Method Article
Nephrotoxin Microinjection in Zebrafish to Model Acute Kidney Injury
W tym Artykule
Podsumowanie
Renal injuries incurred from nephrotoxins, which include drugs ranging from antibiotics to chemotherapeutics, can result in complex disorders whose pathogenesis remains incompletely understood. This protocol demonstrates how zebrafish can be used for disease modeling of these conditions, which can be applied to the identification of renoprotective measures.
Streszczenie
The kidneys are susceptible to harm from exposure to chemicals they filter from the bloodstream. This can lead to organ injury associated with a rapid decline in renal function and development of the clinical syndrome known as acute kidney injury (AKI). Pharmacological agents used to treat medical circumstances ranging from bacterial infection to cancer, when administered individually or in combination with other drugs, can initiate AKI. Zebrafish are a useful animal model to study the chemical effects on renal function in vivo, as they form an embryonic kidney comprised of nephron functional units that are conserved with higher vertebrates, including humans. Further, zebrafish can be utilized to perform genetic and chemical screens, which provide opportunities to elucidate the cellular and molecular facets of AKI and develop therapeutic strategies such as the identification of nephroprotective molecules. Here, we demonstrate how microinjection into the zebrafish embryo can be utilized as a paradigm for nephrotoxin studies.
Wprowadzenie
AKI is an abrupt loss of kidney function that can lead to devastating health consequences1. AKI is a significant healthcare issue worldwide due to its high incidence of approximately 20% among hospitalized patients, with even higher rates of 30-50% in critical care cases and the elderly, and mortality rates of 50-70%1-3. Unfortunately, the prevalence of AKI has been increasing and is projected to escalate further over the next decade, due in part to the diversity of factors that can induce AKI, which include post-operative stress, ischemia, and exposure to nephrotoxins such as antibiotics and chemotherapeutic drugs4.
AKI involves sudden cellular damage within the kidney, commonly occurring in nephrons, which are the essential functional units, and are comprised of a blood filter and a segmented tubule that drains urine into central collecting ducts1. When a significant number of nephrons are damaged during AKI, the immediate effects include an interruption in waste clearance from the circulation, and reduced or abrogated fluid flow through nephrons due to obstruction from dead and dying cells1. Over time, tubular obstruction can lead to degeneration of entire nephrons, which permanently reduces renal function1. Physiological alterations in the kidney following AKI also involve complex inflammatory events that can lead to chronic scarring1.
Despite these outcomes, nephrons have some capacity to undergo regeneration after AKI that reconstitutes the tubular epithelium5,6. While there has been an increasing molecular understanding of nephron regeneration, the mechanisms remain elusive in many regards and necessitate continued investigation7. The degree to which AKI results in permanent renal damage also remains unknown. Current research suggests the regenerative potential for the kidney is the highest following less severe cases of AKI, while more pronounced or repeated episodes lead to chronic kidney disease (CKD) and culminate in end stage renal disease (ESRD) that requires life-saving transplantation or dialysis8,9. Additionally, individuals already suffering from CKD are at an even higher risk of contracting a severe episode of AKI8,9. Taken together, it is clear that continued basic and clinical research is vital to understand, treat and prevent AKI.
Research with animal models has been instrumental in appreciating the progression of local and environmental alterations that occur during AKI10. To expand this understanding as well as develop new therapies, the zebrafish animal model has been employed in a variety of ways11,12. The nephrons of the zebrafish kidney, in both the embryo and adult, display a high degree of conservation with mammals13-16. Further, nephron epithelial injury in zebrafish resembles the process in higher vertebrates, whereby the local destruction of tubular cells is followed by intratubular proliferation and reestablishment of nephron architecture17-19. In the embryo, however, extensive tubule damage from the nephrotoxins like cisplatin is associated with lethality20,21. By comparison, zebrafish adults survive AKI and exhibit substantive regenerative capabilities in the kidney. For example, following exposure to the aminoglycoside antibiotic gentamicin, zebrafish regenerate tubule epithelial damage and grow new nephron units as well22-24. While these gentamicin-induced AKI studies have provided invaluable information, understanding renal damage from diverse nephrotoxins remains critical to appreciate the effects and response to different types of damage25.
The zebrafish embryo, due to its size, transparency, and genetic tractability, has many benefits for nephrotoxin studies25, where the method of microinjection20,21 is used to administer the molecule(s) for investigation. Nephrons are formed by 24 hr post fertilization (hpf) and begin to filter blood by approximately 48 hpf26,27. Thus, the rapid formation and function of the embryonic kidney facilitates experimental analysis. However, the process of microinjection has technical challenges and there can be a steep learning curve to mastering the technique. In this video article, we describe how to perform microinjections and provide troubleshooting tips in order to enhance the rate of successful injections.
Protokół
The procedures for working with zebrafish embryos described in this protocol were approved by the Institutional Animal Care and Use Committee at the University of Notre Dame.
1. Preparation of Solutions
- Make a 50x stock solution of E3 embryo media by mixing 73.0 g NaCl, 3.15 g KCl, 9.15 g CaCl2, and 9.95 g MgSO4 in 5 L of distilled water, and store at RT.
- For culturing of zebrafish embryos, dilute the 50x stock solution of E3 embryo media stock to a 1x working solution with distilled water, then add 200 μl of 0.05% methylene blue to every 1 L of 1x E3 to act as a fungicide, and store at RT.
- Make a pigmentation blocking solution of E3 embryo media with 0.003% 1-phenyl-2-thiourea (PTU) by combining 40 ml of the 50x E3 stock with 0.06 mg PTU. Then bring the volume to a total of 2 L with distilled water.
- Stir the E3/PTU O/N at RT to get the PTU powder into solution. Then store the E3/PTU at RT.
Note: The E3/PTU has a shelf life of approximately one week, and older solutions will be less effective at blocking pigmentation development in zebrafish larvae. - Make an anesthetic solution of 0.2% Tricaine (MS-222) by adding 1 g of Tricaine to 500 ml of distilled water and adjust the pH to 7.2 with 1 M Tris, pH 9.5.
- Prepare to make a 2% methylcellulose solution for imaging by placing a solution of 1x E3 (no methylene blue), with 1/10th volume of 0.2% Tricaine added at 4 °C.
Note: The methylcellulose solution is a thick, clear solution that is used to gently stabilize live embryos for microscopy. After manipulations are performed the embryos can be washed in 1x E3 to dissolve the methylcellulose without injuring the animal and enabling microscopy at subsequent stages in the same specimen. - When cooled to 4 °C, stir the 1x E3/Tricaine solution vigorously on a stir plate and gradually add the appropriate amount of methylcellulose powder while continuing to stir vigorously. Gradually add the powder to the solution to prevent the formation of insoluble methylcellulose aggregates.
- After all the powder is mixed into the 2% methylcellulose/E3/tricaine solution, stir the composite solution in the 4 °C cold room O/N.
Note: The cold temperature is best for fully dissolving the powder. If the solution is not clear after one night, stir for an additional workday and/or a second O/N. - To remove air bubbles from the 2% methylcellulose/E3/tricaine solution, aliquot the mixture into 1.5 ml tubes and spin at high speed (12,000 x g) at 4 °C for 30 min or up to 2 hr.
- Place the 2% methylcellulose/E3/Tricaine solution at 4 °C for long-term storage.
Note: Prior to usage, aliquots should be pre-warmed to RT for working with the zebrafish specimens.
2. Preparation of Tools
- Prepare an embryo manipulator tool, used for positioning embryos for microinjection, by attaching a gel loading tip on the end of a 5" straight dissecting needle, and secure with tape or superglue.
- Prepare microinjection needles using a needle puller, by first placing a fire polished 10 cm borosilicate glass with filament into the instrument and secure the borosilicate glass by tightening the handles.
- Pull the borosilicate glass to fashion fine-tapered needles. Use the following settings: heat 540, pull 245, velocity 200, and time 125.
- Place needles inside a Petri dish, suspending them on a strip of modeling clay to protect the needle tips, and keep the dish covered to prevent dust accumulation.
- To finish preparing the needle for microinjections, use a razor blade or fine forceps edge to cut the pulled end of the needle to create a sharp angular injection tip with a diameter of approximately 0.05-0.1 mm.
- To make the microinjection tray, prepare a 1.5% agarose/E3 solution in a 100 ml Erlenmeyer flask and dissolve the agarose by heating the E3 to a boil in a microwave then allow to cool at RT for approximately 10 min.
- Pour the cooled agarose/E3 solution into a Petri dish to make a foundation with a depth of approximately 0.5 cm. When this has solidified pour a second layer with a depth of approximately 0.3 cm and insert the prefabricated embryo well mold and allow the agarose to solidify at RT.
Note: When inserting the mold into the top agar/E3 layer, placing the mold into the agar at an angle to decrease the number of air bubbles. - When the entire microinjection tray has set, lift the prefabricated embryo well mold out slowly and carefully using a scoopula, then fill the dish with E3 solution and store at 4 °C.
3. Embryo Preparation
- Set up zebrafish mating tanks by placing dividers into mating chambers and fill with system water.
- Place one fish on each side of the divider such that each mating chamber contains one female and one male fish, and cover each mating chamber.
- The next morning, remove the dividers to enable spawning.
- Check the fish after approximately 30 min, and after returning the adults to the aquarium, collect their embryos by passing the mating tank water through a fine wire-mesh strainer tool.
- Invert the strainer over a Petri dish and rinse with E3 to collect the embryos.
- Incubate the embryos at 28.5 °C O/N.
- When embryos have reached the 24-26 somite stage26, decant most of the E3 media and then dechorionate by adding 100 µl of 50 mg/ml pronase to each dish of embryos and incubating the dish at RT (23 °C) for approximately 15 min.
Note: It will take approximately 24-25 hr from the time of fertilization for the embryos to reach the 24-26 somite stage if they are collected in the manner described and incubated O/N at 28.5 °C. - Fill the dish with E3 and then swirl gently to dislodge the chorions from the embryos.
- Decant the E3/pronase and rinse the dish with 25 ml of fresh E3. Repeat rinse step to remove all pronase.
- To block the development of pigmentation, decant the E3 and replace with 25 ml of fresh E3/PTU when the embryos have reached 24 hr post fertilization.
Note: For experiments spanning several days, replace the E3/PTU solution with fresh media once daily to keep the dish clean.
4. Microinjection of Nephrotoxin Solution
- On the day of injection, prepare the desired nephrotoxin solution along with the appropriate vehicle control.
- Vortex or mix gently to insure the drug(s) is/are in solution, checking the sides of the tube and top of the water column.
Note: Nephrotoxin solutions can be prepared to contain trace amounts of fluorescently conjugated dextran to monitor microinjection efficiency and also assess renal clearance at subsequent time points20,28. - Load a trimmed microinjection needle with ~2-3 µl of the nephrotoxin by threading a fine gel loading tip into the back of the needle and suspend the needle vertically with a piece of tape to allow the solution to fill the trimmed needle tip by gravity.
- Once the needle tip is full, secure the loaded needle in the micromanipulator.
- Test the microinjection volume by placing a drop of mineral oil onto a micrometer slide and inject into the oil to evaluate the droplet size. An injection volume of 500 pl has a diameter of 0.1 mm.
- Remove the dish of embryos from the incubator, and anesthetize by adding approximately 5 ml of 0.2% Tricaine to the embryo dish.
- To ensure complete anesthetization, gently touch embryo with the tip of the embryo manipulator tool. Lack of movement indicates sufficient anesthetization.
- After successful anesthetization, transfer embryos to the injection mold with a transfer pipette.
- Maneuver each embryo into a different well of the mold placing the head in the deepest area of the well such that the trunk rests along the depression and the tail sticks up out of the depression.
- Insert the filled needle into the micromanipulator and position the needle tip next to an embryo.
- Gently insert the needle into the tail vessel by moving the joystick forward and depress the foot pedal to deliver the microinjection.
Note: Successful injection can be gauged by watching the liquid enter circulation. Further, co-injection of the nephrotoxicant with fluorescently conjugated dextran enables the researcher to verify successful injection subsequent to the procedure. - Gently pull back on the joystick to remove the needle from the embryo.
- After injection, transfer the embryos to a clean dish and rinse to remove the Tricaine and replace with fresh E3/PTU.
- Incubate the embryo to the desired time point to assess morphology, which can be documented by photography in methylcellulose mounting media, or process for experimental analysis as desired.
Note: Recovery of embryos should be assessed to determine the percentage of individuals with edema, which commonly indicates renal injury.
Wyniki
A microinjection station set up includes a stereomicroscope, micromanipulator and pressure regulator (Figure 1A). Transillumination of the injection plate is preferable to view specimens during this procedure (Figure 1B). Preparation of the injection needle involves pulling the appropriate borosilicate glass, followed by preparing the edge with cutting and finally back-loading the needle. Optimally, the needle tip is beveled rather than blunt (Figure 1C), a cut that is made by sharply angling the edge of the cutting instrument, such as the fine forceps, during the cutting procedure (Figure 1D). This beveled edge is critical and will greatly affect the ability to gently insert the needle into the zebrafish.
On the day of injection, embryos were positioned using sturdy but pliable embryo manipulation tools (Figure 1E). For the injection, embryos were maneuvered, such that the torso rested along the side of the injection tray to provide leverage for the subsequent injection step (Figure 1F). While injecting, one should focus the plane of view on the embryonic torso to visualize the vascular destination for needle insertion and then observe the injected material enter the circulation (Figure 1G).
The transparency of the zebrafish embryo facilitates the microinjection procedure, and can be further enhanced by PTU chemical treatment at 24 hpf to lessen the development of pigmentation during a typical experimental time course (Figure 2A). Here, embryo specimens were allowed to develop through the 72 hpf stage, then were either mock injected, microinjected with saline vehicle, or microinjected with 2.5 mg/ml gentamicin (Figure 2A). Subsequent live observation was conducted at one and two days post injection, using transillumination lighting with a stereomicroscope (Figure 2B). Compared to mock or saline injected embryos, only the gentamicin-injected individuals showed the development of edema (Figure 2B), in this case pericardial edema, suggesting an abrogation of normal kidney functionality consistent with previously published observations11,20,21.
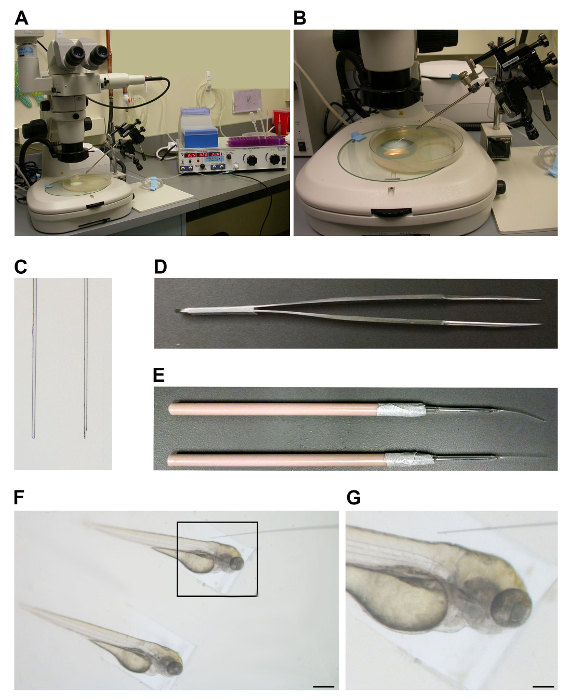
Figure 1. Microinjection apparatus and tools. (A) Injection station set up with stereomicroscope (left), micromanipulator and needle holder (middle) and pressure regulator (right). (B) Transillumination of the injection plate. (C) Left: Blunt cut needle. Right: Beveled cut needle. (D) Fine forceps used to cut needle tips. (E) Manipulation tools used to insert and position the specimens into the injection mold. (F) Embryos arrayed in the microinjection mold. Scale bar = 0.5 mm. (G) Positioning of needle next to the embryo for injection. Scale bar = 0.15 mm. Please click here to view a larger version of this figure.

Figure 2. Experimental timeline and post injection edema assay. (A) Example nephrotoxin experimental timeline in zebrafish, here applied to a gentamicin study. Embryos were dechorionated and treated with PTU solution at 24 hr post fertilization (hpf). Each day, the PTU solution was decanted and replaced with fresh media as indicated by the smaller green arrows. At 72 hpf, embryos were anesthetized, transferred to an injection mold and then treated as follows: mock injected or injected either saline (vehicle control) or gentamicin. After reviving the embryos in fresh media, the embryos were observed at 1 and 2 days post injection (96 and 120 hpf, respectively) for analysis and image acquisition. (B) Top: uninjected zebrafish embryo. Middle: embryo injected at 72 hpf with saline. Bottom: embryo injected with 2.5 mg/ml gentamicin. Scale bar = 0.5 mm. Please click here to view a larger version of this figure.
Dyskusje
A diverse number of therapeutic agents have been associated with AKI29. There have been significant research advances in understanding the damage induced by many individual compounds, such as the aminoglycoside gentamicin30 and the widely used chemotherapeutic cisplatin31,32. Some pathological changes involved in these conditions, however, remain the subject of ongoing study. One emergent challenge remains understanding how multiple drugs adversely affect patients, especially those in high-risk populations such as those in critical care settings and the elderly29. Thus, models that enable further cellular based studies about the effects of drug-induced AKI serve an important role in improving detection of this condition and appreciating dynamic drug interactions.
Work with mammalian models like the mouse and rat have been integral to establish cellular changes in the kidney following AKI, but one limitation with these systems is that direct, real-time observation is limited due to the architectural complexity and internal location of the organ. Simply put, the kidney is not available for direct observation, which necessitates that individuals be sacrificed to analyze the kidney. These limitations can be countered by using zebrafish embryos, which form a simple kidney of two nephrons that have a conserved segment structure with other vertebrates, including humans33. Zebrafish development occurs ex utero and with minor pigmentation, allowing for direct observation of renal organogenesis and monitoring of responses to external cues34. Tools for molecular analysis, such as gene expression35-37, have been utilized with high resolution to determine the features of nephrogenesis38-40. Experimental manipulations for testing of small molecules through chemical genetics are well established in zebrafish and applicable to the kidney41-43. Indeed, in recent years, zebrafish have been applied to model nephrotoxicity of agents ranging from acetominophen to mycotoxins and aristolochic acid44-48. It is important to note that there are also significant limitations to AKI modeling in the zebrafish embryo, because of the very simplicity of renal structure at this stage in development. For example, zebrafish are not suited to addressing multifactorial interactions within renal tissue that is densely packed with nephrons that are surrounded by a complex stroma that contains multiple interstitial cell types, as is the case in the rodent or human metanephros. Thus, zebrafish are best suited to visualizing autonomous cellular and molecular changes in the nephron during AKI.
The method of microinjection described here, while having a steep learning curve, is very useful for studying both developmental and disease states including nephrotoxins. This technique can be used to test drugs singly or in combination. Additionally, injections can be performed during a wide range of development time points. A similar, equally viable method for performing intravenous microinjections in zebrafish larvae has been previously described by Cosentino, et al.21. One significant distinction in their methodology, compared to the methods described here, is that the embryo to be injected is immobilized utilizing a holding pipette21. The use of a holding pipette is a viable alternative to the injection mold. Researchers who seek to implement microinjection as a delivery method for nephrotoxicant agents should be aware of this alternative and may wish to compare the methods to identify which is personally preferable, as learning to manipulate the holding pipette so as not to damage the embryo will involve practice just as it requires practice to successfully maneuver and microinject using a simple injection mold with a depression cavity for the embryos.
When performing this procedure, it is critical to prepare appropriately sized injection needles, and to cut the angle on the needle tip to optimize smooth entry and exit into the embryonic circulation. During the injection procedure, it is critical to monitor the injection volume to assess consistency of the nephrotoxin delivery between samples. Periodic assessment of injection volume with a micrometer can be performed if the researcher is uncertain about changes in volume while performing an experiment. For example, due to the nature of the technique, the needle tip can partially or entirely clog with cellular debris. This complication can be counteracted by clearing the needle periodically to ensure consistency of the ejected fluid. Additionally, a vital dye like phenol red can be added to the mixture to act as a visual marker of the injection and assist in monitoring fluid dispersal49,50. Further, injections of tracer molecules can aid in visualizing specific cell populations, following the injection. For co-injection of fluorescent dextran moieties, specifically 10 kDa dextran conjugates, has a number of applications16,17. In this case, evaluation of fluorescent intensity can be performed immediately following the procedure to confirm successful microinjection with minimal leakage. The intensity can be measured using appropriate image capture photography and then reexamined at subsequent time points to measure the change in fluorescent intensity so as to measure renal clearance51. Further, reabsorption of the dextran in the proximal tubule provides a proxy for functionality of this nephron segment16,17.
Taken together, there are many ways that microinjection can be utilized to investigate AKI with the zebrafish, particularly as this model provides the opportunity to address pharmacokinetics in vivo. Thus, once mastered, microinjection of nephrotoxins into the zebrafish embryo provides a useful paradigm for renal studies.
Ujawnienia
The authors have nothing to disclose.
Podziękowania
This work was supported in part by the NIH grant DP2OD008470. Additionally, RAM was supported in part by funds provided by the University of Notre Dame Graduate School. We thank the staffs of the Department of Biological Sciences, the Center for Zebrafish Research, and the Center for Stem Cells and Regenerative Medicine at the University of Notre Dame. We especially thank the members of lab for engaging discussions about kidney biology and their helpful feedback on this work.
Materiały
| Name | Company | Catalog Number | Comments |
| Sodium Chloride | American Bioanalytical | AB01915 | |
| Potassium Chloride | American Bioanalytical | AB01652 | |
| Calcium Chloride | American Bioanalytical | AB00366 | |
| N-Phenylthiourea (PTU) | Aldrich Chemistry | P7629 | |
| Ethyl 3-aminobenzoate (Tricaine) | Fluka Analytical | A5040 | |
| Borosilicate glass | Sutter Instruments Co. | BF100-50-10 | |
| Flaming/Brown Micropipette puller | Sutter Instruments Co. | Mo. P097 | |
| UltraPure Agarose | Invitrogen | 15510-027 | |
| Magnesium Sulfate | Sigma-Aldrich | M7506 | |
| Methylene Blue | Sigma-Aldrich | M9140 | |
| Falcon Diposable Petri Dishes, Sterile, Corning: | |||
| 60mm x 15mm | VWR | 25373-085 | |
| 100mm x 15mm | VWR | 25373-100 | |
| (microinjection tray) 150mm x 15mm | VWR | 25373-187 | |
| Low Temperature Incubator | Fischer Scientific | 11 690 516DQ | |
| Micro Dissecting Tweezer | Roboz Surgical Instruments Co. | RS-5010 | |
| Micrometer | Ted Pella, Inc. | 2280-24 |
Odniesienia
- Basile, D. P., Anderson, M. D., Sutton, T. A. Pathophysiology of acute kidney injury. Compr. Physiol. 2, 1303-1353 (2012).
- Ostermann, M. Diagnosis of acute kidney injury: kidney disease improving global outcomes criteria and beyond. Curr. Opin. Crit. Care. 20, 581-587 (2014).
- Fluck, R. J. Acute kidney: improving the pathway of care for patients and across healthcare. Curr. Opin. Nephrol. Hypertens. 24, 511-516 (2015).
- Silver, S. A., Cardinal, H., Colwell, K., Burger, D., Dickhout, J. G. Acute kidney injury: preclinical innovations, challenges, and opportunities for translation. Can. J Kidney Health Dis. 2, 30 (2015).
- McCampbell, K. K., Wingert, R. A. Renal stem cells: fact or science fiction?. Biochem. J. 444, 153-168 (2012).
- Li, Y., Wingert, R. A. Regenerative medicine for the kidney: stem cell prospects and challenges. Clin. Transl. Med. 2, 11 (2013).
- Romagani, P., Lasagni, L., Remuzzi, G. Renal progenitors: an evolutionary conserved strategy for kidney regeneration. Nat. Rev. Nephrol. 9, 137-146 (2013).
- Kline, J., Rachoin, J. S. Acute kidney injury and chronic kidney disease: it's a two-way street. Ren. Fail. 35, 452-455 (2013).
- Chawla, L. S., Kimmel, P. L. Acute kidney injury and chronic kidney disease: an integrated clinical syndrome. Kidney Int. 82, 516-524 (2012).
- Sanz, A. B., Sanchez-Niño, M. D., Martìn-Cleary, C., Ortiz, A., Ramos, A. M. Progress in the development of animal models of acute kidney injury and its impact on drug discovery. Expert Opin. Drug Discov. 8, 879-895 (2013).
- McCampbell, K. K., Wingert, R. A. New tides: using zebrafish to study renal regeneration. Transl Res. 163, 109-122 (2014).
- McKee, R. A., Wingert, R. A. Zebrafish renal pathology: emerging models of acute kidney injury. Curr. Pathobiol. Rep. 3, 171-181 (2015).
- Wingert, R. A., et al. The cdx genes and retinoic acid control the positioning and segmentation of the zebrafish pronephros. PLoS Genet. 3, 1922-1938 (2007).
- Wingert, R. A., Davidson, A. J. The zebrafish pronephros: a model to study nephron segmentation. Kidney Int. 73, 1120-1127 (2008).
- Wingert, R. A., Davidson, A. J. Zebrafish nephrogenesis involves dynamic spatiotemporal expression changes in renal progenitors and essential signals from retinoic acid and irx3b. Dev Dyn. 240, 2011-2027 (2011).
- McCampbell, K. K., Springer, K. N., Wingert, R. A. Analysis of nephron composition and function in the adult zebrafish kidney. J. Vis. Exp. (90), e51644 (2014).
- Johnson, C. S., Holzemer, N. F., Wingert, R. A. Laser ablation of the zebrafish pronephros to study renal epithelial regeneration. J. Vis. Exp. (54), e2845 (2011).
- Palmyre, A., et al. Collective epithelial migration drives kidney repair after acute injury. PLoS One. 9, e101304 (2014).
- Fogelgren, B., et al. Exocyst Sec10 protects renal tubule cells from injury by EGFR/MAPK activation and effects on endocytosis. Am. J. Physiol. Renal Physiol. 307, F1334-F1341 (2014).
- Hentschel, D. M., Park, K. M., Cilenti, L., Zervox, A. S., Drummond, I. A., Bonventre, J. V. Acute renal failure in zebrafish: a novel system to study a complex disease. Am. J. Physiol. Renal. Physiol. 288, F923-F929 (2005).
- Cosentino, C. C., Roman, B. L., Drummond, I. A., Hukriede, N. A. Intravenous microinjections of zebrafish larvae to study acute kidney injury. J. Vis. Exp. (42), e2079 (2010).
- Zhou, W., Boucher, R. C., Bollig, F., Englert, C., Hildebrandt, F. Characterization of mesonephric development and regeneration using transgenic zebrafish. Am. J. Physiol. Renal. Physiol. 299, F1040-F1047 (2010).
- Diep, C. Q., et al. Identification of adult nephron progenitors capable of kidney regeneration in zebrafish. Nature. 470, 95-101 (2011).
- McCampbell, K. M., Springer, K. N., Wingert, R. A. Atlas of cellular dynamics during zebrafish adult kidney regeneration. Stem Cell Int. , 547636 (2015).
- Sharma, P., Sharma, S., Patial, V., Singh, D., Padwad, Y. S. Zebrafish (Danio rerio): a potential model for nephroprotective drug screening. Clinical Queries: Nephrol. 3, 97-105 (2014).
- Gerlach, G. F., Wingert, R. A. Kidney organogenesis in the zebrafish: insights into vertebrate nephrogenesis and regeneration. Wiley Interdiscip Rev Dev Biol. 2, 559-585 (2013).
- Kimmel, C. B., Ballard, W. W., Kimmel, S. R., Ullmann, B., Schilling, T. F. Stages of embryonic development of the zebrafish. Dev. Dyn. 203, 253-310 (1995).
- Hanke, N., et al. 'Zebrafishing' for novel genes relevant to the glomerular filtration barrier. Biomed. Res. Int. 2013, 658270 (2013).
- Kane-Gill, S. L., Goldstein, S. L. Drug-induced acute kidney injury: a focus on risk assessment for prevention. Crit. Care Clin. 31, 675-684 (2015).
- Lopez-Novoa, J. M., Quiros, Y., Vicente, L., Morales, A. I., Lopez-Hernandez, F. J. New insights into the mechanism of aminoglycoside nephrotoxicity: an integrative point of view. Kidney Int. 79, 33-45 (2010).
- Ozkok, A., Edelstein, C. L. Pathophysiology of cisplatin-induced acute kidney injury. Biomed. Res. Int. 2014, 967826 (2014).
- Perazella, M. A., Moeckel, G. W. Nephrotoxicity from chemotherapeutic agents: clinical manifestations, pathobiology, and prevention/therapy. Semin. Nephrol. 30, 570-581 (2010).
- Cheng, C. N., Verdun, V. A., Wingert, R. A. Recent advances in elucidating the genetic mechanisms of nephrogenesis using zebrafish. Cells. 4, 218-233 (2015).
- Pickart, M. A., Klee, E. W. Zebrafish approaches enhance the translational research tackle box. Transl. Res. 163, 65-78 (2014).
- Cheng, C. N., Li, Y., Marra, A. N., Verdun, V., Wingert, R. A. Flat mount preparation for observation and analysis of zebrafish embryo specimens stained by whole mount in situ hybridization. J. Vis. Exp. (89), e51604 (2014).
- Galloway, J. L., Wingert, R. A., Thisse, C., Thisse, B., Zon, L. I. Combinatorial regulation of novel erythroid gene expression in zebrafish. Exp. Hematol. 36, 424-432 (2008).
- McKee, R., Gerlach, G. F., Jou, J., Cheng, C. N., Wingert, R. A. Temporal and spatial expression of tight junction genes during zebrafish pronephros development. Gene Expr. Patterns. 16, 104-113 (2014).
- Li, Y., Cheng, C. N., Verdun, V. A., Wingert, R. A. Zebrafish nephrogenesis is regulated by interactions between retinoic acid, mecom, and Notch signaling. Dev. Biol. 386, 111-122 (2014).
- Gerlach, G. F., Wingert, R. A. Zebrafish pronephros tubulogenesis and epithelial identity maintenance are reliant on the polarity proteins Prkc iota and zeta. Dev. Biol. 396, 183-200 (2014).
- Cheng, C. N., Wingert, R. A. Nephron proximal tubule patterning and corpuscles of Stannius formation are regulated by the sim1a transcription factor and retinoic acid in the zebrafish. Dev. Biol. 399, 100-116 (2015).
- Lessman, C. A. The developing zebrafish (Danio rerio): A vertebrate model for high-throughput screening of chemical libraries. Birth Defects Res. C Embryo Today. 93, 268-280 (2011).
- Poureetezadi, S. J., Wingert, R. A. Congenital and acute kidney disease: translational research insights from zebrafish chemical genetics. Gen. Med. 1, 112 (2013).
- Poureetezadi, S. J., Donahue, E. K., Wingert, R. A. A manual small molecule screen approaching high-throughput using zebrafish embryos. J. Vis. Exp. (93), e52063 (2014).
- Peng, H. C., Wang, Y. H., Wen, C. C., Wang, W. H., Cheng, C. C., Chen, Y. H. Nephrotoxicity assessments of acetaminophen during zebrafish embryogenesis. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 151, 480-586 (2010).
- Wu, T. S., Yang, J. J., Yu, F. Y., Liu, B. H. Evaluation of nephrotoxic effects of mycotoxins, citrinin and patulin, on zebrafish (Danio rerio) embryos. Food Chem. Toxicol. 50, 4398-4404 (2012).
- Ding, Y. J., Chen, Y. H. Developmental nephrotoxicity of aristolochic acid in a zebrafish model. Toxicol. Appl. Pharmacol. 261, 59-65 (2012).
- Zennaro, C., et al. Podocyte developmental defects caused by adriamycin in zebrafish embryos and larvae: a novel model of glomerular damage. PLoS One. 9, e98131 (2014).
- Ding, Y. J., Sun, C. Y., Wen, C. C., Chen, Y. H. Nephroprotective role of resveratrol and ursolic acid in aristolochic acid intoxicated zebrafish. Toxins. 7, 97-109 (2015).
- Rosen, J. N., Sweeney, M. F., Mably, J. D. Microinjection of zebrafish embryos to analyze gene function. J. Vis. Exp. (27), e1115 (2009).
- Yuan, S., Sun, Z. Microinjection of mRNA and morpholino antisense oligonucleotides in zebrafish embryos. J. Vis. Exp. (27), e1113 (2009).
- Christou-Savina, S., Beales, P. L., Osborn, D. P. Evaluation of zebrafish kidney function using a fluorescent clearance assay. J. Vis. Exp. (96), e52540 (2015).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone