Method Article
Engineering Skeletal Muscle Tissues from Murine Myoblast Progenitor Cells and Application of Electrical Stimulation
W tym Artykule
Podsumowanie
Engineered muscle tissue has great potential in regenerative medicine, as disease model and also as an alternative source for meat. Here we describe the engineering of a muscle construct, in this case from mouse myoblast progenitor cells, and the stimulation by electrical pulses.
Streszczenie
Engineered muscle tissues can be used for several different purposes, which include the production of tissues for use as a disease model in vitro, e.g. to study pressure ulcers, for regenerative medicine and as a meat alternative 1. The first reported 3D muscle constructs have been made many years ago and pioneers in the field are Vandenburgh and colleagues 2,3. Advances made in muscle tissue engineering are not only the result from the vast gain in knowledge of biochemical factors, stem cells and progenitor cells, but are in particular based on insights gained by researchers that physical factors play essential roles in the control of cell behavior and tissue development. State-of-the-art engineered muscle constructs currently consist of cell-populated hydrogel constructs. In our lab these generally consist of murine myoblast progenitor cells, isolated from murine hind limb muscles or a murine myoblast cell line C2C12, mixed with a mixture of collagen/Matrigel and plated between two anchoring points, mimicking the muscle ligaments. Other cells may be considered as well, e.g. alternative cell lines such as L6 rat myoblasts 4, neonatal muscle derived progenitor cells 5, cells derived from adult muscle tissues from other species such as human 6 or even induced pluripotent stem cells (iPS cells) 7. Cell contractility causes alignment of the cells along the long axis of the construct 8,9 and differentiation of the muscle progenitor cells after approximately one week of culture. Moreover, the application of electrical stimulation can enhance the process of differentiation to some extent 8. Because of its limited size (8 x 2 x 0.5 mm) the complete tissue can be analyzed using confocal microscopy to monitor e.g. viability, differentiation and cell alignment. Depending on the specific application the requirements for the engineered muscle tissue will vary; e.g. use for regenerative medicine requires the up scaling of tissue size and vascularization, while to serve as a meat alternative translation to other species is necessary.
Protokół
1. Culture of Murine Myoblast Progenitor Cells or C2C12 Cells
- Isolate cells according to the protocol initially published by Shefer and colleagues 10 and later adapted by Collins et al. 11 and Boonen et al. 12 and store these in liquid nitrogen. This requires mice, e.g. C57Bl/6. Alternative methods are used in other labs, e.g. a method published in Journal of Visualized Experiments by Li Y et al. 13. For the reagents and equipment you are referred to the table on page 7 and 8. From the muscle from one mouse you will generally obtain enough cells to cryopreserve about 20 vials in liquid nitrogen. Next, we start the protocol to thaw the cells from the liquid nitrogen storage.
- Place the cells from 1 vial into a 25 cm2 Matrigel (1 mg/ml) coated tissue culture flask and add growth medium (GM) consisting of (335 ml DMEM advanced, 100 ml fetal bovine serum (FBS), 50 ml HS, 5 ml penicillin/streptomycin, 5 ml L-glutamin, 5 ml chicken embryo extract (CEE)).
Note: coat for 2 hr at Room Temperature and remove the Matrigel by aspiration.
- Subculture the cells approximately 1:3 every 3 days.
This means: on day 3: Passage cells from one 25 cm2 to a 75 cm2 flask, on day 6: passage cells from one 75 cm2 flask to two 150 cm2 flasks (with 30 min preplating in uncoated flasks). On day 9 transfer one 150 cm2 flask to 1 triple 150 cm2 flask, or if unavailable to three 150 cm2 flasks (if needed preplate again depending on the phenotype of the cells). On day 12 the cells are ready for seeding into a muscle construct.
Notes: - Per triple 150 cm2 the number of cells will be approximately 4.5 x 106 cells.
- Use vials from different isolations to mix them to obtain a mixed population of cells at time of seeding.
Note: If you choose not to work with primary cells, C2C12 myoblasts are a good alternative.
2. Engineering Skeletal Muscle Tissues
- Prepare silicon glue by mixing the"elastomer" with the "curing agent" (10:1). Cut +/- 5 mm square segments of Velcro with one triangular side (house-like shape; Figure 1). Glue the Velcro into the wells of a 6-well culture plate at a space of approximately 12 mm between the squares.
Notes: - Only use the soft side of the Velcro and face this side upwards.
- Make sure that the rooftops face each other.
- Only cover the Velcro with silicon glue, do not spread glue throughout the well.
- For later electrical stimulation it is relevant to align the constructs in vertical direction of the well plate (along the long axis).
- Leave to dry overnight in vacuum oven, not heated, primarily to remove air bubbles. Sterilize by adding 70% EtOH to the wells and incubate for 15 min. Rinse 3x with PBS and put under UV for 15 min. Remove all PBS from the wells and the Velcro and place in incubator until use.
- Thaw Matrigel solution in the fridge and prepare the collagen solution to the desired concentration shortly before making the 3D constructs. Dilute the stock collagen with sterile 0.02% acetic acid (final concentration 3.2 mg/ml).
Note: leave everything on ice.
- Trypsinize the cells, resuspend in GM and count. Leave cells in centrifuge tube in the incubator.
- Add the desired amount of Collagen stock solution for the numbers of constructs that you want to make to a tube (according to Table 1), add 0.5M NaOH to this collagen solution until a light pink color is indicating a pH of 7.5. Mix gently by pipetting up and down and avoid bubble formation. Then add the Matrigel and mix very gently but thoroughly. Finally, add the GM to the Collagen/Matrigel mixture (for the appropriate amounts see Table 1).
Notes: - Perform each step on ice! Matrigel and collagen will readily gel at increasing temperature.
- Mind that the color indeed is pink! An increase in pH will also induce rapid gelation.
- Final concentration of collagen: 1.6 mg/ml.
- Centrifuge the desired amount of cells at 1,000 rpm for 5 min and remove the supernatant.
Note: - Depending on the activity of the cells the number of cells per construct needs to be adjusted. Typically, the number of cells lies between 750,000 and 1,250,000 cells per construct.
- Use some of the gel mixture to resuspend the cell pellet and transfer the cells into the remaining mixture and mix thoroughly, but without introducing air bubbles.
- Take the preheated well plates out of the incubator and pipette 0.35 -0.4 ml of the cell-gel mixture into the Velcro first. Then, begin to pipette the mixture from the center between the attachment sites and extend to the Velcro. Finally, use the remainder of gel to fill up the gap between the two Velcro anchors and pipette around the Velcro pieces.
- Carefully check after 5-10 min if the gels are solid enough to be transferred, i.e. gently tap the dishes and inspect visually if the gel is rigid. If so, carefully place the dishes in an incubator. Usually, they can be picked up after ten minutes. Then, after 1-2 hr, gently overlay each gel with 4 ml of warm GM.
Note: -Do not make any vigorous movements when handling the plates.
- Replace GM by differentiation medium (growth medium without chick embryo extract) after 24 hr, and replace with fresh medium every 2-3 days. On day 7 you should have obtained mature oriented muscle fibers, as can be evaluated with staining for cross striation development in the fused muscle fibers.
3. Electrical Stimulation
- Sterilize the electrodes from the ionoptix plate (see table of reagents and equipment) before use with 70% ethanol and subsequently dry under UV in a safety cabinet. Place the plate with electrodes onto the culture plate with the constructs and cover with the lid from the culture plate and transfer to an incubator. Connect the Ionoptix C-pacer with the appropriate cables.
Note: Electrodes are placed parallel alongside the muscle construct (Figure 2).
- Apply the stimulation protocol as anticipated.
Note: We generally use 4 V/CM, 6ms pulses at a frequency of 2Hz. Change culture medium every 24 hr during stimulation.
Wyniki
The end product will be muscle constructs as indicated in Figure 3. The size of the tissue will be approximately 8 mm long, 2 mm wide and 0.5 mm thick. Electrical stimulation during differentiation will change the expression of myosin heavy chain isoforms, but does not greatly enhance the differentiation process as induced by the differentiation medium 8, but electrical stimulation can also be applied at the end of the process to check for functionality of the muscle, because a muscle with fully developed sarcomeres will be able to contract upon an electrical pulse.
Differentiation and maturation can also be analyzed using quantitative PCR for gene expression evaluation and staining for muscle maturation markers or cross striation development (e.g. staining for alpha actinin) on either sections made from the tissues or whole mount stained tissue samples. Muscle maturation and differentiation related genes and myosin heavy chain expression include e.g. MyoD, myogenin, MFR4 and MLP, and MYH 1, 2, 4 and 8 8. Proof that the muscle tissue that is formed indeed is muscle tissue, based on gene expression, immunohistochemical stainings and contraction induction by electrical stimulation, has been published previously 8 and a figure with stained cross sections of muscle tissues made from myoblast progenitor cells and C2C12 cells from this paper is displayed in Figure 4.
| Solution | Volume (if making 10 muscles) in μl |
| Collagen (3.2 mg/ml, diluted with 0.02% acetic acid) | 2570 (51.3%) |
| NaOH (0.5M) | 10 (0.2%) |
| Matrigel | 430 (8.6%) |
| Growth medium | 2,000 (39.9%) |
| total | 5,010 (should suffice with enough for spilling and pipetting loss) |
Table 1. mixture of cells and gel for the generation of a tissue engineered muscle construct.

Figure 1. Cutting the pieces of Velcro.
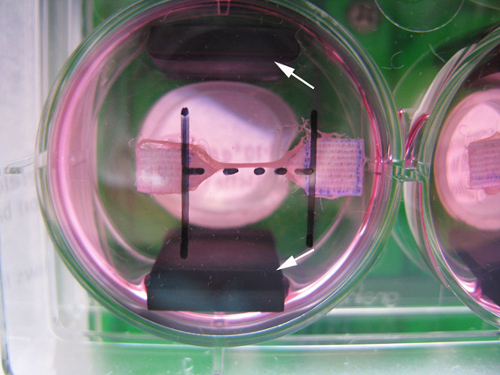
Figure 2. A picture taken from the bottom of a muscle construct in a well of a 6-well plate showing the placement of the electrodes. The electrodes (indicated with the white arrows) are placed parallel to the muscle constructs.

Figure 3. A tissue engineered muscle in between two pieces of Velcro in a 6-well culture plate.
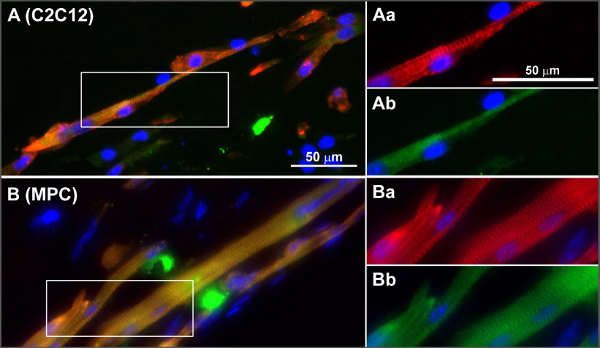
Figure 4. Typical examples of cross-striations in C2C12 (A) and MPC (B) in engineered skeletal muscle tissues. Frozen sections of non-stimulated mBAMs (day 8 of differentiation) were stained for sarcomeric α-actinin (red), sarcomeric myosin (green) and nuclei (blue). (Aa-Ab and Ba-Bb) Magnifications of boxed areas in (A, B) α-Actinin (red) and sarcomeric myosin (green). Reprinted with permission from 8. Click here to view larger figure.
Dyskusje
The engineering of muscle tissues has great potential for the use as a disease model, for drug screening, in regenerative medicine and for meat production. However, the requirements for these applications vary. We chose to work with a combination of collagen and matrigel, because collagen allows for cell alignment and because the myoblast progenitor cells require the presence of basement membrane derived proteins as determined in previous 2D studies 12. Moreover, fibrin gels have been tested in our laboratory and seem not to support the C2C12 and myoblast progenitor cells as does the mixture of collagen and Matrigel™ and especially do not lead to tissue compaction 14. The model as described here has been used already in studies on pressure ulcer damage while using a cell line 15. For regenerative medicine applications, the translation to human satellite cells has recently been made 6,16. In addition, as an alternative source for meat consumption the technique has great potential 1. In our opinion however, the current technology needs to advance to a next stage to further its use in the clinic as well as for consumption. For example, the differentiation stops at around the neonatal stage and also force production is much less than a muscle produces in vivo. Additionally, although mouse, rat and human stem cells can be implemented in the 3D constructs, the isolation and especially the differentiation and maturation of farm animal derived muscle stem cells is not developed that far yet1. Also, the use of Matrigel and animal derived serum needs to be omitted 1. To further the application in regenerative medicine, the tissue size has to be increased, and requires (neo) vascularization and use of perfusion bioreactor systems to overcome oxygen and nutrient diffusion limitations. Some initial vascularization studies on small constructs have been done in the past years 9,17,18.
Ujawnienia
The authors have nothing to disclose.
Podziękowania
The authors want to thank Yabin Wu for culturing the tissues presented in Figure 2, the picture was taken by Bart van Overbeeke. The work was financially supported by SenterNovem, grant ISO 42022.
Materiały
| Name | Company | Catalog Number | Comments |
| Matrigel-growth factor reduced | Beckton and Dickinson | ||
| DMEM (high glucose)* | Gibco | 42430 | |
| Advanced DMEM | Gibco | 12491 | |
| Horse serum | Gibco | 65050-122 | |
| Fetal bovine serum | Greiner | 758075 | |
| 0.45 and 0.22 μm syringe filter* | Whatmann (Schleicher and Scheull) | 10462100 | |
| L-glutamine | Gibco | 25030024 | |
| Penicillin/streptomycin | Gibco | 10378016 | |
| Amphotericin | Gibco | 15290-018 | |
| Culture plastic | Greiner | Includes culture flasks and pipets | |
| Chick embryo extract | United States Biological | C3999 | |
| Pasteur pipet* | Hilgenberg | Pasteur pipettes, with constriction, with cotton, open tip L: 230 mm with tip diameter of 0,9 - 1,1 mm | |
| Pasteur pipet* | Hilgenberg | Pasteur pipettes, with constriction, with cotton, open tip L: 230 mm with tip diameter of 1,4 - 1,6 mm | |
| Pasteur pipet | VWR | 612-1702 | |
| Collagenase type I* | Sigma | C0130-16 | |
| 40 μm cell strainer* | BD Falcon | 352340 | |
| 19G needle | |||
| Elastomer | Dow Corning corporation | 3097358-1004 | Silastic MDX 4-4210# |
| Curing agent | Dow Corning corporation | Silastic MDX 4-4210# | |
| Velcro | Regular store | You can buy this at a regular store, only use the soft side | |
| Collagen type I, rat tail | BD Biosciences | 3544236 | |
| C-Pace EP Culture Pacer | Ionoptix | ||
| 6-well culture dishes for electrical stimulation | Beckton Dickinson-Falcon | BD Falcon #353846 | |
| C-Dish culture dish electrodes | Ionoptix | ||
| * Needed for the isolation of cells (point 1.1) # Together in one kit | |||
Odniesienia
- Langelaan, M. L. P., Boonen, K. J. M., Polak, R. B., et al. Meet the new meat: tissue engineered skeletal muscle. Trends Food Sci. Tech. 21 (2), 59-66 (2010).
- Shansky, J., Chromiak, J., Tatto, M., Vandenburgh, H. A simplified method for tissue engineering skeletal muscle organoids in vitro. In Vitro Cell Dev. Biol. Animal. 33 (9), 659-661 (1997).
- Vandenburgh, H., Del Tatto, M., Shansky, J., et al. Tissue-engineered skeletal muscle organoids for reversible gene therapy. Hum. Gene Ther. 7 (17), 2195-2200 (1996).
- Yaffe, D. Retention of differentiation potentialities during prolonged cultivation of myogenic cells. Proc. Natl. Acad. Sci. U.S.A. 61 (2), 477-483 (1968).
- Rando, T. A., Blau, H. M. Primary mouse myoblast purification, characterization, and transplantation for cell-mediated gene therapy. J. Cell Biol. 125 (6), 1275-1287 (1994).
- Koning, M., Werker, P. M. N., vander Schaft, D. W. J., Bank, R. A., Harmsen, M. C. MicroRNA-1 and MicroRNA-206 Improve Differentiation Potential of Human Satellite Cells: A Novel Approach for Tissue Engineering of Skeletal Muscle. Tissue Eng. Part A. , (2011).
- Darabi, R., Pan, W., Bosnakovski, D., et al. Functional myogenic engraftment from mouse iPS cells. Stem Cell Rev. 7 (4), 948-957 (2011).
- Langelaan, M. L. P., Boonen, K. J. M., Rosaria-Chak, K. Y., et al. Advanced maturation by electrical stimulation: Differences in response between C2C12 and primary muscle progenitor cells. J. Tissue Eng. Regen. Med. 5 (7), 529-539 (2011).
- van der Schaft, D., van Spreeuwel, A. C., van Assen, H. C., Baaijens, F. Mechanoregulation of vascularization in aligned tissue engineered muscle; a role for VEGF. Tissue Eng. Part A. , (2011).
- Shefer, G., Wleklinski-Lee, M., Yablonka-Reuveni, Z. Skeletal muscle satellite cells can spontaneously enter an alternative mesenchymal pathway. J. Cell. Sci. 117 (Pt. 22), 5393-5404 (2004).
- Collins, C. A., Olsen, I., Zammit, P. S., et al. Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell. 122 (2), 289-301 (2005).
- Boonen, K. J. M., Rosaria-Chak, K. Y., Baaijens, F. P. T., van der Schaft, D. W. J., Post, M. J. Essential environmental cues from the satellite cell niche: optimizing proliferation and differentiation. Am. J. Physiol. Cell Physiol. 296 (6), C1338-C1345 (2009).
- Li, Y., Pan, H., Huard, J. Isolating Stem Cells from Soft Musculoskeletal Tissues. J. Vis. Exp. (41), e2011 (2010).
- Boonen, K. J. M., Langelaan, M. L. P., Polak, R. B., et al. Effects of a combined mechanical stimulation protocol: Value for skeletal muscle tissue engineering. J. Biomech. 43 (8), 1514-1521 (2010).
- Gawlitta, D., Boonen, K. J. M., Oomens, C. W. J., Baaijens, F. P. T., Bouten, C. V. C. The influence of serum-free culture conditions on skeletal muscle differentiation in a tissue-engineered model. Tissue Eng. Part A. 14 (1), 161-171 (2008).
- Koning, M., van Luijn, M., van der Schaft, D. W. J., et al. Human skeletal muscle formation and engraftment In vivo is independent of preconditioning In vitro with HUVEC. , (2013).
- Levenberg, S., Rouwkema, J., Macdonald, M., et al. Engineering vascularized skeletal muscle tissue. Nat. Biotechnol. 23 (7), 879-884 (2005).
- Koffler, J., Kaufman-Francis, K., Yulia, S., et al. Improved vascular organization enhances functional integration of engineered skeletal muscle grafts. Proc. Natl. Acad. Sci. U.S.A. 108 (36), 14789-14794 (2011).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaPrzeglądaj więcej artyków
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone