Method Article
Characterizing DNA Repair Processes at Transient and Long-lasting Double-strand DNA Breaks by Immunofluorescence Microscopy
* Wspomniani autorzy wnieśli do projektu równy wkład.
W tym Artykule
Podsumowanie
Repair of double-strand DNA breaks is a dynamic process, requiring not only formation of repair complexes at the breaks, but also their resolution after the lesion is addressed. Here, we use immunofluorescence microscopy for transient and long-lasting double-stranded breaks as a tool to dissect this genome maintenance mechanism.
Streszczenie
The repair of double-stranded breaks (DSBs) in DNA is a highly coordinated process, necessitating the formation and resolution of multi-protein repair complexes. This process is regulated by a myriad of proteins that promote the association and disassociation of proteins to these lesions. Thanks in large part to the ability to perform functional screens of a vast library of proteins, there is a greater appreciation of the genes necessary for the double-strand DNA break repair. Often knockout or chemical inhibitor screens identify proteins involved in repair processes by using increased toxicity as a marker for a protein that is required for DSB repair. Although useful for identifying novel cellular proteins involved in maintaining genome fidelity, functional analysis requires the determination of whether the protein of interest promotes localization, formation, or resolution of repair complexes.
The accumulation of repair proteins can be readily detected as distinct nuclear foci by immunofluorescence microscopy. Thus, association and disassociation of these proteins at sites of DNA damage can be accessed by observing these nuclear foci at representative intervals after the induction of double-strand DNA breaks. This approach can also identify mis-localized repair factor proteins, if repair defects do not simultaneously occur with incomplete delays in repair. In this scenario, long-lasting double-strand DNA breaks can be engineered by expressing a rare cutting endonuclease (e.g., I-SceI) in cells where the recognition site for the said enzyme has been integrated into the cellular genome. The resulting lesion is particularly hard to resolve as faithful repair will reintroduce the enzyme's recognition site, prompting another round of cleavage. As a result, differences in the kinetics of repair are eliminated. If repair complexes are not formed, localization has been impeded. This protocol describes the methodology necessary to identify changes in repair kinetics as well as repair protein localization.
Wprowadzenie
Each day, every cell in the human body is bombarded with an estimated 10,000 DNA lesions1. This existential threat puts us at risk for mutations, oncogenesis as well as cell death. To protect genome fidelity, mammalian cells have evolved to respond to DNA damage with a complex series of protein associations and modifications. This response is organized into multiple pathways, collectively known as the DNA damage response (DDR)2,3. The DDR consists of the accumulation of DNA repair proteins at DNA lesions, coordinated both temporally and spatially. DDR frequently induces cell cycle arrest to avoid the propagation or intensification of damage that can occur during the replication of damaged DNA2,4,5. In turn, it is also necessary for the cellular viability to turn off cell cycle arrest by disassociating repair complexes after repair has been completed.
Among the various types of DNA damage, DSBs are the most deleterious. Failure to repair DSBs can result in chromosome rearrangements or large-scale deletions such as the loss of entire chromosome arms. The repair of DSBs is divided into two pathways6,7,8. Homologous recombination (HR) requires a sister chromosome to use as a DNA template and thus is limited to late S and G2/M phases of the cell cycle9,10. Non-homologous end joining (NHEJ) does not have these restrictions but can cause small deletions when repairing DSBs11,12.
DSB repair specifically and the DDR in general are active areas of investigation. Despite being organized into conveniently separated pathways, there is a great deal of redundancy. Indeed, many proteins (BRCA1, BRCA2, and the RPA complex for example) are involved in multiple pathways13,14,15,16. The repair of a lesion by one pathway, can lead to a damage intermediate that must be repaired by another pathway14. The intertwining of these pathways, combined with their complex task of recruiting the right proteins to the correct place for the precise amount of time necessary, requires a multi-tiered regulatory process.
A recent report highlights the intricacies of DDR by demonstrating that repair complex formation, resolution, and localization can each separately be impaired17. The overall goal of the following protocol is to definitively dissect the ability of cells to repair DSB. Using immunofluorescence microscopy, accumulation of repair proteins at the sites of damage can be visualized at the representative time points following the induction of DSBs.
This technique has several advantages to commonly used approaches. Frequently, repair is investigated at single time points and incapable of representing the dynamic process of assembly and dissociation of repair complexes. Observing the full range of repair from the initial activation to the full resolution ensures that a delay in repair is not misidentified as complete inhibition. Conversely, it assures that the induction of a repair response that is unable to inactivate said response is not misidentified as the normal or the excessive activation.
Delayed protein complex formation and the mis-localization of repair proteins, however, may not be unambiguously distinguished with this approach. To determine if repair proteins are mis-localized versus delayed in their localization, a "long-lasting" DSB can be introduced through enzymatic cleavage of cellular DNA. The resulting lesion is recut each time it is repaired, resulting in a distinct large nuclear repair focus and removing the temporal restriction from recruitment. This can be achieved by modifying the existing approach with the use of a rare cutting endonuclease (e.g., I-SceI) to induce a long-lasting DSB. The longevity of DSBs enables the visualization of elusive repair proteins by immunofluorescence microscopy. The enhanced abundance could also improve the visualization when detection is hindered by limitation in the antibody quality, a feature that could be useful when lesser studied proteins are identified as having an impact on DNA repair.
Notably, we provide explicit instructions for a free image processing and analysis software (e.g., ImageJ). This removes a major financial barrier in image analysis, opening the interrogation of DNA damage repair to a wider audience.
Protokół
Please note, this protocol is written for U2OS cells containing an I-SceI recognition site18. The cells need not be U2OS but must contain the I-SceI site. The protocol may need to be adjusted (e.g., number of cells seeded and incubation times) depending on the type of cells used.
1. Defining the Kinetics of DSB Repair Complex Formation
- Grow U20S-DRGFP cells on a 10 cm tissue culture plate until 85–90% confluent.
- Remove the media and incubate at room temperature (RT) in 3 mL of EDTA for 2 min.
- Replace EDTA with 1 mL of trypsin and incubate at 37 °C for 5 min. Ensure that the cells are detached from the surface of the plate by viewing under a microscope. Neutralize trypsin with 5 mL of Dulbecco's Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin.
- Determine the concentration of cells in this suspension using a hemocytometer and the trypan blue exclusion method.
- Seed 6 x 103 cells/well in 200 µL of a glass-bottom 96-well plate. Alternatively, seed 4 x 106 cells in 8 mL on 12 mm coverslips placed in a 10 cm plate.
NOTE: Each well or coverslip represents a single time point, including the control. - Grow the cells overnight at 37 °C and 5% CO2.
- Inducing DSBs
- Replace the media from the 96-well plate/10 cm dish with H2O2 diluted to a concentration of 25 µM with DMEM + 10% FBS and incubate at 37 °C for 1 h.
- Wash the cells 3x with 1x PBS and replace with fresh DMEM supplemented with 10% FBS and 1% penicillin/streptomycin. Incubate the cells at 37 °C for 0, 1, 4, 8, 24, and 48 h. Ensure that fresh dilutions of H2O2 are used for each experiment.
NOTE: To avoid killing cells at the H2O2 concentration used here, ensure that the dose of damage is low enough such that the bulk of cells will avoid apoptosis and senescence. It is critical to observe the recovery process. This is best achieved by measuring sensitivity to a range of doses. Perform an MTT assay or other cell viability assay to determine the minimal concentration of hydrogen peroxide that can be used.
- Fixing and permeabilizing cells
- Wash the cells 3 times with 1x PBS. Remove a coverslip from the 10 cm plate for each time point and place it in a single well of a 24-well plate for fixing. Use the needle and forceps to carefully remove the coverslip and avoiding scratching cells off the coverslip surface.
- Fix the cells by incubating for 15 min with 4% paraformaldehyde (PFA) at designated times after 1 h of H2O2 exposure and fresh DMEM replacement (0, 1, 4, 8, 24, and 48 h).
- Wash the fixed coverslips 3 times with PBS. Permeabilize the cells with 0.5% non-ionic detergent diluted in 1x PBS. Incubate for 15 min at RT, before washing 3x with PBS.
NOTE: After permeabilization, storage of coverslips is possible in PBS at 4 °C for at least a week.
- Repair complex visualization
- Block the 96-well plate/coverslips in a solution of 3% bovine serum albumin (BSA) and 0.1% non-ionic detergent diluted in 1x PBS (3% BSA solution) for 1 h at RT.
- Incubate with primary antibodies that target the repair protein of interest, diluted according to the manufacturer's specifications in 3% BSA solution for 1 h at RT, before washing 3 times with 1x PBS.
- Incubate the 96-well plate/coverslips with secondary antibody diluted according to the manufacturer’s protocol in 3% BSA solution. Confirm that the secondary fluorophores do not have overlapping excitation or emission spectra.
- After washing the cells with PBS 3 times add DAPI, diluted according to the manufacturer’s specifications in 1x PBS and incubate at RT for 5 min.
- Mount the coverslips onto slides with a drop of mounting agent, making sure the cell-side faces down. Press the coverslips carefully and soak up any excess mounting agent with a wipe.
- Seal the coverslips with fast-drying transparent nail polish and ensure a complete seal of the coverslip with the slide.
- Take the confocal microscope images by focusing on the DAPI channel, before other channels (with signals from DNA repair gene of interest) to avoid introducing bias into the experiment.
- Alternatively, images can be acquired by taking Z-sections of the desired region and condensing the image into a single plane to visualize all detectable foci.
NOTE: If DSB resolution or protein of interest is not observed at time points chosen, determine when DSBs resolve by selecting a wide range of time points initially (0–48 h post DSB induction). The time points of interest will vary by the method of DSB induction and the repair protein of interest.
- Use free ImageJ software to define the nuclei in the immunofluorescence microscopy images.
NOTE: To open microscope images, the Bio-Formats plugin must be installed in ImageJ.- Open the confocal images (.czi or .tif) by dragging the image file into the ImageJ window, or by selecting "Bio-Formats Importer" from the "Plugin" toolbar in ImageJ.
NOTE: The "Bio-Formats Import Option" window will appear. - Select "Split Channels" to split the image into constitutive DAPI and fluorescent images within the "Bio-Formats Import Option" window.
- Select "Colorized" in "Color options" from the drop-down menu. Select "OK" to open the DAPI and Histone H2AX (H2AX) images as separate windows and choose the DAPI image.
- Select the "Image" toolbar and "Adjust Threshold" option to select nuclei.
NOTE: A "Threshold" window will appear. - Adjust the image threshold by sliding the bottom bar of the "Threshold" window completely to the right. Adjust the top bar until the nuclei appear completely red with distinct outlines, and the background black. DO NOT select apply. Close the "Adjust Threshold" window.
- Select the "Analyze" toolbar and "Analyze Particles" option. See an "Analyze Particles" window appearing on the screen.
- Input the minimum size of a nucleus.
NOTE: Particles below the size inputted will not be counted as nuclei. - From the "Show" dropdown menu, select "Outlines". Select the "Add to Manager" option. Click "OK" to open an "ROI Manager" window.
NOTE: Outlines of nuclei will appear in a separate window. - Assign numbers to the nuclei that can be selected from the "ROI Manager" window.
- Open the confocal images (.czi or .tif) by dragging the image file into the ImageJ window, or by selecting "Bio-Formats Importer" from the "Plugin" toolbar in ImageJ.
- Use ImageJ to quantify H2AX foci in immunofluorescence microscopy images.
- Select the "H2AX" foci window (stained with fluorescently conjugated secondary antibody). Then, select the "Process" toolbar and choose "Find Maxima" to find the foci within the nuclei.
NOTE: A "Find Maxima" window will open. - Choose the "Single Points" option from the "Output Type" dropdown menu and select "Preview point selection" to visualize the maxima/foci. Input a "Noise Tolerance" value depending on the fluorescence intensity of the image (Figure 1). Check for the appearance of a white window with small black dots.
NOTE: These dots represent the H2AX foci/maxima that have been detected. The noise tolerance value must be maintained constant for all images being analyzed. A low/high noise tolerance will depict too many/few maxima (foci) within the nucleus and will not be an accurate representation of the foci within the nucleus. - Select the nuclei for which H2AX foci must be quantified for from the "ROI Manager" window. Select all the nuclei by checking the "show all" option.
- Select "Measure" to measure the number of maxima/foci within the selected nuclei.
NOTE: The number of maxima/foci appear as multiples of 255, i.e., one maximum has a "RawIntDen" (Integrated density) reading of 255. - Copy the "RawIntDen" values and paste them into software for data analysis.
- Select the "H2AX" foci window (stained with fluorescently conjugated secondary antibody). Then, select the "Process" toolbar and choose "Find Maxima" to find the foci within the nuclei.
2. Analyzing Localization to a Long-lasting Double-strand DNA Break
- Seed cells on 96-well plates/coverslips as described in sections 1.1 and 1.2.
- Induction of double-strand DNA breaks
- Transfect cells with the I-SceI expression vector using a lipid-based transfection reagent according to the manufacturer's specifications. Wait 24–72 h after transfection to maximize the endonuclease expression. Determine if the transfection was successful by locating cells with a singular large nuclear γ-H2AX focus.
- Acquisition of images
- Fix, permeabilize, block, and wash the 96-well plate/coverslips as described in section 1.8.
- Co-incubate cells with an antibody against γ-H2AX and the repair protein of interest (here, a primary antibody against RAD51 was used) diluted according to the manufacturer's specifications in 3% BSA solution.
- Wash the 96-well plate/coverslips 3 times with 1x PBS. Incubate the 96-well plate/coverslips with secondary antibody diluted according to the manufacturer's protocol in 3% BSA solution. Confirm that the secondary fluorophores do not have overlapping excitation or emission spectra.
- Identify cells that have a large nuclear γ-H2AX focus. Only take pictures of these cells to avoid bias.
- Analysis of long-lasting I-SceI induced foci
- Analyze the long-lasting foci manually, by counting both the number of cells that have the large γ-H2AX foci and co-localized foci of the repair protein of interest.
Wyniki
Figure 1 depicts the selection of the correct noise discrimination for maxima/foci quantification using ImageJ. The merged images of DAPI and the repair protein of interest are on the left panel. Figure 1A shows a noise discrimination of 90 and marks the correct number of foci. Nuclei on the edge (depicted with a pink arrow) and foci outside the nuclei (depicted with a yellow arrow) are not counted during the quantification. Figure 1B depicts a low noise tolerance of 50. Numerous maxima are identified outside the nucleus and within the nucleus. Figure 1C shows a high noise tolerance of 220. Only a single focus (a white arrow) was detected. To offer the reader a sense of "positive" and "negative" results, we have also compiled representative results in Figure 2. Specifically, Figure 2A depicts co-localization between a γ-H2AX focus (green) and a repair protein of interest (red). Similarly, an untransfected cell is shown in the upper left of this panel and a cell with a non-nuclear (false) γ-H2AX focus is shown in the upper right. Please note the micronuclei associate with DNA damage machinery are a likely source of these non-nuclear foci19. Figure 2B provides a higher magnification of the two right-most cells from Figure 2A to provide clarity in how they were determined to be interior and exterior to the nucleus, respectively. An example of a nuclear γ-H2AX focus without co-localization of a repair protein is shown in Figure 2C. A schematic of this protocol is provided in Figure 3 that summarizes this approach to characterizing DNA repair.

Figure 1: Representative analysis of double-strand DNA break repair kinetics. Representative images of γ-H2AX foci quantification using ImageJ (with Bio-Formats plugin) software. The left panels are composite images of nuclei (40X magnification), stained blue for DAPI and green for γ-H2AX. Center panels show reference un-analyzed nuclei with γ-H2AX foci/maxima. (A) The right panel shows maxima/foci detection with a noise tolerance of 90 and is representative of the actual estimation of the number of foci within the nucleus. (B) The right panel shows maxima quantification with a low noise tolerance (50). Maxima/foci can be detected outside the nucleus (yellow arrow), while majority of the maxima located within the nucleus are non-specific background signals. (C) The right panel shows maxima quantification with high noise tolerance (220). Only a single maximum/focus (white arrow) is detected within the nucleus and is not representative of the foci within the nucleus. Please click here to view a larger version of this figure.
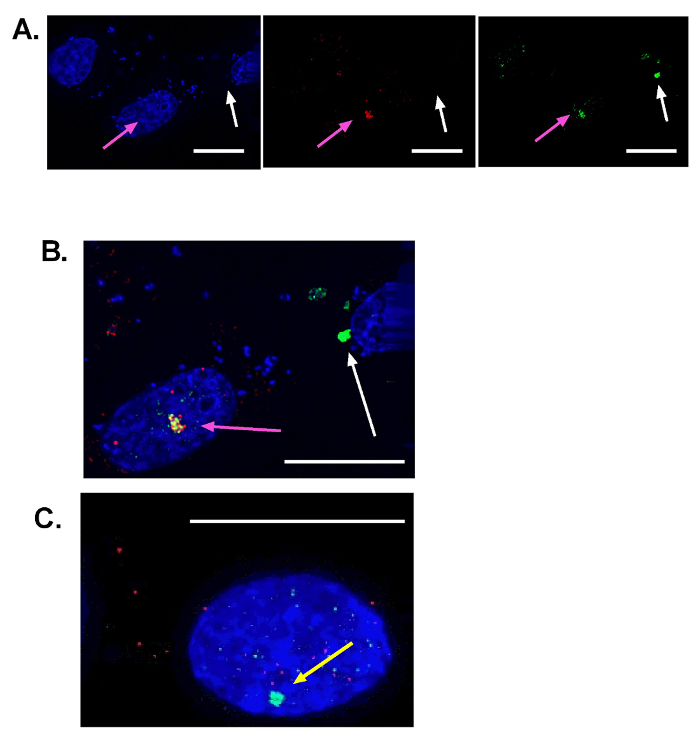
Figure 2: Representative I-SceI induced double-strand DNA breaks. Representative images of nuclei from cells grown on coverslips (40X magnification). Nuclei are stained blue. A repair protein (RAD51) is stained red and γ-H2AX is stained green. (A) The three panels show the same three cells. In the left most panel, nuclei are visible. In the middle panel, staining for RAD51 is shown. On the right panel, γ-H2AX staining is shown. The nucleus in the upper right corner does not show any sign of I-SceI induced DSBs. The nucleus in the center (pink arrow) represents a true positive event as it has both a large γ-H2AX nuclear focus and a co-localized RAD51 nuclear focus. The nucleus on the right side has a false large γ-H2AX focus as it is extranuclear. Foci outside of the nucleus should not be used for co-localization analysis. (B) This is a higher magnification of the merged image in part A (center and right most cells). The large nuclear yellow focus indicating overlap of RAD51 and H2AX signal is denoted by the pink arrow. (C) A representative image of a nuclear γ-H2AX focus without co-staining of the DNA repair protein of interest. Unlike the focus denoted with a white arrow in (A) and (B), the yellow arrow illustrates typical results when repair proteins are prevented from localizing to sites of damage. Scale bar = 15 µm. Please click here to view a larger version of this figure.

Figure 3: Visual depiction of protocol for analyzing double-strand DNA break repair. (1) Representation of protocol steps 1.1 for seeding cells onto coverslips/glass bottom plates. (2) Illustration of the induction of double-strand breaks by treating with hydrogen peroxide (right) or inducing long-lasting double-strand breaks by transfecting 3 µg of I-SceI. (3) Representation of protocol steps 1.4 to 1.5.3 for visualization of double-strand breaks. (4a) Image representing confocal microscopy of cell stained with DAPI (blue) and γ-H2AX foci (green). (4b) Image of singular large γ-H2AX foci (green) again nuclei stain DAPI (blue). (5a) Depiction of the analysis of γ-H2AX foci using ImageJ (protocol steps 1.10.1–1.11.5). (5b) Depiction of manual count of large long-lasting γ-H2AX foci (protocol step 2.4). Please click here to view a larger version of this figure.
Dyskusje
The analysis of DNA damage repair in general and the repair of double-stranded DNA breaks specifically is an active area of research because its consequences span tumorigenesis to basic biology6,20. This manuscript details an approach that accurately dissects the contribution of RAD51 and γ-H2AX proteins to the resolution of DSBs through HR. Looking forward, this method can be used to elucidate additional functions of repair proteins at DSBs14. The comparison of repair kinetics and the recruitment of repair machinery to I-SceI induced DSBs between control cells and cells with the protein of interest knocked out, would define the contribution of that protein to the recruitment, localization, and resolution of repair factors to DSBs.
Visualizing repair kinetics has several advantages over commonly used approaches. Frequently, repair is investigated at single time points, which is incapable of representing the dynamic process of assembly and dissociation of repair complexes. Observing the full range of repair from initial activation to full resolution ensures that a delay in repair is not misidentified as complete inhibition. Conversely, it assures that induction of a repair response without the ability to inactivate that response is not misidentified as normal or excessive activation. These are largely changes in the application of an established approach, but the use of a rare cutting endonuclease to induce a long-lasting DSB is a novel tool. It allows the investigator to unambiguously determine if the localization of a repair protein is inhibited instead of its recruitment being delayed a short period of time beyond the conclusion of the experiment. I-SceI induced DSB can last for days and theoretically as long as the enzyme is expressed (should the cell remain viable). The kinetics of DSB repair can also be observed via live cell imaging. However, although live cell imaging allows detection of repair events in real time, its application is hindered by the requirement that proteins be tagged with a fluorophore such as GFP. Such genetic manipulation has the potential to alter protein function and represents an additional technical barrier.
Image analysis via ImageJ software can be cumbersome to master. The step-by-step instruction for repair focus recognition and quantification using this software is provided in hopes of removing this obstacle for other groups. This could lead to increased utilization of the powerful software and eliminate the cost of more expensive programs at a time of shrinking pools of federal grant support.
In principle, the duration of these DSBs should enable the visualization of elusive repair proteins by immunofluorescence microscopy. This could be the case for proteins that are difficult to view because they do not accumulate in a high enough concentration to be detected, e.g., the persistence of the I-SceI induced DSB results in a greater than typical accumulation of repair proteins at the lesion. The enhanced abundance could also improve the visualization when detection is hindered by a limitation in antibody quality; a feature that could be particularly useful when less studied proteins are identified as having an impact on DNA repair. To date, this approach has been verified for 4 DNA repair proteins, namely RAD51, RPA70, BRCA1, and BRCA2 (data not shown, Figure 2, and reference17)
Finally, there are modifications that could be made to this protocol to expand the types of DNA damage that can be interrogated as well as the cellular environments. The most obvious modification would be changing the source or type of induced DNA damage. The proof of principle for using UVB, ionizing radiation, or radiomimetics (phleomycin, bleomycin, and etoposide) to study the kinetics of DNA repair has already been published18,19,23,24,25. With slight modification, this approach can also elucidate repair protein response by NHEJ to DSBs. Alternatively, Britton et al. describe a pre-extraction technique that could be used26. Further, there are homing nicking endonucleases (I-HmuI or I-BasI) that have extensive recognition sites, similar to I-SceI27. Distinguishing them from I-SceI, these enzymes cause single-strand DNA breaks (SSBs). Introducing the recognition site for these enzymes into a cell line would allow the analysis of SSB repair that parallels the protocol described in this manuscript for interrogating long-lasting DSBs. Notably, the process of integrating the I-SceI recognition site and verifying the introduction of the site can be laborious. Fortunately advances in genome editing technology (such as CRISPER/Cas9 systems) have eased this burden28. There is also a field of active research that has succeeded in engineering homing endonucleases to cut at a desired genomic location, which suggests that in the future, these engineering advancements and the use of CRISPR/Cas9 may lead this cloning step to be dispensable28,29.
Ujawnienia
The authors have nothing to disclose.
Podziękowania
We thank Joel Sanneman and Dr. Philine Wangemann of the Confocal Microscopy Core, funded by the Kansas State University College of Veterinary Medicine, for their support of efforts to develop this technique. pCBASceI was a gift from Maria Jasin (Addgene plasmid # 26477)30. U2OS DR-GFP cells were a kind gift from Maria Jasin18.
Materiały
| Name | Company | Catalog Number | Comments |
| 12mm Coverslips | VWR | 89015-725 | |
| 16% Paraformaldehyde (PFA) | ThermoFisher Scientific | 28908 | |
| 24-well plate | VWR | 82050-892 | |
| 96-well glass bottom plate | Cellvis | P96-1.5H-N | |
| Anti-H2AX Alexa488 | EMD Millipore | 05-636-AF488 | |
| Anti-Rad51 (D4B10) | Cell Signaling Technology | 8875S | |
| Bio-formats plugin for ImageJ | National Institute of Health (NIH) | https://imagej.nih.gov/ij/plugins/index.html | |
| Bovine Serum Albumin (BSA) | VWR | 97061-416 | |
| DAPI | ThermoFisher Scientific | D1306 | |
| DMEM, High Glucose | ThermoFisher Scientific | 12100046 | |
| EDTA | Invitrogen | 15576-028 | |
| Fetal Bovine Serum (FBS) | VWR | 89510-194 | |
| Goat Anti-Rabbit IgG Alexa594 | ThermoFisher Scientific | A-11012 | |
| Hydrogen Peroxide | sigma-Aldrich | 216763-100ML | |
| ImageJ Software | National Institute of Health (NIH) | https://imagej.nih.gov/ij/ | |
| I-SceI Expression Vector | Addgene | 26477 | |
| Nail Polish- Insta Dri | Sally and Hansen | Clearly Quick (103) | |
| Phosphate Buffered Saline (PBS) | Bio Basic | PD8117 | |
| ProLong Gold Antifade Reagent | Life Technologies | P36930 | |
| Triton X-100 | Sigma-Aldrich | X100-100ML | |
| Trypsin-EDTA | Sigma-Aldrich | T4049-500ML | |
| TurboFect Transfection Reagent | ThermoFisher Scientific | R0531 | |
| Tween-20 | Fisher Scientific | BP337-500 |
Odniesienia
- Lindahl, T. Instability and decay of the primary structure of DNA. Nature. 362 (6422), 709-715 (1993).
- Branzei, D., Foiani, M. Regulation of DNA repair throughout the cell cycle. Nat Rev Mol Cell Biol. 9 (4), 297-308 (2008).
- Ciccia, A., Elledge, S. J. The DNA damage response: making it safe to play with knives. Mol Cell. 40 (2), 179-204 (2010).
- Auclair, Y., Rouget, R., Drobetsky, E. A. ATR kinase as master regulator of nucleotide excision repair during S phase of the cell cycle. Cell Cycle. 8 (12), 1865-1871 (2009).
- Awasthi, P., Foiani, M., Kumar, A. ATM and ATR signaling at a glance. J Cell Sci. 128 (23), 4255-4262 (2015).
- Ceccaldi, R., Rondinelli, B., D'Andrea, A. D. Repair Pathway Choices and Consequences at the Double-Strand Break. Trends Cell Biol. 26 (1), 52-64 (2016).
- Chapman, J. R., Taylor, M. R. G., Boulton, S. J. Playing the End Game: DNA Double-Strand Break Repair Pathway Choice. Mol Cell. 47 (4), 497-510 (2012).
- Daley, J. M., Sung, P. 53BP1, BRCA1, and the Choice between Recombination and End Joining at DNA Double-Strand Breaks. Mol Cell Biol. 34 (8), 1380-1388 (2014).
- Hartlerode, A., Odate, S., Shim, I., Brown, J., Scully, R. Cell Cycle-Dependent Induction of Homologous Recombination by a Tightly Regulated I-SceI Fusion Protein. PLOS ONE. 6 (3), e16501 (2011).
- Brandsma, I., Gent, D. C. Pathway choice in DNA double strand break repair: observations of a balancing act. Genome Integr. 3, 9 (2012).
- Davis, A. J., Chen, D. J. DNA double strand break repair via non-homologous end-joining. Transl Cancer Res. 2 (3), 130-143 (2013).
- Reid, D. A., et al. Organization and dynamics of the nonhomologous end-joining machinery during DNA double-strand break repair. Proc Natl Acad Sci. 112 (20), E2575-E2584 (2015).
- Chen, J. J., Silver, D., Cantor, S., Livingston, D. M., Scully, R. BRCA1, BRCA2, and Rad51 Operate in a Common DNA Damage Response Pathway. Cancer Res. 59 (7 Supplement), 1752s-1756s (1999).
- D'Andrea, A. D. BRCA1: A Missing Link in the Fanconi Anemia/BRCA Pathway. Cancer Discov. 3 (4), 376-378 (2013).
- Gudmundsdottir, K., Ashworth, A. The roles of BRCA1 and BRCA2 and associated proteins in the maintenance of genomic stability. Oncogene. 25 (43), 5864-5874 (2006).
- Liu, S., et al. Distinct roles for DNA-PK, ATM and ATR in RPA phosphorylation and checkpoint activation in response to replication stress. Nucleic Acids Res. 40 (21), 10780-10794 (2012).
- Wallace, N. A., Khanal, S., Robinson, K. L., Wendel, S. O., Messer, J. J., Galloway, D. A. High Risk Alpha Papillomavirus Oncogenes Impair the Homologous Recombination Pathway. J Virol. , (2017).
- Pierce, A. J., Johnson, R. D., Thompson, L. H., Jasin, M. XRCC3 promotes homology-directed repair of DNA damage in mammalian cells. Genes Dev. 13 (20), 2633-2638 (1999).
- Luzhna, L., Kathiria, P., Kovalchuk, O. Micronuclei in genotoxicity assessment: from genetics to epigenetics and beyond. Front Genet. 4, (2013).
- Jasin, M. Homologous repair of DNA damage and tumorigenesis: the BRCA connection. Oncogene. 21 (58), 8981-8993 (2002).
- Wallace, N. A., Robinson, K., Howie, H. L., Galloway, D. A. β-HPV 5 and 8 E6 Disrupt Homology Dependent Double Strand Break Repair by Attenuating BRCA1 and BRCA2 Expression and Foci Formation. PLOS Pathog. 11 (3), e1004687 (2015).
- Wallace, N. A., Robinson, K., Howie, H. L., Galloway, D. A. HPV 5 and 8 E6 Abrogate ATR Activity Resulting in Increased Persistence of UVB Induced DNA Damage. PLoS Pathog. 8 (7), (2012).
- Mah, L. J., Vasireddy, R. S., Tang, M. M., Georgiadis, G. T., El-Osta, A., Karagiannis, T. C. Quantification of γH2AX Foci in Response to Ionising Radiation. J Vis Exp. (38), e1957 (2010).
- Popp, H. D., Brendel, S., Hofmann, W. K., Fabarius, A. Immunofluorescence Microscopy of γH2AX and 53BP1 for Analyzing the Formation and Repair of DNA Double-strand Breaks. J Vis Exp. (129), e56617 (2017).
- Burma, S., Chen, B. P., Murphy, M., Kurimasa, A., Chen, D. J. ATM Phosphorylates Histone H2AX in Response to DNA Double-strand Breaks. J Biol Chem. 276 (45), 42462-42467 (2001).
- Britton, S., Coates, J., Jackson, S. P. A new method for high-resolution imaging of Ku foci to decipher mechanisms of DNA double-strand break repair. J Cell Biol. 202 (3), 579-595 (2013).
- Landthaler, M., Shen, B. W., Stoddard, B. L., Shub, D. A. I-BasI and I-HmuI: two phage intron-encoded endonucleases with homologous DNA recognition sequences but distinct DNA specificities. J Mol Biol. 358 (4), 1137-1151 (2006).
- Lackner, D. H., et al. A generic strategy for CRISPR-Cas9-mediated gene tagging. Nat Commun. 6, 10237 (2015).
- Chan, S. H., Stoddard, B. L., Xu, S. Y. Natural and engineered nicking endonucleases--from cleavage mechanism to engineering of strand-specificity. Nucleic Acids Res. 39 (1), 1-18 (2011).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone