Determination Of NOx in Automobile Exhaust Using UV-VIS Spectroscopy
Przegląd
Source: Laboratories of Margaret Workman and Kimberly Frye - Depaul University
In the troposphere, ozone is naturally formed when sunlight splits nitrogen dioxide (NO2):
NO2 + sunlight → NO + O
O + O2 → O3
Ozone (O3) can go on to react with nitric oxide (NO) to form nitrogen dioxide (NO2) and oxygen:
NO + O3 → NO2 + O2
This results in no net gain of ozone (O3). However, with the anthropogenic production of ozone forming precursors (NO, NO2, and volatile organic compounds) through the combustion of fossil fuels, elevated levels of ozone in the troposphere have been found. Motor vehicle exhaust is a significant source of these ozone forming precursors: NO, NO2, and volatile organic compounds (VOCs). For example, mobile sources make up nearly 60% of NO + NO2 emissions.
At the high temperatures found in a car’s combustion chamber, nitrogen and oxygen from the air react to form nitric oxide (NO) and nitrogen dioxide (NO2):
N2(g) + O2 (g)→ 2 NO(g)
2 NO(g) + O2(g)→ 2 NO2(g)
The nitric oxide (NO) emitted in the car exhaust is gradually oxidized to nitrogen dioxide (NO2) in ambient air. This mixture of NO and NO2 is often referred to as NOx. When NOx reacts with volatile organic compounds in the atmosphere in the presence of sunlight, tropospheric ozone forms, as seen in this simplified chemical reaction:
NOx + VOCs + sunlight → O3 + other products
This noxious mixture of air pollution, which can include aldehydes, peroxyacetyl nitrates, ozone, VOCs, and NOx, is called photochemical smog. Ozone is the largest component of photochemical smog. This smog is found in all modern cities, but it’s found especially in cities with sunny, warm, dry climates and large numbers of motor vehicles. The yellow-brown color of smog in the air is due in part to the nitrogen dioxide present, since this gas absorbs visible light near 400 nm (Figure 1).
Short-term NO2 exposure (30 min to 1 day) leads to adverse respiratory effects in healthy people and increased respiratory symptoms in people with asthma. NOx reacts with ammonia and other compounds to form particulates. These small particles can penetrate the lungs and cause respiratory problems, including emphysema and bronchitis. Individuals who spend a lot of time on the road or who live near a roadway experience considerably higher exposure to NO2.
Due to the impact it has on human health and the environment, the U.S. Environmental Protection Agency (EPA) has classified NO2 as a criteria pollutant and has set the primary standard at 100 ppb (98th percentile of 1-h daily maximum concentrations, averaged over 3 years) and 53 ppb (annual mean). Considering that on-road vehicles account for approximately 1/3 of NOx emissions in the U.S., automobile emissions are therefore regulated through the Clean Air Act. The U.S. EPA established emission standards that automobile manufacturers must follow when producing cars. Currently, Tier 2 emission standards set that manufacturers must have fleet average NOx emissions of no more than 0.07 g/mile.
One way manufacturers meet this standard is by using catalytic converters on their cars. This device is placed between the engine and the tailpipe. The exhaust stream passes through the catalytic converter and is exposed to a catalyst. A reduction catalyst of platinum and rhodium is used to reduce the NOx concentration in the exhaust. When an NO or NO2 molecule in the exhaust contacts the catalyst, the nitrogen atom is grabbed off the molecule and held onto by the catalyst. The oxygen is freed and forms O2. The nitrogen atom on the catalyst binds with another nitrogen atom held on the catalyst to form N2.
Catalytic converters have greatly reduced the emissions of NOx from car exhaust – up to 80% reduction, when performing properly. However, they only work when they have reached a fairly high temperature. Therefore, when doing a cold start of a car, the catalytic converter is removing virtually no NOx. It isn’t until the catalytic converter reaches higher temperatures that it effectively removes the NOx from the exhaust stream. Catalytic converters do not work on diesel passenger cars due to the lean conditions under which they operate. In addition, the sulfur in diesel fuel also deactivates the catalyst. The NOx in diesel engines are reduced mainly through the exhaust gas recirculation (EGR) valve, which cools the temperature of the combustion gases. As a result, diesel cars generally emit more NOx than gasoline cars.

Figure 1. Characteristic coloration for smog in California in the beige cloud bank behind the Golden Gate Bridge. The brown coloration is due to the NOx in the photochemical smog.
Zasady
In this experiment, the concentration of NOx in the exhaust stream of various brands of automobiles are measured twice (upon start-up and after 10 min) to study the ability of the car’s catalytic converter to remove NOx from the exhaust. The catalytic converter requires a high temperature to be effective. Therefore, the measurement of the exhaust upon start-up of the car represents the amount of NOx in the exhaust without the catalytic converter working. The measurement of the exhaust after 10 min represents the amount of NOx in the exhaust after the catalytic converter is in effect.
The NOx concentration is determined colorimetrically by diazotization of sulfanilic acid and subsequent reacting with N-(1-naphthyl)-ethylenediamine and measuring the color intensity of the resulting azo dye molecule using a UV-VIS spectrophotometer set at 550 nm.
In solution, NO and NO2 undergo the following reactions to form NO2-:
2 NO2(g) + H2O(l) → 2H+(aq) + NO2-(aq) + NO3-(aq)
4NO(g) + O2(g) + 2 H2O(l) → 4 NO2-(aq) + 4 H+(aq)
Although the expected ratio between NO2 and NO2- is 2:1 based on the first equation listed previously, it has been determine empirically to be 1.39:1.
When sulfanilic acid and N-(1-naphthyl)-ethylenediamine are added to the solution, a pink-colored molecule develops (Figure 2).
The concentration of this pink-colored molecule is directly proportional to the concentration of the NOx in the solution. The concentration of the azo dye molecule is measured using a UV-VIS spectrophotometer set at 550 nm.
UV-VIS spectroscopy is based on the measurement of the absorbance (A) of solutions held in a transparent container of width b (in cm). The concentration of the absorbing species is directly proportional to the absorbance, as seen in the following equation:
A =  b c
b c
where  is the molar absorptivity. This equation is known as Beer’s Law. The molar absorptivity is a measure of how strongly a substance absorbs light at a given wavelength and is a constant for a given substance.
is the molar absorptivity. This equation is known as Beer’s Law. The molar absorptivity is a measure of how strongly a substance absorbs light at a given wavelength and is a constant for a given substance.
To measure the absorbance of a solution, a beam of light with intensity Io is aimed at the solution in a cuvette (Figure 3). The intensity of the entering beam (Io) and the emerging beam (I) are measured, and the absorbance is calculated by:


Figure 2. A pink-colored molecule that develops when sulfanilic acid and N-(1-naphthyl)-ethylenediamine are added to the solution.

Figure 3. A beam of light with intensity Io aimed at the solution in a cuvette.
Procedura
1. Preparation of Nitrite (NO2-) Stock Solution
- Weigh out 1.500 g NaNO2 and add to a 1-L volumetric flask.
- Dilute to the mark using nanopure water. (Check the distilled water from the tap – it may contain enough nitrite to interfere with the measurements.) This produces a 1,000 µg NO2-/mL stock solution.
- To make a 5.0 µg NO2-/mL solution, take 1 mL of the 1,000 µg NO2-/mL solution and dilute to 200 mL in a volumetric flask.
2. Preparation of NOx Indicator Solution
- Weigh out 5.0 g of anhydrous sulfanilic acid and add to a 1-L volumetric flask.
- Add 500 mL of nanopure water.
- Add 140 mL of glacial acetic acid.
- Using a stir bar, stir the solution until the sulfanilic acid dissolves. This takes approximately 30 min.
- Weigh out 0.020 g of N-(1-naphthyl)-ethylenediamine dihydrochloride and add to the volumetric flask.
- Dilute to the mark using nanopure water.
- Transfer to a dark bottle (to prevent photodecomposition) and stopper tightly (to prevent reaction with air).
3. Preparation of Calibration Standards
- Put 1.0 mL of the 5.0 µg NO2-/mL solution in a 25 mL volumetric flask and dilute with the NOx indicator solution to the mark. This makes a 0.2 µg NO2-/mL standard solution.
- Put 2.0 mL of the 5.0 µg NO2-/mL solution in a 25 mL volumetric flask and dilute with the NOx indicator solution to the mark. This makes a 0.4 µg NO2-/mL standard solution.
- Put 3.0 mL of the 5.0 µg NO2-/mL solution in a 25 mL volumetric flask and dilute with the NOx indicator solution to the mark. This makes a 0.6 µg NO2-/mL standard solution.
- Put 4.0 mL of the 5.0 µg NO2-/mL solution in a 25 mL volumetric flask and dilute with the NOx indicator solution to the mark. This makes a 0.8 µg NO2-/mL standard solution.
- Put 5.0 mL of the 5.0 µg NO2-/mL solution in a 25 mL volumetric flask and dilute with the NOx indicator solution to the mark. This makes a 1.0 µg NO2-/mL standard solution.
4. Creation of the Standard Curve
- Using a UV-VIS spectrophotometer, set the instrument to read Absorbance.
- Set the wavelength to 550 nm on the spectrophotometer.
- Using the NOx indicator solution, zero the spectrophotometer.
- Measure the absorbance of the 5 standard solutions. Record values on the data table (Table 1).
5. Automobile Exhaust Sample Measurement
- Start the diesel-powered automobile.
- Using a 60 mL gas-tight syringe, insert it a few inches into the tailpipe. Avoid burns and don’t breathe in fumes. Draw in and expel the exhaust twice to condition the syringe.
- Draw 25 mL of the NOx indicator solution into the syringe. Expel any air from the syringe without spilling the indicator solution.
- Draw 35 mL of exhaust into the syringe, pulling the plunger to the 60 mL mark.
- Cap the syringe. Shake the solution in the syringe for 2 min. Cover the syringe with aluminum foil.
- Measure the air temperature at the tailpipe when collecting the samples.
- Repeat steps 5.1 – 5.6 using a gasoline-powered automobile. These steps can be repeated as many times as desired, using various models of automobiles.
- Repeat steps 5.1 – 5.6 after the automobiles have been running at least 10 min.
- Wait 45 min to allow the color to develop, before measuring the absorbance of the solution.
- After the 45 min are up, expel the gas from the syringe, put the solution into a cuvette, and measure the absorbance using the spectrophotometer set at 550 nm. Record values on the data table (Table 1).
| Sample | Absorbance |
| 0.2 µg NO2-/mL standard | |
| 0.4 µg NO2-/mL standard | |
| 0.6 µg NO2-/mL standard | |
| 0.8 µg NO2-/mL standard | |
| 1.0 µg NO2-/mL standard | |
| Diesel Car Exhaust (upon startup) | |
| Diesel Car Exhaust (after running 10 min) | |
| Gasoline Car Exhaust (upon startup) | |
| Gasoline Car Exhaust (after running 10 min) |
Table 1. Blank data table to record values of absorption.
Wyniki
Table 2 provides an example of proper results. Using the absorbance measurements of the standard solutions, a plot of Absorbance vs. Concentration of NO2- can be made (Figure 4). Then, the best fit line of the data can be determined. Using the best-fit line of the standard curve, the concentration of NO2- in each unknown solution (µg/mL) can be calculated. This value can be converted to the concentration of NO2 in the exhaust gaseous sample using the following equation:
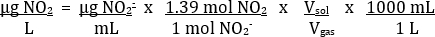
Based on the balanced equation of NO2 in H2O seen previously, a 2 mol NO2/1 mol NO2- ratio is expected. In empirical experiments, it has been found to be nearer a 1.39:1 ratio. The volume of solution used was 25 mL. The volume of the gas sample was 35 mL.
The concentration of NO2 calculated actually represents all of the NOX in the exhaust sample (Table 3). The equation for conversion between ppmV and µg/L depends on the temperature and pressure at which the samples were collected. The conversion equation is:

Where R = universal gas constant = 0.08206 atm·L/mol·K, P = atmospheric pressure in atm, T = temperature in K, and MW = molecular weight of NOx (as NO2) = 46.01 g/mol. Therefore,
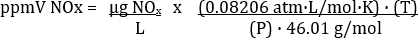
It’s important to input T in K and P in atm.
| Sample | Absorbance |
| 0.2 µg NO2-/mL standard | 0.22 |
| 0.4 µg NO2-/mL standard | 0.43 |
| 0.6 µg NO2-/mL standard | 0.60 |
| 0.8 µg NO2-/mL standard | 0.79 |
| 1.0 µg NO2-/mL standard | 1.05 |
| Diesel Car Exhaust (upon startup) | 1.03 |
| Diesel Car Exhaust (after running 10 min) | 1.03 |
| Gasoline Car Exhaust (upon startup) | 0.10 |
| Gasoline Car Exhaust (after running 10 min) | 0.04 |
Table 2. Data table with representative results of absorption.

Figure 4. A standard curve plot of Absorbance vs. Concentration of NO2-.
| Vehicle | NOx Concentration (ppm) |
| Diesel Car (upon startup) | 500 |
| Diesel Car (after running 10 minutes) | 500 |
| Gasoline Car (upon startup) | 48 |
| Gasoline Car (after running 10 minutes) | 21 |
Table 3. NOx concentration (ppm) per vehicle.
Wniosek i Podsumowanie
The measurement of nitrite using the modified Saltzman reaction is very common and useful in many different fields. As described, the method can be used to measure NOx concentrations in air samples – car exhaust, laboratory rooms, air quality of cities, etc. In addition, this method can be used to monitor NOx in cigarette smoke. The procedure would be very similar to this experiment, except instead of drawing car exhaust into the syringe, cigarette smoke would be drawn in. There is often a higher concentration of NOx in cigarette smoke than coming out of the tailpipe of automobiles, which tends to be surprising to many. Typical values for NOx in cigarette smoke range from 500-800 ppm.
This method can also be used to test the levels of nitrate produced in the presence of nitrification bacteria. Nitrification bacteria are found in soil and water and play an important role in the nitrogen cycle – oxidizing ammonia to nitrite and then nitrate. The nitrate in the sample is first converted to nitrite by the enzyme nitrate reductase. Then the nitrite is measured using the modified Saltzman reaction. Lastly, this method can be used to determine the concentration of nitrates and nitrites in food products. Nitrites and nitrates are added to food mainly to preserve meats and meat products. A typical value for nitrite in cured meats is approximately 125 µg/mL.
Przejdź do...
Filmy z tej kolekcji:

Now Playing
Determination Of NOx in Automobile Exhaust Using UV-VIS Spectroscopy
Environmental Science
30.1K Wyświetleń

Tree Identification: How To Use a Dichotomous Key
Environmental Science
81.3K Wyświetleń

Tree Survey: Point-Centered Quarter Sampling Method
Environmental Science
49.4K Wyświetleń

Using GIS to Investigate Urban Forestry
Environmental Science
12.6K Wyświetleń

Proton Exchange Membrane Fuel Cells
Environmental Science
22.1K Wyświetleń

Biofuels: Producing Ethanol from Cellulosic Material
Environmental Science
53.3K Wyświetleń

Testing For Genetically Modified Foods
Environmental Science
89.8K Wyświetleń

Turbidity and Total Solids in Surface Water
Environmental Science
35.9K Wyświetleń

Dissolved Oxygen in Surface Water
Environmental Science
55.8K Wyświetleń

Nutrients in Aquatic Ecosystems
Environmental Science
38.9K Wyświetleń

Measuring Tropospheric Ozone
Environmental Science
26.5K Wyświetleń

Lead Analysis of Soil Using Atomic Absorption Spectroscopy
Environmental Science
125.5K Wyświetleń

Carbon and Nitrogen Analysis of Environmental Samples
Environmental Science
29.5K Wyświetleń

Soil Nutrient Analysis: Nitrogen, Phosphorus, and Potassium
Environmental Science
216.0K Wyświetleń

Analysis of Earthworm Populations in Soil
Environmental Science
16.5K Wyświetleń
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone