Method Article
Establishment and Validation of a Rat Model of Pulmonary Arterial Hypertension Associated with Pulmonary Fibrosis
* These authors contributed equally
In This Article
Summary
Here, the method of inducing pulmonary arterial hypertension associated with pulmonary fibrosis (PF-PH) rat model by injecting bleomycin into the airway is introduced. We also provide a step-by-step approach to validate this animal model.
Abstract
Patients with pulmonary fibrosis are at a higher risk of developing pulmonary hypertension, a complication with poor prognosis. At present, the mechanism of this link is still poorly understood. A major obstacle to progress in this area is the lack of a reliable animal model to replicate PF-PH. This study aimed to establish a stable PF-PH rat model. Rats were fasted overnight prior to intervention. Under sodium pentobarbital anesthesia (45 mg/kg), the trachea was intubated with a PE50 tube inserted to a depth of 3 cm (the distance from the glottis to the tube). Bleomycin (BLM) was administered intratracheally as a single dose (5 mg/kg, dissolved in 0.2 mL of 0.9% NaCl). Following the injection, the rats were immediately rotated to ensure even distribution of the BLM. At 35 days after the BLM injection, the rats exhibited progressive impairment of lung function and increased right ventricular systolic pressure and right ventricular hypertrophy, revealing the pathological characteristics of pulmonary hypertension. We provide a general and reliable method to establish a rat model of PF-PH.
Introduction
Pulmonary hypertension (PH) due to interstitial lung disease (ILD) is common clinically, with an estimated prevalence of 10% to 80% in patients with idiopathic pulmonary fibrosis (IPF), and it is also frequently seen in other fibrotic ILDs1,2. Numerous studies have shown that the development of PH is linked to substantial morbidity and reduced survival3,4,5. Compared to Group 1 pulmonary arterial hypertension (PAH), the pathogenesis of pulmonary arterial hypertension associated with pulmonary fibrosis (PF-PH) remains poorly understood6. The purpose of establishing an animal model of PF-PH in rats is to provide a reliable framework for scientific research on pulmonary fibrosis associated with pulmonary hypertension and to explore potential avenues for clinical therapeutic applications.
Bleomycin is a classic inducer of pulmonary fibrosis widely used in animal models7. Further research by Blackburn et al.8 and our laboratory9 has revealed that bleomycin can also trigger typical pathological features of pulmonary hypertension, such as increased right ventricular systolic pressure (RVSP) and right ventricular hypertrophy. Mechanistically, bleomycin induces pulmonary parenchymal fibrosis, hypoxic vasoconstriction, and a reduction in pulmonary vascular bed density, thereby leading to the development of pulmonary hypertension6. Additionally, we observed a significant loss of pulmonary vascular endothelial cells starting from day 7 of bleomycin treatment, with this loss progressively worsening over the course of the experiment9. This phenomenon suggests that bleomycin-induced pulmonary vascular endothelial dysfunction may play a potential role in the initiation and progression of pulmonary hypertension.
Due to pulmonary interstitial fibrosis, IPF patients are in a state of hypoxia for a long time, and compensatory changes occur in cardiopulmonary vessels, which leads to pulmonary hypertension6. The use of animal models can help us further understand the underlying mechanisms of human idiopathic pulmonary fibrosis associated with pulmonary hypertension. Although this model cannot fully simulate the pathological features of human disease, this model can still provide valuable insights. There are many experimental models simulating pulmonary fibrosis, such as single-dose airway infusion of bleomycin, viral vector delivery of transforming growth factors, and exposure to silica8,10. At present, the BLM model is the most widely used and characterized model because it can be easily induced in a short time and has high reproducibility. In addition, temporal changes in pulmonary fibrosis have been evaluated in a bleomycin mouse model, where increased expression of fibrosis markers and genes associated with disease pathology, such as Col1A1 and Col1A2, were observed from days 15-218. Cardiovascular changes, such as right ventricular hypertrophy and a significant increase in RVSP, were detected on or after day 3311. At the same time, our laboratory has previously evaluated the changes in PH and PF parameters of rat models induced by bleomycin9. We found that in addition to pulmonary fibrosis (PF) characteristics such as progressive lung function impairment and collagen deposition in rats, typical features of pulmonary hypertension (PH) gradually emerged within 7 to 35 days after a single airway instillation of bleomycin. RVSP and Fulton index showed an increase in time dependence. Currently, various animals with pulmonary fibrosis have been reported in the literature. Some experts have suggested that rat models exhibited a more pronounced fibrotic response than the mouse models12. Therefore, in order to better study the progression of pulmonary fibrosis combined with pulmonary hypertension, a bleomycin-induced rat model is the key.
Protocol
The animal experiments described in this study were approved by the Animal Care and Use Committee of The First Affiliated Hospital of Guangzhou Medical University (ethical approval number: 2018-456).
1. Procurement of experimental rats
- Divide rats into two groups: the normal control group and the model group, with 7 rats in each group. Use 10-week-old male Sprague-Dawley (SD) rats weighing 200 ± 20 g for the study
2. Induction of PF-PH rat model
- In order to prevent regurgitating aspirations during the procedure, fast the rats overnight prior to modeling.
- Anesthetize rats by intraperitoneal injection of pentobarbital sodium (45 mg/kg) to ensure procedural accuracy and safety and minimize animal pain and stress. Confirm surgical anesthesia levels by a lack of response to toe pinching. At the same time, apply an ointment to the rats' eyes to prevent dryness.
- Intubate the trachea of the rat with a PE50 tube inserted to a depth of 3 cm (the distance from the glottis to the tube).
- Using a 1 mL syringe (26G), draw 0.2 mL of Bleomycin (5 mg/kg, dissolved in 0.2 mL of 0.9% NaCl). Inject the syringe containing BLM into the airway through a cannula as a single dose. For rats in the control group, perform intratracheal administration of sterile normal saline (0.2 mL). During this process, rats do not need pain relief.
- To ensure even distribution of bleomycin, place the rat in a supine position, gently hold, and slowly rotate. The recommended rotation angle is 30°-45°, alternating between sides. Each rotation should be maintained for 10-15 s, repeated 3x-4x.
- Place the rats on heating pads to help maintain body temperature and closely monitor their status. Observe when the rats begin to move on their own and transfer the animals from the heating pad to a separate cage for recovery.
- After 5 weeks of BLM injection, evaluate the rats to determine whether the PF-PH model could be successfully established using methods such as echocardiographic monitoring, hemodynamic measurements, and histological analysis.
3. Echocardiographic monitoring
- Anesthetize rats using 4% isoflurane. Remove the rat's fur with a hair removal cream and apply ultrasonic gel to its chest.
- Evaluate the functional and structural parameters of the right heart in rats with a 250 MHz ultrasonic probe. To obtain the best long-axis view of the ventricle, place the probe on the left side of the median line of the animal's chest.
- According to the individual anatomy, rotate the probe 15° counterclockwise relative to the left parasternal line, with the incisor pointing towards its right shoulder, and adjust the x and y axes in B-Mode so that the entire heart is in the center of the visual field.
- Select Color to display the blood flow color to distinguish the pulmonary artery (the pulmonary artery blood flow signal in this position is blue).
- Select PW (PW Doppler) mode, place the sampling line under the pulmonary valve, and rotate the PW angle knob to adjust the direction of the sampling line so that the sampling direction is parallel to the direction of pulmonary artery blood flow (about 25°). Press PW key again and obtain the pulmonary artery blood flow acceleration time/ejection time (PAT/PET). Measure at least five cardiac cycles and average. Use Cine to save the image.
- Adjust the animal to a low head and a high foot, place the ultrasonic probe at the apex of the animal's heart, and drive the ultrasound along the apex of the heart to obtain the four-chamber incisal surface. Adjust the x and y axes in B mode to ensure that the four heart chambers are visible.
- Display the measurement line 2x by holding down the M mode and place it at the junction of the tricuspid valve ring and the right ventricular free wall. Measure the tricuspid valve displacement distance (TAPSE) within at least three cardiac cycles and preserve it in Cine.
- Place the probe at the midline of the right clavicle of the animal's chest and adjust the angle between the probe and the animal's chest notch to about 45°. Adjust the image in B-Mode so the right ventricular ventricle and interventricular septum are visible.
- Select M-Mode to display measurement line 2x and place the measurement line in the middle of the interventricular septum. The sampling line was perpendicular to the right ventricular free wall. Save the image to obtain the thickness of the right ventricular free wall.
4. Estimation of lung function
- Open the PFT software and check that the device is recognized. Calibrate according to equipment instructions to ensure the accuracy of flow sensors and pressure sensors. Set test parameters in the PFT software, such as respiratory rate, tidal volume, test duration, etc.
- The animal detection mode is the mode for rat studies. Turn the switch of the main control instrument on and ensure that the switch of each signal amplifier is in the off position.
- Set the airflow according to the animal's body weight. Turn on the suction airflow regulating nut and set the inspiration at 5. Turn on the expiratory airflow regulating nut and set the slow expiration to -4. Set the pressure value to 60 cm H2O and then calibrate the instrument according to the order of FRC flow, flow, high flow, and lung pressure with an error of less than 0.5%.
- Anesthetize rats by intraperitoneal injection with pentobarbital sodium (45 mg/kg) as in step 2.2. Cut the skin along the center of the neck and remove the muscle layer by layer.
- At the exposed upper portion of the cervical trachea, make a horizontal incision between the tracheal cartilage rings. Quickly insert the metal cannula into the tracheal incision, ensuring the cannula is correctly positioned and secure. Use 4-0 silk thread sutures to fix the cannula to the trachea, preventing it from slipping or dislodging. Place the rat in the PFT animal compartment and connect the tracheal cannula to the airflow channel interface outside the instrument.
- Click the Start button and examine the forced vital capacity (FVC) and dynamic lung compliance. To ensure data consistency, record each indicator at least 3x.
- After the test is complete, the data is saved and exported to the analysis software. Use statistical software to analyze the data and compare the lung function parameters of different groups.
5. Hemodynamic and histological measurements
- Detection of Right ventricular systolic pressure (RVSP)
- Anesthetize rats by intraperitoneal injection with pentobarbital sodium (45 mg/kg) as in step 2.2. Confirm that the rat has been properly anesthetized by pinching the toe to ensure that the rat does not respond. Fix the abdomen of the rat upward on the experimental bench and apply eye cream to the rat's eyes to prevent dryness.
- Make an incision (about 4 cm) on the right side of the neck through surgical scissors and separate the external jugular vein through microsurgical forceps. Separate the veins with a length of about 1 cm gently with micro-forceps and insert two surgical lines at the distal and proximal ends.
- Before intubation, soak the PE50 tube in normal saline containing 1% heparin sodium or EDTA for at least 30 minutes. Adjust the physiological recorder to bring the pressure back to the 0 line and adjust the pressure range to 0-150 mmHg.
- Ligate the distal end of the external jugular vein with 4-0 silk thread, gently lift the proximal surgical line, and place it on the distal vein wall. Use small scissors to make a small incision (about 0.3 mm). After inserting PE50 tube into the external jugular vein, use 4-0 silk thread to ligate the catheter together with the external jugular vein to prevent blood leakage.
- Observe the venous pressure waveform on the recorder. Push the catheter slowly into the right atrium, and the right atrium waveform is visible, with an amplitude of about 0-5 mmHg. After the catheter continues to advance from the right atrium to the right ventricle, the right ventricle pressure waveform can be seen. When the pressure is stable, record RVP for 5 min and the data is saved and analyzed with analysis software.
- Detection of Fulton index
- After detection of RVSP, following isoflurane anesthesia (5%, 2 L/min O2), euthanize the rats through exsanguination.
- After thoracotomy, remove the intact heart of the rat with tweezers. Cut off the auricle and the connective tissue near the heart with scissors.
- Cut the free wall of the right ventricle along the pulmonary artery. This tissue is the right ventricle(RV). The remaining tissues are left ventricle plus interventricular septum(LV+S). After separation, drain the surface of the right ventricle (RV) and left ventricle plus interventricular septum (LV+S) with filter paper and weigh separately using an analytical balance. After recording the weight of the right ventricle (RV) and the left ventricle plus ventricular septum (LV+S), calculate the weight ratio of the right ventricle to the left ventricle plus ventricular septum (RV/(LV+S)).
- Histological staining
- After opening the thoracic cavity, use a 10 mL syringe to draw 3 mL of saline. Insert the syringe into the pulmonary artery and slowly inject the saline to flush the lungs, removing blood and other residues.
- Use scissors to dissect the right accessory lobe and the right anterior lobe of the lung. Place these two lung lobes into 4% formalin for fixation to preserve tissue morphology and structure. Quickly place the remaining three lobes of the lung (such as the left lung, right middle lobe, and right posterior lobe) into liquid nitrogen for frozen storage, ready for subsequent experiments.
- Remove excess fat and connective tissue from the surface of the lung tissue to ensure the tissue blocks are clean and neat. Trim the lung lobes into small blocks measuring 5 mm x 5 mm x 3 mm. Place the trimmed tissue blocks into the dehydrator and gradually dehydrate them using a gradient series of alcohol (60%, 70%, 80%, 90%, 100%).
- Immerse the lung tissue in xylene for 2 h to achieve transparency. After removal, soak it in melted paraffin at 56-58 °C for 2 h to ensure complete infiltration. Then, place the tissue into a mold, positioning it with the cutting surface facing downward for subsequent sectioning.
- Slice the wax block on the microtome with a thickness of 4-8 µm13, and store it at room temperature for pathological staining.
- Perform Eosin and Masson's trichrome staining14.
6. Detection of hydroxyproline (HYP)
- Weigh 30-100 mg lung tissue in a glass tube. Add 2 mL of hydrochloric acid solution (6 mol/L) and digest at 110 °C in an oven for 2-6 h until no large clumps are visible.
- After cooling, adjust the pH value to the range of 6-8 using 1 mL of NaOH solution (10 mol/L), then condense the distilled water to 4 mL solution. Finally, centrifuge at 28,620 x g at 25 °C for 20 min and take the supernatant to be.
- Preheat the microplate reader for 30 min, adjust the wavelength to 560 nm, and zero the instrument using distilled water.
- Dilute the standard solution with distilled water to prepare standard solutions with concentrations of 30, 15, 7.5, 3.75, 1.875, 0.938, 0.469, and 0.234 µg/mL.
- Take 60 µL of the sample supernatant and mix it with 60 µL of Reagent A, then let it stand at room temperature for 20 min.
- Add 60 µL of Reagent B and 120 µL of distilled water. Place the mixed solution in 60 °C water bath for 20 min. Follow the same steps for each concentration of the standard solutions (e.g., 30, 15, 7.5, 3.75, 1.875, 0.938, 0.469, 0.234 µg/mL) and perform the operations accordingly.
- After standing at room temperature for 15 min, take 200 µL of the solution in the 96-well plate to detect the light absorption value at 560 nm. Plot the standard curve with the concentration of the standard solutions as the x-axis and the measured values as the y-axis, obtaining the equation y=kx+b. Substitute the measured value of the sample into the equation to determine the concentration of the sample.
Results
Bleomycin-induced pulmonary fibrosis in rats
Bleomycin has been reported as a classic inducer of pulmonary fibrosis in animal models7. Here, indexes of pulmonary fibrosis were assessed following BLM stimulation. First, after 35 days of BLM treatment, we conducted lung function tests and found that both FVC (Figure 1E) and dynamic lung compliance (Figure 1F) in the model group were significantly reduced. These results clearly indicate that lung function was markedly impaired. Second, to evaluate collagen deposition in lung tissue, the study employed Masson's trichrome staining and HYP assay. Masson's trichrome staining revealed a significant increase in blue-stained areas around pulmonary arteries in the model group, suggesting pronounced collagen accumulation (Figure 1D). Meanwhile, the HYP assay results demonstrated that HYP levels in the model group were significantly higher than those in the control group (Figure 2E), further confirming increased collagen deposition. Additionally, we assessed the weights of the liver (Figure 3A) and kidneys (Figure 3B) using a weighing method and found no significant differences between the two groups.
BLM-stimulated progression of pulmonary hypertension
Next, characteristic hemodynamic changes of pulmonary hypertension were measured. After 35 days of BLM treatment, right ventricular systolic pressure (RVSP) was assessed using right heart catheterization. The results showed that RVSP (Figure 1A,C) in the model group was significantly higher than that in the control group. Meanwhile, by calculating the Fulton index of rat hearts, it was found that the ratio of right ventricle to left ventricle plus septum weight (RV/(LV+S); Figure 1B) significantly increased after 35 days of BLM treatment. The elevated RVSP (Figure 1A,C) and increased RV/(LV+S) (Figure 1B) collectively indicated the successful establishment of the pulmonary fibrosis combined with the pulmonary hypertension (PF-PH) model. Additionally, histological analysis revealed typical histopathological changes related to pulmonary vascular remodeling in the model group (Figure 1D). To further evaluate cardiac function, we performed echocardiography, which showed that the model group exhibited significantly reduced ratios of PAT/PET (Figure 2A), TAPSE (Figure 2B), RVFAC (Figure 2C), and CO (Figure 2D). In summary, these results demonstrate that BLM is an effective inducer for successfully constructing the PF-PH rat model.

Figure 1: BLM-treated rat exhibited increased hemodynamic indexes, vascular remodeling and dysfunction of lung function. (A) RVSP, (B) RV/LV+S, and (C) representative traces show hemodynamic indexes for the control and model groups. (D) Eosin and Masson's trichrome staining, exhibiting the histological changes in the pulmonary arteries within the lung sections of each group. (E) Bar graphs display the FVC (F) and dynamic lung compliance. Results are expressed as mean ± SEM, and significance was assessed by Student t-test. n = 8 in each group (A, B); n = 6 in each group (E, F). *p < 0.05, indicating a significant difference. Please click here to view a larger version of this figure.
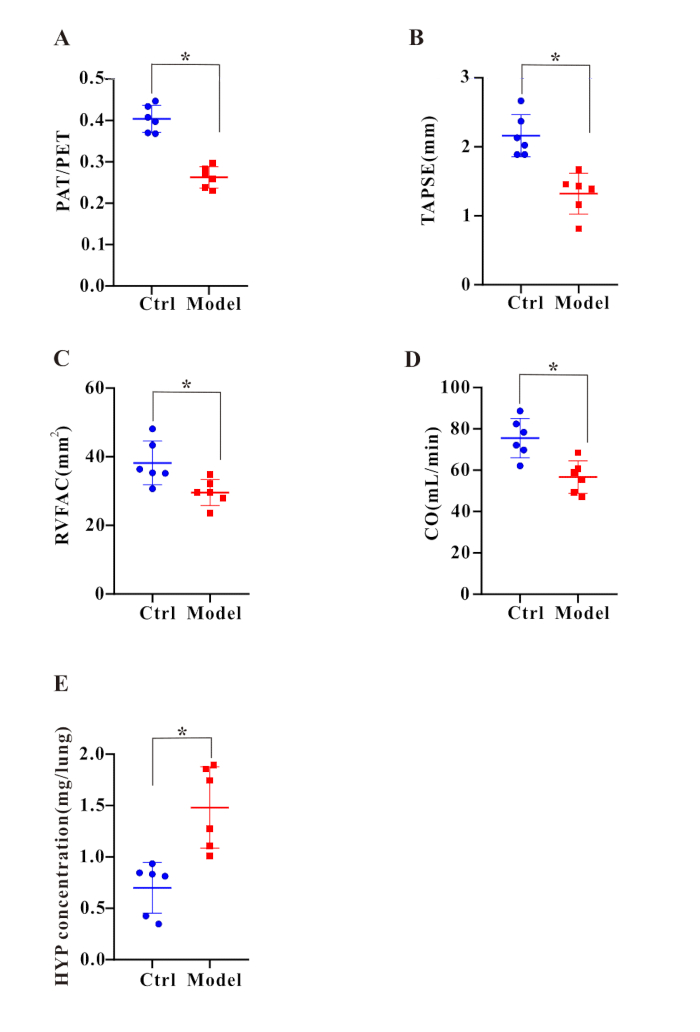
Figure 2: Right heart dysfunction and collagen deposition were detected after BLM treatment. Bar graphs display the ratio of (A) PAT/PET, (B) TAPSE, (C) RVFAC, (D) CO, and (E) HYP concentration. Results are expressed as mean ± SEM, and significance was assessed by Student t-test. n = 6 in each group. *p < 0.05, indicating a significant difference. Please click here to view a larger version of this figure.
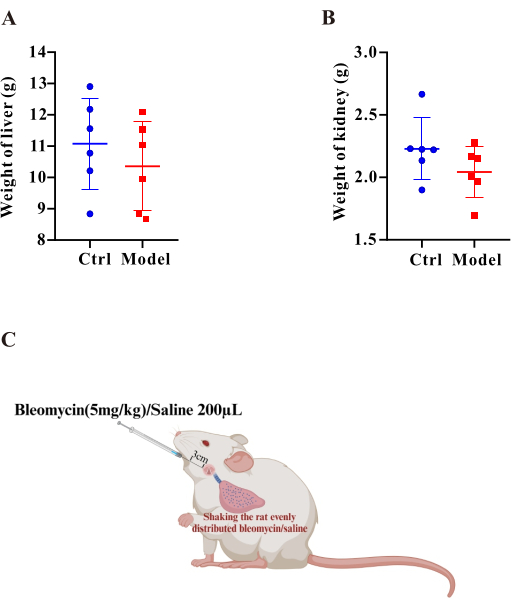
Figure 3: The liver and kidney weights were comparable between the model and control group. Bar graphs display the weights of the (A) liver and (B) kidney. Results are expressed as mean ± SEM, and significance was assessed by Student t-test. *p < 0.05, indicating a significant difference. (C) Schematic of the protocol for the establishment of the PF-PH rat model. Please click here to view a larger version of this figure.
Discussion
Idiopathic pulmonary fibrosis is a progressive, fatal disease with a median survival of 2-3 years from diagnosis, suggesting a bleak prognosis9. Pulmonary hypertension is a common comorbidity of IPF, which rapidly deteriorates IPF with a worsened prognosis15. What's more, there were limited therapeutic options for IPF-PH7. Thus, it is essential to gain a deeper understanding of the underlying molecular mechanisms of PF-PH, which may provide a potential therapeutic approach for the disease.
The proposed protocol outlines a well-established and stable method for generating a PF-PH rat model. The model is essential for studying the pathophysiological mechanisms of diseases and plays a crucial role in verifying novel therapeutic approaches. PF animal models can be established through various methods, including drug administration, irradiation, silica exposure, viral vectors, transgenics, and the transfer of human fibroblasts16. Each modeling method has its strengths and weaknesses. For instance, irradiation is expensive and time-consuming (taking more than 30 weeks); silica treatment is more likely to cause pneumoconiosis rather than PF; the procedures for viral vectors and transgenic animal models are relatively complicated and require advanced skills; and the transfer of human fibroblasts results in low reproducibility and high costs16. In contrast, drug-induced PF models, such as those using bleomycin, amiodarone, methotrexate, and nitrogen mustard, are easy to implement, effective, and reproducible7.
The model has three key steps, including dose and mode of administration, sex selection, and time to make the model. Although a single dose of BLM (0.1-6 mg/kg), delivered by intratracheal or oropharyngeal routes, is a commonly used approach for PF modeling17. We have made some improvements. In the present study, the optimal dose and mode of BLM administration were explored, and a dose of 5 mg/kg delivered intratracheally was selected. Because female Sprague-Dawley rats exhibited less severe pulmonary hypertension (PH) than male rats18 and male rats are more sensitive to pulmonary arteriole remodeling; we used male rats in this study. According to previous studies by us, the RVSP and Fulton index in the Bleomycin group increased gradually within 7-35 days after bleomycin treatment, significantly higher than that in the control group. Therefore, the modeling time of the model is recommended to be about 35 days.
Our previous studies successfully established pulmonary hypertension rat models using various methods, including hypoxia19, Sugen-hypoxia20, monocrotaline19, pulmonary artery ligation21, and nephrectomy15. Characteristic changes observed in these pulmonary hypertension animal models include increased RVSP, elevated RV/(LV+S) ratios, pulmonary vascular remodeling, and right heart dysfunction, in line with the findings of the present study
Despite the easy access to BLM, this animal model has several limitations. First, airway infusions of BLM cause uneven distribution of drug concentrations, leading to local fibrosis of the lungs. Second, BLM is a chemotherapy agent that poses health risks to the researchers. Third, research indicates that the fibrotic process induced by BLM may be self-limiting16.
In conclusion, we successfully established a PF-associated PH rat model using BLM. This animal model exhibited typical features of PF, including impaired lung function and elevated collagen deposition. Additionally, we observed representative hemodynamic changes, such as increased RVSP and RV/(LV+S) ratios, along with vascular remodeling and right heart dysfunction in this BLM-induced rat model. Importantly, establishing a PF-PH rat model is valuable for understanding the mechanisms underlying PF-PH and providing novel therapeutic directions.
Disclosures
The authors have no relevant financial disclosures.
Acknowledgements
This work was supported by the grants from in part by the grants from the National Natural Science Foundation of China (82370063, 82170069, 82120108001, 82241012), R&D Program of Guangzhou National Laboratory (GZNl2023A02013), National Key R&D Program of China(2022YFE0131500), Guangdong Department of Science and Technology (2024A1515011208, 2022A1515012052, 2024A1515013104, 202102020019, 202201020538, 202201010069, 2023A03J0334), the Independent Project of State Key Laboratory of Respiratory Disease (SKlRD-Z-202513), Guangdong Medical Research Foundation(A2023379) Guangzhou Medical University and Plan on enhancing scientific research in GMU and Open Research Funds from The Sixth Affiliated Hospital of Guangzhou Medical University (Qingyuan People's Hospital) (202201-101).
Materials
| Name | Company | Catalog Number | Comments |
| Bleomycin | MedChemExpress | HY-17565A | |
| Coupling agent | HYNAUT | BX-CSRH | |
| Formalin fixative | Biosharp) | BL401B | |
| Hair removal cream | LUSEN | LS-B-TMG-50 | |
| Hematoxylin eosin (HE) staining kit | Beyotime | C0189S | |
| Isoflurane | RWD Life Science(China) | R510-22-10 | |
| Masson Tri-color dyeing kit | Beyotime | C0189S | |
| Normal saline | KERONG | SLYS-001 | |
| syringe | Beyotime | FS701-50pcs |
References
- Sm, N., et al. Clinical significance of pulmonary hypertension in interstitial lung disease: A consensus statement from the Pulmonary Vascular Research Institute's innovative drug development initiative-Group 3 pulmonary hypertension. Pulm Circ. 12 (3), e12127 (2022).
- Nathan, S. D., et al. Pulmonary hypertension in chronic lung disease and hypoxia. Eur Resp J. 53 (1), 1801914 (2019).
- King, C. S., Shlobin, O. A. The Trouble With Group 3 Pulmonary Hypertension in Interstitial Lung Disease. Chest. 158 (4), 1651-1664 (2020).
- Meyer, K. C. Pulmonary fibrosis, part I: epidemiology, pathogenesis, and diagnosis. Expert Rev Respir Med. 11 (5), 343-359 (2017).
- Raghu, G., et al. Idiopathic Pulmonary Fibrosis (an Update) and Progressive Pulmonary Fibrosis in Adults: An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am J Respir Critic Care Med. 205 (9), e18-e47 (2022).
- Ruffenach, G., Hong, J., Vaillancourt, M., Medzikovic, L., Eghbali, M. Pulmonary hypertension secondary to pulmonary fibrosis: clinical data, histopathology and molecular insights. Respir Res. 21 (1), 303 (2020).
- Li, S., Shi, J., Tang, H. Animal models of drug-induced pulmonary fibrosis: an overview of molecular mechanisms and characteristics. Cell Biol Toxicol. 38 (5), 699-723 (2022).
- Karmouty-Quintana, H., et al. Deletion of ADORA2B from myeloid cells dampens lung fibrosis and pulmonary hypertension. FASEB J. 29 (1), 50-60 (2015).
- Jiang, Q., et al. Dysregulation of BMP9/BMPR2/SMAD signalling pathway contributes to pulmonary fibrosis and pulmonary hypertension induced by bleomycin in rats. Br J Pharmacol. 178 (1), 203-216 (2021).
- Degryse, A. L., Lawson, W. E. Progress toward improving animal models for idiopathic pulmonary fibrosis. Am J Med Sci. 341 (6), 444-449 (2011).
- Collum, S. D., et al. Inhibition of hyaluronan synthesis attenuates pulmonary hypertension associated with lung fibrosis. Br J Pharmacol. 174 (19), 3284-3301 (2017).
- Ye, X., et al. Animal models of acute exacerbation of pulmonary fibrosis. Respir Res. 24 (1), 296 (2023).
- León-Mancilla, B., et al. Three-Dimensional Collagen Matrix Scaffold Implantation as a Liver Regeneration Strategy. J Vis Exp. (172), e62697 (2021).
- Oldham, S., Rivera, C., Boland, M. L., Trevaskis, J. L. Incorporation of a Survivable Liver Biopsy Procedure in Mice to Assess Non-alcoholic Steatohepatitis (NASH) Resolution. J Vis Exp. (146), e59130 (2019).
- Jiang, Q., et al. Nephrectomy and high-salt diet inducing pulmonary hypertension and kidney damage by increasing Ang II concentration in rats. Respir Res. 25 (1), 288 (2024).
- Moore, B. B., Hogaboam, C. M. Murine models of pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 294 (2), L152-L160 (2008).
- Jenkins, R. G., et al. An Official American Thoracic Society Workshop Report: Use of Animal Models for the Preclinical Assessment of Potential Therapies for Pulmonary Fibrosis. Am J Respir Cell Mol Biol. 56 (5), 667-679 (2017).
- Lahm, T., et al. The effects of estrogen on pulmonary artery vasoreactivity and hypoxic pulmonary vasoconstriction: Potential new clinical implications for an old hormone. Crit Care Med. 36 (7), 2174-2183 (2008).
- Chen, Y., et al. Tetramethylpyrazine: A promising drug for the treatment of pulmonary hypertension. Br J Pharmacol. 177 (12), 2743-2764 (2020).
- Zheng, Q., et al. Established pulmonary hypertension in rats was reversed by a combination of a HIF-2α antagonist and a p53 agonist. Br J Pharmacol. 179 (5), 1065-1081 (2022).
- Chen, J., et al. Upregulation of mechanosensitive channel Piezo1 involved in high shear stress-induced pulmonary hypertension. Thrombo Res. 218, 52-63 (2022).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved