Method Article
Adult Mouse Venous Hypertension Model: Common Carotid Artery to External Jugular Vein Anastomosis.
W tym Artykule
Podsumowanie
We describe a method for creating a reliable model of cerebral venous hypertension in the adult mouse. This model has been widely described and tested in the rat. This new counterpart in the mice opens the possibility of using genetic modified animals and thereby broadens the applications of the model.
Streszczenie
The understanding of the pathophysiology of brain arteriovenous malformations and arteriovenous fistulas has improved thanks to animal models. A rat model creating an artificial fistula between the common carotid artery (CCA) and the external jugular vein (EJV) has been widely described and proved technically feasible. This construct provokes a consistent cerebral venous hypertension (CVH), and therefore has helped studying the contribution of venous hypertension to formation, clinical symptoms, and prognosis of brain AVMs and dural AVFs. Equivalent mice models have been only scarcely described and have shown trouble with stenosis of the fistula. An established murine model would allow the study of not only pathophysiology but also potential genetic therapies for these cerebrovascular diseases.
We present a model of arteriovenous fistula that produces a durable intracranial venous hypertension in the mouse. Microsurgical anastomosis of the murine CCA and EJV can be difficult due to diminutive anatomy and frequently result in a non-patent fistula. In this step-by-step protocol we address all the important challenges encountered during this procedure. Avoiding excessive retraction of the vein during the exposure, using 11-0 sutures instead of 10-0, and making a carefully planned end-to-side anastomosis are some of the critical steps. Although this method requires advanced microsurgical skills and a longer learning curve that the equivalent in the rat, it can be consistently developed.
This novel model has been designed to integrate transgenic mouse techniques with a previously well-established experimental system that has proved useful to study brain AVMs and dural AVFs. By opening the possibility of using transgenic mice, a broader spectrum of valid models can be achieved and genetic treatments can also be tested. The experimental construct could also be further adapted to the study of other cerebrovascular diseases related with venous hypertension such as migraine, transient global amnesia, transient monocular blindness, etc.
Wprowadzenie
Animal models of cerebral venous hypertension have proved to be a key tool in the understanding of the pathophysiology of brain arteriovenous malformations and arteriovenous fistulas1-7. The most widely used is the rat model created through an artificial fistula between the common carotid artery (CCA) and the external jugular vein (EJV), which provokes a consistent cerebral venous hypertension (CVH) in the rat1,8-10. Equivalent mice models, by opening the possibility of using different transgenic mice strains, would allow further study on not only pathophysiology but also potential genetic therapies for these cerebrovascular diseases. Furthermore, the experimental construct could also be further adapted to the study of other cerebrovascular diseases related with venous hypertension such as migraine, transient global amnesia, transient monocular blindness, etc.11 However, previous attempts to construct these mice models have demonstrated the difficulties with patency of the fistula due to the diminutive anatomy5,12. Here, we describe our step-by-step protocol for a successful anastomosis of the murine CCA and EJV that translates into a long-term patent fistula and a durable venous hypertension in the mouse.
Protokół
1. Preparing the Mouse
- Induce general anesthesia in the mouse with isoflurane gas. Inject 0.15 ml of intraperitoneal brupenorphine for pain management. Before proceeding, check if the anesthesia level is satisfactory by pricking the mouse's paws.
- Put the mouse in dorsal recumbency with the four limbs fixed by adhesive tape. Remove the hair of the neck and the upper chest with scissors. Via subcutaneous injection, administer 0.2-0.4 ml of 0.9% saline to keep the mouse hydrated during the surgical procedure.
- Prepare the operative field following a strict sterile method. The area of the skin incision should be cleaned with 90% alcohol.
2. Dissecting the Common Carotid Artery and the External Jugular Vein
- Make a horizontal midline cervical incision across the lower neck area of the mouse. After deepening the wound, elevate the salivary glands and cervical soft tissue by using a traction suture (Figure 1). Expose the right external jugular vein (EJV) lateral to the sternocleidomastoid muscle (SCM). This step should be performed under the microscope, since excessive traction over the vein might damage it and induce its thrombosis.
- Carefully dissect the right EJV along its course from the clavicle to the skull base. Usually electric bipolar forceps, coagulate and divide any branches to prepare an adequate length for the later temporary clip placement and anastomosis.
- Lateral to the trachea and medial to the SCM, explore the common carotid artery (CCA). It should be carefully exposed from the clavicle to just beyond its bifurcation into the external and internal carotid arteries. During this step, attention should be given again to avoid excessive traction to the EJV that could later compromise the patency of the anastomosis.
3. Preparing the Anastomosis
- Ligate the CCA with 10-0 Nylon just proximal to its bifurcation. Next, apply a temporary clip over the proximal CCA as close to the clavicle as possible.
- Once the flow has been interrupted, transect the artery just under the bifurcation ligature and irrigate it with saline to wash out any remaining blood inside the lumen. Avoid bipolar coagulation in this step, since thermal injury to the artery wall could put the future anastomosis in jeopardy.
- In order to improve the visibility of the edges of the EJV, the medial wall is marked with a blue marking pen along the course of the planned venotomy. Once the EJV is marked, use a 10-0 suture to ligate the distal end as caudal as possible and apply a temporary vascular clip on the proximal end as cranial as possible.
- With a fine 30 G needle and 0.5 ml syringe, make an initial aperture over the marked area of the EJV and immediately irrigate the lumen with saline to avoid thrombus formation. Next, extend the venotomy with the microscissors until the length is about 2-3 times the diameter of the CCA. Avoid violent stretching and pay attention to keep a sharp and neat cutting edge.
- Approximate the end of the CCA to the EJV. Make a side-cut incision in the donor end of the CCA to adjust the diameter to the length to the venotomy size (Figure 2).
4. End-to-side Anastomosis
- Use a 11-0 monofilament nylon suture for the CCA-to-EJV end-to-side anastomosis. Suturing the medial wall of the anastomosis in a craneo-caudal direction is the initial step. Every stitch should be placed from outside-in the venous wall first (Figure 3) and from inside-out the arterial wall (Figure 4) next. This will keep the knot on the outside surface of the anastomotic vessels at all times (Figure 5). Either interrupted or continuous sutures can be used, but all continuous sutures should be tightened as the final step.
- Once the medial wall has been sutured, repeat the procedure from caudal to cranial with the lateral wall. Now every stitch should be placed from outside-in the arterial wall first and from inside-out the venous wall next. Saline irrigation will help keep the lumen of the anastomosis visible at all times during the procedure.
- After finishing all the steps of the anastomosis, remove the temporary clip from the vein first and from the artery next. The arterial blood will flow into the EJV with no or little oozing from the anastomosis (see videos VH model and VH model2). The minimal bleeding should stop without using cotton compression over the anastomosis. This must be avoided in order to prevent thrombosis of the delicate vein.
- Once pulsatile flow through the anastomosis is confirmed and no apparent bleeding is observed, irrigate the surgical field with saline and close the cervical incision with a 6-0 nylon suture. Finally, administer 0.15 ml more of intraperitoneal brupenorphine for postoperative pain management and 0.2-0.4 ml of subcutaneous 0.9% saline to replenish any blood loss during the surgery.
Wyniki
A successful outcome of the model is a patent arteriovenous fistula that induces venous hypertension in the murine brain. To validate the model we initially measured the intracranial venous pressure in the sagital sinus of the mice at 2, 3 and 4 weeks after surgery. 6 different mice were assigned to every time group. The sinus pressure was 8.8 ± 1.2 mmHg in the group measured two weeks after the surgery. In the 6 mice measured 3 weeks after surgery, the sinus pressure was 4.7 ± 1.4 mmHg. Finally, the 6 mice measured 4 weeks after surgery had a sinus pressure of 3.9 ± 0.6 mmHg (Figure 6). Postoperative 2 week sinus pressure was significantly higher than sinus pressure at 3 and 4 weeks (both p <0.001).
However, the complex technique required to measure the sinus pressure is not necessary to ensure fistula patency and venous hypertension on a regular basis. Instead, the desired outcome can be checked by direct inspection of the fistula and by clinical signs of venous hypertension in the mouse.
The patency of the fistula can be directly inspected at the end of the surgical procedure by two methods. The first one consists of temporary obstructing the EJV cranial to the anastomsis area with a jeweler’s forceps. This point is now the only outflow of the blood coming from the CCA. If the end-to-side anastomosis is patent, the distensible EJV will immediately balloon out as shown in the patency test video 1. The second method is performed by occluding the vein graft distal to the anastomosis and slowly emptying it or “milking” it with a pair of jeweler's forceps. As shown on the second video for patency test, once the occlusion is released, a patent anastomosis should quickly refill the emptied segment.
If the model is working, enlargement of the mouse eye should be noted 24 hr after the surgery. This may be due to comprised venous drainage of the head and neck. Although both eyes get enlarged after the surgery, the phenomenon is more prominent on the operated side as can be observed in Figure 7.

Figure 1: Skin incision. A horizontal mid-line cervical incision across the lower neck area of the mouse and a soft traction of the salivary glands exposes the right external jugular vein (EJV). Note the superficial location of the EJV that warrants a careful skin incision to prevent any injuries to the delicate vein.
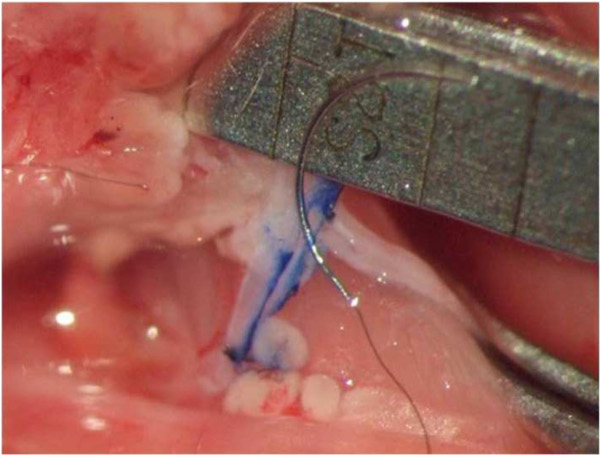
Figure 2: Preparing the anastomosis. The medial wall of the EJV has been marked with a blue line along the course of the planned venotomy. A side-cut incision in the donor end of the CCA is performed to adjust the diameter of the artery to the length to the venotomy.

Figure 3: Outside-in. The medial wall of the anastomosis is being sutured in a craneo-caudal direction with the 11-0 stitch first placed from outside-in the venous wall.

Figure 4: Inside-out. The needle is driven here from inside-out the arterial wall to complete a suture in the medial wall in the appropriate way to keep the knot on the outside surface of the anastomosis.
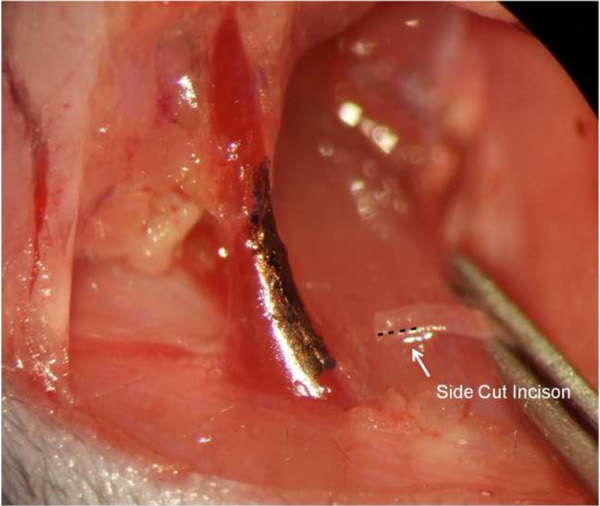
Figure 5: Keeping the knot outside. Note how the knot of the interrupted suture chosen in this case stays outside the lumen of the vessels to avoid thrombus formation that would occlude the fistula.
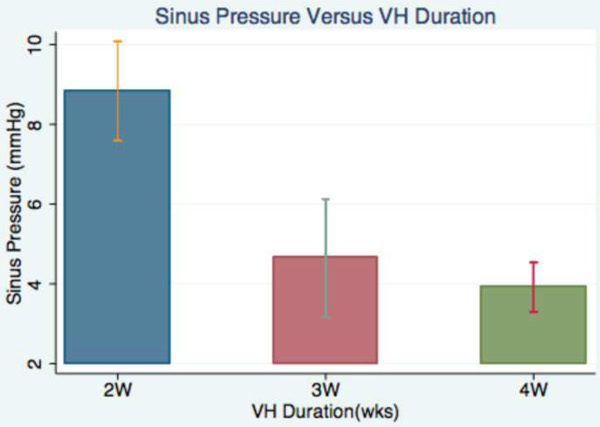
Figure 6: Sinus pressure analysis. This bart chart graphically displays the difference in the intracranial sinus pressure measured in mmHg at 2, 3 and 4 weeks after surgery.
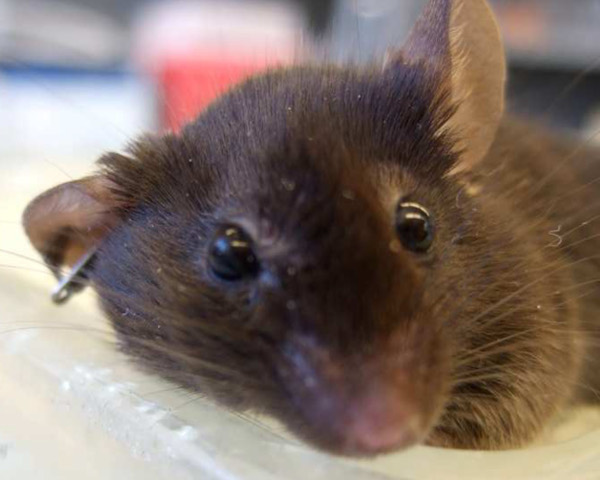
Figure 7: Proptosis. One day after surgery, both eyes are enlarged but the right eye enlargement (ipsilateral to the surgery) is more prominent.
Dyskusje
Sustained cerebral venous hypertension has been closely related with more severe clinical manifestations and poor prognosis in patients with dural AVFs and brain AVMs3. These effects of CVH have been widely studied in rat models1,2,8. An equivalent model in the mouse would allow the use of genetically modified animals which would ultimately allow the analysis of molecular pathways involved on the pathogenesis of venous hypertension and its relationship with dural AVF and brain AVM.
Here, we report a method for creating cerebral venous hypertension in the adult mouse through an end-to-side carotid-jugular fistula. This new model enhances the well-described carotid–jugular fistula models in the rat by translating it to the mouse and thereby allowing the aforementioned possibility of investigating molecular pathways and gene therapies.
Common Problems Encountered and Suggestions
The CCA-to-EJV end-to-side anastomosis for mice is relatively difficult due to the diminutive size and fragility of the involved vessels. The diameter of the EJV is about 2 mm and the diameter of the CCA is about 0.6 mm. Using the appropriate instruments and sutures is a critical point to achieve a successful model. We use number 55 forceps as a needle holder in one hand and as a forceps to handle tissues in the other. These delicate forceps often get their tips damaged and can injure the vessels’ walls because of it. This can be easily avoided by viewing under the microscope the tips of all micro-instruments before each procedure. The suture is equally important. The anastomosis must be performed with 11-0 sutures (AROS Micro suture, Aurosurgical, Newport Beach, CA, USA). Attempts using 10-0 resulted in excessive bleeding of the anastomosis site or damage to the vessel walls that may promote thrombus formation.
Another common problem is damaging the vein with excessive traction, compression, or dissection along its course. Therefore, the EJV exposure must be carefully performed under the microscope to identify the appropriate plane of dissection. All the small branches that drain into it should also be identified and coagulated. This will avoid uncontrolled bleeding requiring compression of the vein and will provide enough redundancy to avoid excessive tension.
It is not uncommon to have poor function of the fistula after removing the temporary clips. In this case, the first step should be look for possible points of tension or angulation in the CCA. Further dissection of the artery or partial resection of the sternocleidomastoid muscle will solve this problem. However, if poor function continues after these maneuvers, thrombosis of the anastomosis site must be suspected. In that case, instead of reopening the anastomosis site and removing the thrombus, we recommend performing a new anastomosis cranial to the previous one. In our experience, a third anastomosis is impossible. There is not enough length of the EJV and the mouse does not tolerate anesthesia for such a long period of time.
Limitations of the Technique
The main limitations of this technique include: (1) the diminutive anatomy of the vessels requires advanced microsurgical skills and a longer learning curve than for the equivalent model in the rat; (2) the patency of the anastomosis is often compromised by thrombus formation and the length of the mouse EJV will only allow one extra attempt to solve this issue; and (3) surgery may take up to three hours, exposing the mouse to a long anesthesia period. However, following the technical suggestions proposed above and keeping the mouse warm and hydrated during the procedure, a patent CCA-to-EJV can be successfully performed and a high survival rate of the animals achieved in our experience.
Applications of the Technique
A reliable and durable cerebral venous hypertension model in mice is an important tool that can serve a broad number of purposes in cerebrovascular research13-16. By adding knockout technology of transgenic mice, this new model should facilitate further gene-related in vivo research for all the pathologies associated with cerebral venous hypertension.
Previously, other authors have described the venous hypertension model in the rat and have used it to study the pathophysiology of dural AVF and brain AVM4,5,8,9. We now describe the method to successfully translate this same model to the mice, therefore opening its potential applications to all kinds of genetic treatment testing.
Conclusion
We demonstrate here a protocol to achieve a patent CCA-to-EJV anastomosis in the adult mouse. This fistula provides a durable brain venous hypertension that has been a useful tool to develop different cerebrovascular disease models in the rat. By providing this initial step to develop those same models in the mice, we open the possibility of testing genetic treatments for those cerebrovascular diseases.
Ujawnienia
The experimental procedures with laboratory animals were approved by the Institutional Animal Care and Use Committee of the University of California, San Francisco (UCSF).
The authors have no potential conflicts of interest related to the drugs and materials used in this procedure.
Podziękowania
This project is partially supported by NIH T32 GM008440 to Espen Walker, R01 NS27713 to William L.Young, P01 NS44155 to William L.Young and Hua Su, R21 NS070153 to Hua SU and by the American Heart Association AHA 10GRNT3130004 to Hua Su. Dr Ana Rodríguez-Hernández is supported by a grant from “Obra Social La Caixa”
Materiały
| Name | Company | Catalog Number | Comments |
| 10-0 Sterile Microsuture | Arosurgical Ic. | VT5A010Q10 | |
| 11-0 Sterile Microsuture | Arosurgical Ic | VT4A00N07 | |
| DUROTIP Scissors | Aesculap | BC210R | |
| Micro-Adson Tissue Forceps | Aesculap | BD510R | |
| Microscissors | Aesculap | OC496R | |
| Micro Forceps #5 Jewelers | Aesculap | BD331R | |
| Angled Jewelers Forceps | Aesculap | BD329R | |
| Micro Suture Forceps | Aesculap | BD338R | |
| DUROGRIP Needle Holder | Aesculap | BM009R |
Odniesienia
- Bederson, J. B., Wiestler, O. D., Brüstle, O., Roth, P., Frick, R., Yaşargil, M. G. Intracranial venous hypertension and the effects of venous outflow obstruction in a rat model of arteriovenous fistula. Neurosurgery. 29, 341-350 (1991).
- Gao, P., Zhu, Y., Ling, F., Shen, F., Lee, B., Gabriel, R. A., Hao, Q., Yang, G. Y., Su, H., Young, W. L. Nonischemic cerebral venous hypertension promotes a pro-angiogenic stage through HIF-1 downstream genes and leukocyte-derived MMP-9. J. Cereb. Blood Flow Metab. 29, 1482-1490 (2009).
- Kim, H., Su, H., Weinsheimer, S., Pawlikowska, L., Brain Young, W. L. arteriovenous malformation pathogenesis: a response-to-injury paradigm. Acta Neurochir. Suppl. 111, 83-92 (2011).
- Lawton, M. T., Arnold, C. M., Kim, Y. J., Bogarin, E. A., Stewart, C. L., Wulfstat, A. A., Derugin, N., Deen, D., Young, W. L. Radiation arteriopathy in the transgenic arteriovenous fistula model. Neurosurgery. 62, 1129-1138 (2008).
- Lawton, M. T., Stewart, C. L., Wulfstat, A. A., Derugin, N., Hashimoto, T., Young, W. L. The transgenic arteriovenous fistula in the rat: an experimental model of gene therapy for brain arteriovenous malformations. Neurosurgery. 54, 1463-1471 (2004).
- Schaller, B., Graf, R., Sanada, Y., Tolnay, M., Rosner, G., Wienhard, K., Heiss, W., D, Hemodynamic changes after occlusion of the posterior superior sagittal sinus: an experimental PET study in cats. AJNR Am J Neuroradiol. 24, 1876-1880 (2003).
- Zhu, Y., Lawton, M. T., Du, R., Shwe, Y., Chen, Y., Shen, F., Young, W. L., Yang, G. Y. Expression of hypoxia-inducible factor-1 and vascular endothelial growth factor in response to venous hypertension. Neurosurgery. 59, 687-696 (2006).
- Herman, J. M., Spetzler, R. F., Bederson, J. B., Kurbat, J. M., Zabramski, J. M. Genesis of a dural arteriovenous malformation in a rat model. J. Neurosurg. 83, 539-545 (1995).
- Terada, T., Higashida, R. T., Halbach, V. V., Dowd, C. F., Tsuura, M., Komai, N., Wilson, C. B., Hieshima, G. B. Development of acquired arteriovenous fistulas in rats due to venous hypertension. J. Neurosurg. 80, 884-889 (1994).
- Yassari, R., Sayama, T., Jahromi, B. S., Aihara, Y., Stoodley, M., Macdonald, R. L. Angiographic, hemodynamic and histological characterization of an arteriovenous fistula in rats. Acta Neurochir (Wien). 146, 495-504 (2004).
- Solheim, O., Skeidsvoll, T. Transient global amnesia may be caused by cerebral vein thrombosis. Med. Hypotheses. 65, 1142-1149 (2005).
- Yang, B., Shergill, U., Fu, A. A., Knudsen, B., Misra, S. The mouse arteriovenous fistula model. J Vasc Interv Radiol. 20, 946-950 (2009).
- Choi, E. J., Choi, E. J., Walker, E. J., Shen, F., Oh, S. P., Arthur, H. M., Young, W. L., Su, H. Minimal Homozygous Endothelial Deletion of Eng with VEGF Stimulation Is Sufficient to Cause Cerebrovascular Dysplasia in the Adult Mouse. Cerebrovascular diseases. 33, 540-547 (2012).
- Hao, Q., Su, H., Marchuk, D. A., Rola, R., Wang, Y., Liu, W., Young, W. L., Yang, G. Y. Increased tissue perfusion promotes capillary dysplasia in the ALK1-deficient mouse brain following VEGF stimulation. Am. J. Physiol. Heart Circ. Physiol. 295, H2250-H2256 (2008).
- Su, H., Hao, Q., Shen, F., Zhu, Y., Lee, C. Z., Young, W. L., Yang, G. Y. Development of a cerebral microvascular dysplasia model in rodents. Acta Neurochir. Suppl. 105, 185-189 (2008).
- Walker, E. J., Su, H., Shen, F., Degos, V., Jun, K., Young, W. L. Bevacizumab Attenuates VEGF-Induced Angiogenesis and Vascular Malformations in the Adult Mouse Brain. Stroke; a journal of cerebral circulation. , (2012).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone