Method Article
A Laser-induced Mouse Model of Chronic Ocular Hypertension to Characterize Visual Defects
W tym Artykule
Podsumowanie
Chronic ocular hypertension is induced using laser photocoagulation of the trabecular meshwork in mouse eyes. The intraocular pressure (IOP) is elevated for several months after laser treatment. The decrease of visual acuity and contrast sensitivity of experimental animals are monitored using the optomotor test.
Streszczenie
Glaucoma, frequently associated with elevated intraocular pressure (IOP), is one of the leading causes of blindness. We sought to establish a mouse model of ocular hypertension to mimic human high-tension glaucoma. Here laser illumination is applied to the corneal limbus to photocoagulate the aqueous outflow, inducing angle closure. The changes of IOP are monitored using a rebound tonometer before and after the laser treatment. An optomotor behavioral test is used to measure corresponding changes in visual capacity. The representative result from one mouse which developed sustained IOP elevation after laser illumination is shown. A decreased visual acuity and contrast sensitivity is observed in this ocular hypertensive mouse. Together, our study introduces a valuable model system to investigate neuronal degeneration and the underlying molecular mechanisms in glaucomatous mice.
Protokół
Procedures
C57BL/6J mice (Jackson Laboratory, Bar Harbor, ME) are raised at Northwestern University's Animal Care Facility. All animals are used in accordance with protocols approved by Northwestern University Institutional Animal Care and Use Committee and conformed to the guidelines on the Use of Animals in Neuroscience Research from the NIH.
1. Laser Photocoagulation
The procedure of laser photocoagulation is modified from previously published protocols 5-7.
- Anesthetize a 40-60 day old mouse by an intraperitoneal injection of ketamine (100 mg/kg, Butler Schein Animal Health, OH) and xylazine (10 mg/kg, Lloyd Inc. of Iowa, Shenandoah, IA).
- Dilate the pupil of the right eye of the experimental animal by topical treatment with one or two drops of 1% atropine sulfate solution (Alcon Labs, Inc., Fort Worth, TX).
- After mydriasis, flatten the anterior chamber to enhance laser induction 6. Insert a glass micropipette with sharp tip (World Precision Instruments Inc, Sarasota, FL) into the anterior space under the slit lamp (SL-3E, Topcon, Oakland, NJ) to drain out the fluid in the anterior chamber.
- Restrain the mouse in a plastic cone holder (Braintree Sci Inc., MA) and tied up on a homemade platform (See Figure 1A). Hold the mouse with restrainer and exposes the right eye of the mouse to the light source behind the slit lamp. Align the right eye of the anesthetized mouse under the slit lamp.
- While holding the mouse restrainer with both hands, apply the laser illumination to the corneal limbus using an Argon laser (Ultima 2000SE, Coherent, Santa Clara, CA). Deliver about 80-100 laser spots (514 nm, 100 mW, 50 msec pulse, and 200 μm spot) perpendicularly around the circumference of the trabecular meshwork. The C57BL/6 mice have pigmented iris which serves as a barrier for any potential stray energy 7.
- Instill topical 0.5% moxifloxacin (Alcon Labs, Inc., Fort Worth, TX) on the ocular surface to disinfect the laser-treated area and 0.5% Proparacaine (Bausch & Lomb, Rochester, NY) to relieve pain.
- Keep the animal on a heating pad (Sunbeam Products Inc, Boca Raton, FL) for recovery for about an hour until it is fully awake.
- The left eye is untreated to serve as a control.
2. IOP Measurements
- Place the awake mouse into a tube to load into the plastic cone holder and then restrain it on the platform (See Figure 2A).
- Allow five to ten minutes to let the mouse get adapted to the holder position. Approach the rebound tonometer (TonoLab, Colonial Medical Supply, Franconia, NH) to the mouse eye until the probe tip is 2-3 mm away from the surface of cornea 14.
- Press the measurement button to let the probe tip hit the center surface of cornea gently. Three consecutive sets of six-measurements of IOP of the same eye are acquired and averaged as the IOP of the eye. The untreated control eye is always measured first to get a baseline reading for the laser-treated eye that is measured next.
3. Optomotor Test
Visual acuity and contrast sensitivity are tested 14,15 . The two eyes of individual mice are examined separately by reversing the drifting grating direction; i.e. a clockwise drifting grating is used to identify the visual function of the left eye and a counter-clockwise drifting grating for the right eye 16. Each test takes about 15 min and is repeated by two observers independently.
- Place the mouse and allow the mouse to move freely on an elevated platform surrounded by four computer monitors (Figure 3A-B).
- Set up the monitors so that they display horizontally drifting sinusoidal gratings as visual stimuli with mean luminance of 39 cd/m2. The moving direction of the grating should alternate consecutively between clockwise and counterclockwise.
- Analyze the animal's movements. The animal's movements in-concert with the drifting gratings are considered "positive" within 15 sec after the visual stimulus is on and then gradually increased. The highest response-eliciting visual stimulus is defined as the animal's visual acuity 17.
- Examine the contrast sensitivity at three pre-selected spatial frequencies: 0.075, 0.16, and 0.3 cycles per degree (cpd). The contrast threshold for each eye is defined as the lowest contrast that elicits visual responses at the pre-fixed frequency. The contrast sensitivity is the reciprocal of the threshold 17.
Wyniki
As described in the Procedures, laser illumination is aimed at the trabecular meshwork in the limbal region to photocoagulate the aqueous outflow, inducing angle closure (Figure 1). Most lasered eyes exhibited no significant physical damage, pigment detachment or infection, consistent with previous findings 6. When a small group of mice (less than 5% of all lasered animals) exhibited physical signs of severe damage such as deflated eye balls, severe cataract, significant pigment detachment, or bleeding, we euthanized them immediately. Around 30% of lasered eyes developed minor corneal scars, and most of them recovered within 1-2 weeks after laser treatment.
We found elevated IOP in nearly every laser-treated eyes from more than one hundred mice. The IOP of experimental animals is monitored using a rebound tonometer (Figure 2). Figure 2B shows one example of the changes of IOP before and after the laser treatment. Before laser treatment, the IOP baselines of the two eyes of the mouse showed no difference: 15.7 mmHg (right) vs. 14.7 mmHg (left, Day 0, Figure 2B). Seven days after laser-treatment, the IOP of the treated (right) eye increased almost 2-fold to 30.7 mmHg, compared to the untreated eye (15.7 mmHg). The IOP of laser-treated eye remained elevated at 26-28 mmHg for about 4 months: at 4 months after treatment, the mean IOP of treated eye was 26 mmHg, significantly higher than that of untreated left eye (16.3 mmHg). Subsequently, the IOP of the treated eye slowly decreased and reached 18.7 mmHg at 6 months (24 weeks) after treatment (untreated eye: 15.3 mmHg). Our data demonstrate that sustained IOP elevation is achieved for more than 4 months.
We next confirmed the vision loss using the optomotor test (Figure 3). The optomotor test measures aspects of spatial vision via reflexive head-tracking movements. Figure 3C shows the decrease of visual acuity of the animal examined in Figure 2B. Before the laser-treatment, both eyes exhibited normal acuity (Left: 0.375 cpd; Right: 0.397 cpd; Figure 3C). At two-months after laser treatment, the acuity of the right eye (elevated IOP) decreased significantly compared with the left control eye (Left: 0.45 cpd; Right: 0.228 cpd; Figure 3C). The acuity of the eye with elevated IOP remained low at 5-6 months after laser treatment (Left: 0.378 cpd; Right: 0. 258 cpd; Figure 3C). Similarly, a lower contrast sensitivity of the right eye with elevated IOP was observed (Figure 3D). At two-months after laser treatment, the contrast sensitivity of the control left eye was 6.13, while the right eye was 1.91 at 0.075 cpd. The contrast sensitivity of the control left eye was 5.53 and 2.67 at 0.16 and 0.3 cpd, while the right eye was 4.28 and 1.45, respectively (Figure 3D).
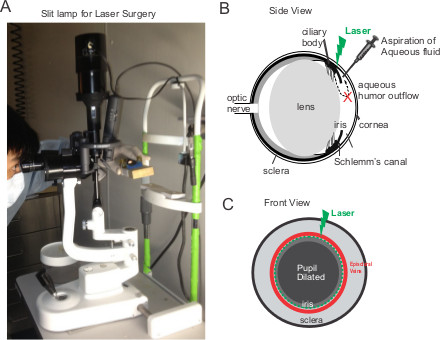
Figure 1. Laser photocoagulation of the aqueous humor outflow in mouse eyes. (A) A photo of the slit lamp for laser treatment. The operator holds the mouse with restrainer and then aligns the right eye of the mouse to the light source of the slit lamp. (B-C) Schematic side-view and front-view of the eye. The operator holds the mouse restrainers with both hands while 80-100 laser spots are applied to the area between the episcleral veins and the dilated pupil.
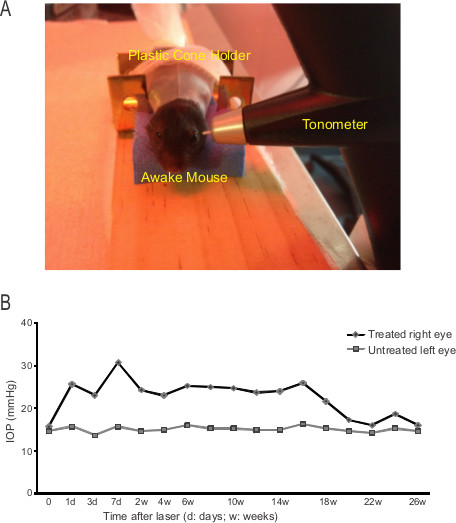
Figure 2. IOP increased after laser treatment. (A) The setup to measure IOP using a rebound tonometer. (B) Changes of IOP of one experimental mouse after laser treatment. Each point is the average of three consecutive sets of six-measurements of IOP.

Figure 3. Decreases of visual acuity and contrast sensitivity with IOP elevation. (A) Schematic drawing of the optomotor setup. (B) A mouse on the center platform in the optomotor apparatus with gratings displayed on four surrounding monitors. (C) The acuity and contrast sensitivity of the mouse examined in Figure 2B.
Dyskusje
We report above that sustained ocular hypertension can be induced by laser illumination in mouse eyes. Compared to the saline injection model 18 and the vein cautery model 11 both of which require extensive microsurgical skills, the laser illumination is relatively simple and easy to perform. Usually we can perform the laser illumination for 4-6 mice in 2-3 hr. The critical steps to achieve sustained IOP elevation are the anterior chamber flattening before laser and the parameters for laser illumination. Draining out the fluid in the anterior chamber facilitated to target the laser to the trabecular meshwork area and minimize injury to nearby ciliary body and blood vessels 6. Different types of laser have also been reported; for example, some studies used a diode laser with wavelength of 532 nm 5,6 and others used 810 nm energy pulses 7 to target the trabecular meshwork and episcleral veins in the limbal region. In order to maximize the angle damage, we have increased the number of laser spots compared to the previously reported laser models 5-7. With our experimental setup, almost every laser-treated mouse had more than 50% increase of IOP by the first week after laser treatment, among which about 60% had elevated IOP for more than 2 months. By contrast, one intraocular injection of microbeads into mouse eyes can elicit an ~30% elevation in IOP for a couple weeks 8 (another study suggested a longer effect of IOP elevation 9,10), and the occlusion of limbal and episcleral veins in albino CD-1 mice only induced an acute IOP elevation for a few days 13.
The accurate measurement of IOP is important in determining the laser effects on mouse eyes. Anesthesia significantly altered the IOP measurement and behavioral training of mice reduced IOP variation in awake animals 9,19. Here, the experimental animals were given a few minutes to rest and adapt to the restrained position before measurement in order to get consistent readings of IOP. To confirm the IOP measurement is reliable and not dependent on the person who performed the test, the same animals were examined by two or three different testers and their differences of IOP readings are generally within 5-15%.
Because of the variability of the duration and degree of IOP elevation, different RGC loss has been reported in different animal models. For example, a 20% loss of axons has been observed in mouse eyes with microbeads injection 8. Around 20% of RGCs died in rat eyes at six weeks after laser illumination at the trabecular meshwork, while around 60% of RGCs died with laser illumination at both the trabecular meshwork and the episcleral veins 5. Our data showed a 20-30% of RGC loss at 2 months after laser treatment in mouse eyes. Nevertheless, all these different animal models of chronic ocular hypertension without significant inflammation or damage to other parts of the eyes provide us the potential to measure the long-term effects of ocular hypertension on retinal structure and visual function over time.
Taking advantage of the non-invasive nature of the visual behavioral assay that allows serial tests as a function of changing conditions, the changes of visual acuity and contrast sensitivity can be monitored for months after the induction of ocular hypertension. The optomotor test provides a rapid assessment of visual function; moreover, the two eyes can be tested separately, which greatly facilitates our experiments because one eye of the target mouse is laser-treated and the other is left intact as the control. At the same time, it is noted that the optomotor reflex sometimes is difficult to use due to the high activity and wandering attention of some mice 12.
Combined with the power of mouse genetics, our model provides a superb readout with which to investigate the pathological mechanisms in high-tension glaucoma. For example, using Thy-1-YFP transgenic mice, which has a small number of RGCs labeled 10,20-22, the dendritic structural changes of individual RGCs can be imaged in eyes with sustained ocular hypertension. We have demonstrated that the dendritic degeneration of RGCs depends on location and subtypes in ocular hypertensive eyes 23. Cell apoptosis or neuroprotective signaling pathways can be further manipulated in vivo to identify the underlying molecular mechanisms of RGC degeneration and survival in glaucoma.
Ujawnienia
The authors declare that they have no competing financial interests.
The authors are full-time employees of Northwestern University.
The authors received NO funding that was provided by companies which produce reagents and instruments used in this article.
Podziękowania
The work contained in this paper has been supported by the Dr. Douglas H. Johnson Award for Glaucoma Research from the American Health Assistance Foundation (XL), the William & Mary Greve Special Scholar Award from the Research to Prevent Blindness (XL), the Illinois Society for the Prevention of Blindness (HC) and NIH grant R01EY019034 (XL).
Materiały
| Name | Company | Catalog Number | Comments |
| Reagent | |||
| moxifloxacin | Alcon Labs, Inc. | NDC 0065-4013-03 | 0.5 %, Rx only |
| Proparacaine Hydrochloride | Bausch & Lomb | NDC 24208-730-06 | 0.5 %, Rx only |
| Ophthalmic Solution USP | Bausch & Lomb | NDC 24208-730-06 | .5 %, Rx only |
| ketamine | Butler Schein Animal Health | NDC 11695-0550-1 | 100 mg / kg |
| xylazine | LLOYD Inc. of Iowa | NADA 139-236 | 10 mg / kg |
| atropine sulfate solution | Alcon Labs, Inc. | NDC 61314-303-02 | 1 %, Rx only |
| Equipment | |||
| Slit Lamp, TOPCON | Visual Systems Inc | SL-3E | powered by PS-30A |
| OptoMotry 1.8.0 virtual | CerebralMechanics Inc. | ||
| opto-kinetic testing system | CerebralMechanics Inc. | ||
| Tonometer, TonoLab, for mice | Colonial Medical Supply | ||
| Heating pad | Sunbeam Products Inc | 722-810 | |
| Argon laser | Coherent Inc | Ultima 2000SE | |
| DECAPICONE Plastic cone holder | Braintree Sci Inc. | MDC-200 | for mouse |
Odniesienia
- Gupta, N., Yucel, Y. H. Glaucoma as a neurodegenerative disease. Curr. Opin. Ophthalmol. 18, 110-114 (2007).
- Quigley, H. A. Neuronal death in glaucoma. Prog. Retin. Eye Res. 18, 39-57 (1999).
- McKinnon, S. J., Schlamp, C. L., Nickells, R. W. Mouse models of retinal ganglion cell death and glaucoma. Experimental Eye Research. 88, 816-824 (2009).
- Pang, I. H., Clark, A. F. Rodent models for glaucoma retinopathy and optic neuropathy. J. Glaucoma. 16, 483-505 (2007).
- Levkovitch-Verbin, H., et al. Translimbal laser photocoagulation to the trabecular meshwork as a model of glaucoma in rats. Investigative Ophthalmology & Visual Science. 43, 402-410 (2002).
- Aihara, M., Lindsey, J. D., Weinreb, R. N. Experimental mouse ocular hypertension: establishment of the model. Investigative Ophthalmology & Visual Science. 44, 4314-4320 (2003).
- Grozdanic, S. D. Laser-induced mouse model of chronic ocular hypertension. Investigative ophthalmology & visual science. 44, 4337-4346 (2003).
- Sappington, R. M., Carlson, B. J., Crish, S. D., Calkins, D. J. The microbead occlusion model: a paradigm for induced ocular hypertension in rats and mice. Investigative ophthalmology & visual science. 51, 207-216 (2010).
- Ding, C., Wang, P., Tian, N. Effect of general anesthetics on IOP in elevated IOP mouse model. Experimental Eye Research. 92, 512-520 (2011).
- Kalesnykas, G., et al. Retinal ganglion cell morphology after optic nerve crush and experimental glaucoma. Investigative Ophthalmology & Visual Science. 53, 3847-3857 (2012).
- Shareef, S. R., Garcia-Valenzuela, E., Salierno, A., Walsh, J., Sharma, S. C. Chronic ocular hypertension following episcleral venous occlusion in rats. Experimental Eye Research. 61, 379-382 (1995).
- Chiu, K., Chang, R., So, K. F. Laser-induced chronic ocular hypertension model on SD rats. J. Vis. Exp. (10), e549 (2007).
- Fu, C. T., Sretavan, D. Laser-induced ocular hypertension in albino CD-1 mice. Investigative Ophthalmology & Visual Science. 51, 980-990 (2010).
- Rangarajan, K. V. Detection of visual deficits in aging DBA/2J mice by two behavioral assays. Curr. Eye Res. 36, 481-491 (2011).
- Wang, L., et al. Direction-specific disruption of subcortical visual behavior and receptive fields in mice lacking the beta2 subunit of nicotinic acetylcholine receptor. J. Neurosci. 29, 12909-12918 (2009).
- Douglas, R. M., et al. Independent visual threshold measurements in the two eyes of freely moving rats and mice using a virtual-reality optokinetic system. Visual Neuroscience. 22, 677-684 (2005).
- Prusky, G. T., Alam, N. M., Beekman, S., Douglas, R. M. Rapid quantification of adult and developing mouse spatial vision using a virtual optomotor system. Investigative Ophthalmology & Visual Science. 45, 4611-4616 (2004).
- Morrison, J. C., et al. A rat model of chronic pressure-induced optic nerve damage. Experimental Eye Research. 64, 85-96 (1997).
- Cone, F. E., et al. The effects of anesthesia, mouse strain and age on intraocular pressure and an improved murine model of experimental glaucoma. Experimental Eye Research. 99, 27-35 (2012).
- Liu, X., et al. Brain-derived neurotrophic factor and TrkB modulate visual experience-dependent refinement of neuronal pathways in retina. J. Neurosci. 27, 7256-7267 (2007).
- Liu, X., et al. Regulation of neonatal development of retinal ganglion cell dendrites by neurotrophin-3 overexpression. The Journal of Comparative Neurology. 514, 449-458 (2009).
- Sun, W., Li, N., He, S. Large-scale morphological survey of mouse retinal ganglion cells. The Journal of Comparative Neurology. 451, 115-126 (2002).
- Feng, L., et al. Sustained Ocular Hypertension Induces Dendritic Degeneration of Mouse Retinal Ganglion Cells that Depends on Cell-type and Location. Investigative Ophthalmology & Visual Science. , (2013).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone