle | VWR | BD-305175 | For tail snip. |
| Sterile saline | Lifeshield | 1261699 | For injections. |
| Surgical scissors | Fine Science Tools | 14088-10 | For tail snip. |
| Test strips | Accu-Chek | 06908217001_us | To measure blood glucose. |
References
- Ayala, J. E., et al. Standard operating procedures for describing and performing metabolic tests of glucose homeostasis in mice. Disease Models & Mechanisms. 3 (9-10), 525-534 (2010).
- Ayala, J. E., et al. Hyperinsulinemic-euglycemic clamps in conscious, unrestrained mice. Journal of Visualized Experiments. (57), e3188 (2011).
- Balcombe, J. P., Barnard, N. D., Sandusky, C. Laboratory routines cause animal stress. Contemporary Topics in Laboratory Animal Science. 43 (6), 42-51 (2004).
- Tabata, H., Kitamura, T., Nagamatsu, N. Comparison of effects of restraint, cage transportation, anaesthesia and repeated bleeding on plasma glucose levels between mice and rats. Lab Animal. 32 (2), 143-148 (1998).
- Olfe, J., Domanska, G., Schuett, C., Kiank, C. Different stress-related phenotypes of BALB/c mice from in-house or vendor: alterations of the sympathetic and HPA axis responsiveness. BMC Physiology. 10, 2 (2010).
- Ghalami, J., Zardooz, H., Rostamkhani, F., Farrokhi, B., Hedayati, M. Glucose-stimulated insulin secretion: Effects of high-fat diet and acute stress. Journal of Endocrinological Investigation. 36 (10), 835-842 (2013).
- Thorens, B. Brain glucose sensing and neural regulation of insulin and glucagon secretion. Diabetes, Obesity and Metabolism. 13, 82-88 (2011).
- Pekow, C. Defining, measuring, and interpreting stress in laboratory animals. Contemporary Topics in Laboratory Animal Science. 44 (2), 41-45 (2005).
- Chan, O., Inouye, K., Riddell, M. C., Vranic, M., Matthews, S. G. Diabetes and the hypothalamo-pituitary-adrenal (HPA) axis. Minerva Endocrinol. 28 (2), 87-102 (2003).
- Rosmond, R. Role of stress in the pathogenesis of the metabolic syndrome. Psychoneuroendocrinology. 30 (1), 1-10 (2005).
- Nonogaki, K. New insights into sympathetic regulation of glucose and fat metabolism. Diabetologia. 43 (5), 533-549 (2000).
- McGuinness, O. P., Ayala, J. E., Laughlin, M. R., Wasserman, D. H. NIH experiment in centralized mouse phenotyping: the Vanderbilt experience and recommendations for evaluating glucose homeostasis in the mouse. American Journal of Physiology-Endocrinology and Metabolism. 297 (4), 849-855 (2009).
- Zahorsky-Reeves, J., LW, C. Housing mice on corncob bedding versus hardwood chip may confound research models. American Association for Laboratory Animal Science. , (2010).
- Niswender, K. D., Shiota, M., Postic, C., Cherrington, A. D., Magnuson, M. A. Effects of increased glucokinase gene copy number on glucose homeostasis and hepatic glucose metabolism. Journal of Biological Chemistry. 272 (36), 22570-22575 (1997).
- Ghosal, S., et al. Mouse handling limits the impact of stress on metabolic endpoints. Physiology & Behavior. 150, 31-37 (2015).
- Hurst, J. L., West, R. S. Taming anxiety in laboratory mice. Nature Methods. 7 (10), 825-826 (2010).
- Ayala, J. E., Bracy, D. P., McGuinness, O. P., Wasserman, D. H. Considerations in the design of hyperinsulinemic-euglycemic clamps in the conscious mouse. Diabetes. 55 (2), 390-397 (2006).
- Barrett, A. M., Stockham, M. A. The effect of housing conditions and simple experimental procedures upon the corticosterone level in the plasma of rats. Journal of Endocrinology. 26, 97-105 (1963).
- Heinrichs, S. C. Neurobehavioral consequences of stressor exposure in rodent models of epilepsy. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 34 (5), 808-815 (2010).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved