Method Article
Laparoscopic Left Approach Resection of the Caudate Lobe
In This Article
Summary
Here, we present a surgical protocol describing the laparoscopic resection of a tumor situated near the paracaval portion within the caudate lobe, utilizing a left-sided approach.
Abstract
Laparoscopic caudate lobectomy (LCL) is one of the most challenging types of laparoscopic liver resections. The main difficulty lies in the deep anatomical position of the caudate lobe, which is closely adjacent to the first and second hepatic ports and the inferior vena cava, increasing the risk of major bleeding during surgery. In addition to a thorough understanding of the anatomy of the caudate lobe tumor, comprehensive imaging assessment, and three-dimensional reconstruction, the flexible choice of surgical approach is also key to reducing surgical difficulty and improving safety. We perform laparoscopic caudate lobectomy using a left-sided approach, especially when the tumor is located in the area of the caudate lobe close to the inferior vena cava. This method avoids the step of splitting the liver substance to expose the field of view required by the traditional anterior approach, with the advantages of larger operating space and shorter operation time. At the same time, combined with preoperative three-dimensional reconstruction technology, we have significantly reduced the risk of damaging important blood vessels and increased the success rate of resection of tumors in the caudate lobe.
Introduction
The caudate lobe is situated deep within the liver, with its specific coverage extending from the front of the inferior vena cava, reaching behind the left, middle, and right hepatic veins, upward to where the three main hepatic veins converge into the inferior vena cava, and downward to the hepatic hilum1.
Since the pioneering report by Dulucq et al. in 2006 on laparoscopic resections of the hepatic caudate lobe, numerous cases involving isolated resections of the caudate lobe and segmental resections have been documented2,3,4,5,6,7. However, the technical complexity of laparoscopic caudate lobectomy is subject to a multitude of factors. These include stringent adherence to surgical indications, comprehensive preoperative assessment of imaging data, mastery of laparoscopic surgical techniques, intimate knowledge of the local anatomy of the caudate lobe, and the judicious selection of surgical approaches. At present, there are four prevalent approaches for the resection of the caudate lobe: the left-sided approach, the right-sided approach, the combined left-right approach, and the anterior approach. The left-sided approach is typically employed for the resection of the Spiegel lobe or when a combined resection of the left lateral segment or left hemi liver is required. Conversely, the right-sided approach is favored for the resection of the caudate process or, in cases necessitating a right hemi liver resection. In instances where the tumor is disproportionately large or extensively infiltrates the caudate lobe, thereby complicating exposure via the left or right approach, a combined left-right approach may become indispensable. For the resection of tumors near the inferior vena cava, the anterior approach is generally preferred, as it facilitates optimal exposure and visualization.
The paracaval segment, a crucial part of the caudate lobe, is widely recognized as Couinaud's segment IX. It is strategically positioned behind segment IV, essentially the epicenter of the liver's anatomy. Its dorsal surface is in intimate contact with the inferior vena cava, while its lower boundary is closely aligned with the first hepatic port. The cephalic end of this segment is contiguous with the origins of the middle and right hepatic veins, and its ventral aspect is in direct apposition to the main trunk of the middle hepatic vein. Given its concealed location and its adjacency to the liver's principal vascular structures, surgical interventions in this area are inherently risky and technically challenging. Historically, surgical procedures targeting the paracaval segment of the caudate lobe have predominantly utilized laparoscopic anterior or right-sided approaches8,9,10. There is a dearth of literature on left-sided approaches, likely due to the profound depth of this region and the restricted field of view from the left, compounded by the intricate vascular architecture in the vicinity. Such complexities demand that the operating surgeon possess a profound understanding of anatomy and a wealth of surgical expertise. Advancements in three-dimensional (3D) visualization reconstruction technology have enabled the creation of precise and vivid three-dimensional liver models11. These models offer a clear representation of the liver, the tumor, the hepatic vascular system, and the spatial relationships between the liver and neighboring organs. This technology is instrumental in providing a comprehensive preoperative understanding of the liver's specific conditions, the precise location of the tumor, and the intricate interplay of the blood vessels.
In this paper, we introduce an innovative surgical strategy: leveraging preoperative three-dimensional assessments and employing a laparoscopic left-sided approach for the excision of tumors in the paracaval region. This approach aims to enhance surgical precision and safety, capitalizing on the detailed anatomical insights provided by modern imaging techniques.
A 30-year-old female patient was admitted to Zhujiang hospital with an incidental finding of a space-occupying lesion in the liver, detected over a month ago. Enhanced computed tomography (CT) scans revealed a mass with a low-density shadow, measuring approximately 37 mm x 34 mm. Preliminary diagnoses included focal nodular hyperplasia or hepatic adenoma, which require further differentiation (Figure 1). Preoperative three-dimensional visualization reconstruction is depicted in Figure 2. The patient's complete blood count, coagulation profile, and liver function tests were all within normal limits. She had no significant medical history, and preoperative assessments confirmed no contraindications to surgery. Given the potential risk of malignancy associated with hepatic adenomas, the surgeons engaged in detailed discussions with the patient and her family, ensuring they were fully informed. After a thorough understanding of the surgical risks and the possible therapeutic outcomes, the patient and her family made an informed decision to proceed with the surgical treatment. They have provided consent by signing the surgical informed consent form, demonstrating their clear understanding of the procedure and commitment to the patient's health and well-being.
Protocol
The surgical procedure received clearance from the Ethics Committee at Zhujiang Hospital, Southern Medical University. Furthermore, the patient and her family provided informed consent for publicly sharing information and data pertinent to the treatment process. This ensures transparency and respects the patient's autonomy in the medical decision-making process.
1. Preoperative preparation
- Have the patient fast for 8 h and refrain from drinking for 4 h before surgery.
- Administer general anesthesia and perform endotracheal intubation12.
NOTE: Evaluate the effectiveness of anesthesia by assessing the patient's condition both post-anesthesia and intraoperatively. Key indicators include achieving a complete block of sensation, requiring no additional medication during the procedure, and maintaining stable vital signs throughout the surgery. - Disinfect the surgical area, from the nipples to the pubic symphysis and the upper third of the thighs, with iodine tincture three times, extending to the mid-axillary line.
2. Surgical technique
- Place the patient supine with legs apart, head elevated, and a 15° tilt to the right.
- Establish pneumoperitoneum with a Veress needle13. Set the pneumoperitoneum pressure to 12-14 mmHg (1 mmHg = 0.133 kPa).
- Make a 1 cm incision with a No.10 surgical scalpel blade at the umbilical edge for an observation port, with a 10 mm trocar inserted (see Table of Materials).
- Insert the trocars, measuring 5 mm and 10 mm in diameter, at the following anatomical landmarks: 2 cm below the rib edge along the right anterior axillary line, 8 cm below the rib edge along the right midclavicular line, and 2 cm and 6 cm below the rib edge along the left midclavicular line, respectively (Figure 3).
- Perform the initial laparoscopic assessment to determine the feasibility of a left-sided approach to caudate lobe resection. Observe the degree of intraperitoneal adhesion of the omentum, the surface texture of the liver, and the position of the left and right triangular ligaments and falcate ligaments.
- Use the ultrasonic scalpel (see Table of Materials) to transect the falciform ligament, revealing the gallbladder triangle. Subsequently, ligate the cystic duct and dissect the gallbladder.
- Use the ultrasonic scalpel to clear the small omental foramen, expose the hepatic hilum, and allow for the establishment of a hepatic hilar occlusion tape.
- Flip the right liver lobe to expose the inferior vena cava, and ligate and transect the hepatic short veins with 3-0 Hem-o-lok (see Table of Materials)(Figure 4).
- Expose the Spiegel area of the caudate lobe, followed by hepatic hilar occlusion, and then transect the liver parenchyma along the caudate lobe notch using the ultrasonic scalpel (Figure 5).
- Use the ultrasonic scalpel and operating forceps to resect liver parenchyma to expose the tumor adjacent to the inferior vena cava (Figure 6 and Figure 7).
- Carefully dissect the tumor, use an ultrasonic scalpel with freeze gear to coagulate small veins, and expose the middle hepatic vein (Figure 8).
- Expose and ligate the portal vein to the right caudate lobe with 3-0 Hem-o-lok, followed by tumor mobilization (Figure 9, Figure 10, and Figure 11).
- Use bipolar electrocoagulation for hemostasis and specimen retrieval. Use 800 mL of 0.9% NaCl solution to irrigate the abdominal cavity (Figure 12) and close with the drainage tube placed inside the abdominal cavity (see Table of Materials).
- Close the wound layer by layer using non-absorbable suture material (see Table of Materials).
3. Postoperative nursing and monitoring
- Within the first 24 h post-surgery, perform cardiac monitoring (see Table of Materials) and provide continuous low-flow oxygen therapy at 3 L/min, closely monitoring vital signs.
- On the first postoperative day, give the patient a semi-solid diet and instruct the patient to perform turning and in-bed exercises.
NOTE: Postoperative care includes hepatoprotective, anti-infective, anti-hemorrhagic, analgesic, albumin, and gastroprotective treatments. - Monitor the drainage tubes for color, volume, and changes in bilirubin levels. Remove the drainage tube once bilirubin levels return to normal and drainage is less than 50 mL.
Results
The surgery (Video 1) lasted for 372 min with a blood loss of 300 mL, and no blood transfusion was required. There were five instances of hepatic portal occlusion totaling 70 min. The patient experienced no postoperative complications and had a smooth recovery, staying in the hospital for 8 days post-surgery. Pathological examination indicated focal nodular hyperplasia (FNH) of the liver. The level of bilirubin in the drainage fluid decreased from 30.1 µmol/L on the third postoperative day to 21.4 µmol/L on the fifth postoperative day (see Table 1), with the drainage tube removed on the sixth postoperative day. Postoperative imaging and pathological images are shown in Figure 13.
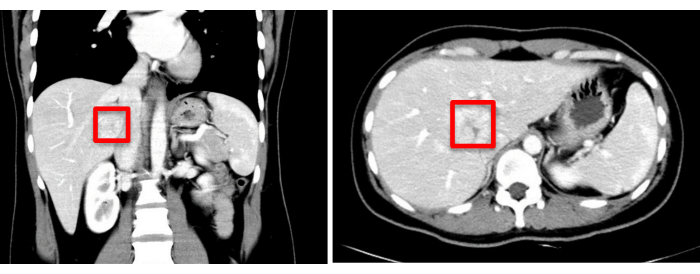
Figure 1: Preoperative imaging assessment (CT) indicates the tumor location, marked with a red square. Please click here to view a larger version of this figure.
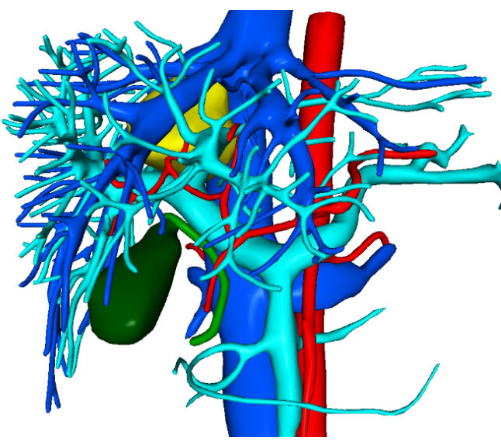
Figure 2: Preoperative three-dimensional visualization reconstruction images. Please click here to view a larger version of this figure.
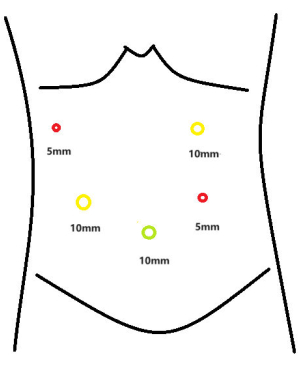
Figure 3: Trocar distribution map. Please click here to view a larger version of this figure.
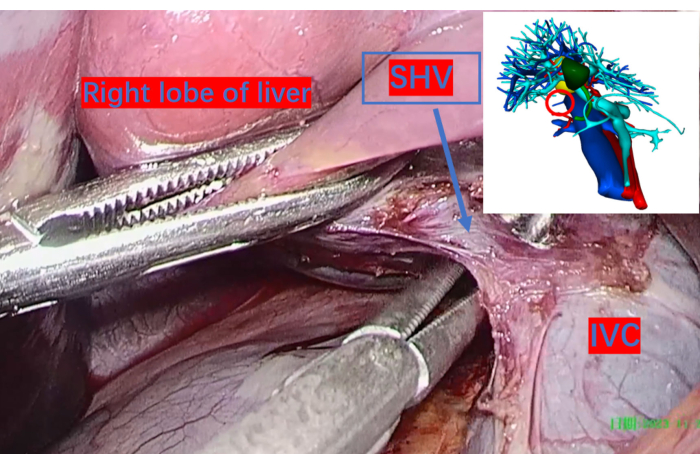
Figure 4: The inferior vena cava exposed from the right hepatic space and the larger short hepatic veins ligated with Hem-o-lok clips and transected. The red circle on the three-dimensional reconstruction image indicates their corresponding positions. Abbreviation: SHV: short hepatic vein. IVC: inferior vena cava. Please click here to view a larger version of this figure.

Figure 5: The liver parenchyma transected along the caudate process using an ultrasonic scalpel. Please click here to view a larger version of this figure.

Figure 6: The portal vein exposed up to the first branch of the left caudate lobe (G1L). G1L is the portal vein's first branch to the left caudate lobe. Please click here to view a larger version of this figure.

Figure 7: Carefully exposed tumor, providing a clear view of its location and facilitating subsequent surgical maneuvers. Please click here to view a larger version of this figure.
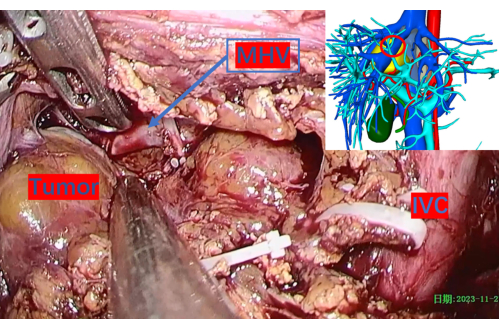
Figure 8: Cautiously exposed middle hepatic vein. The red circle indicates its corresponding position on the 3D reconstruction image. Abbreviations: MHV: middle hepatic vein, IVC: inferior vena cava. Please click here to view a larger version of this figure.
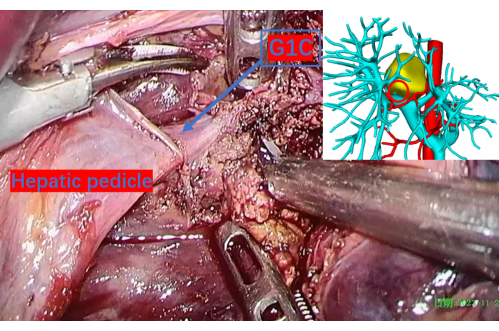
Figure 9: Carefully transected liver parenchyma revealing the portal vein up to the first branch of the right caudate lobe, denoted as G1C. A red circle on the 3D reconstruction image corresponds to this location, providing a precise reference point. G1C is the portal vein's first branch to the right caudate lobe. Please click here to view a larger version of this figure.

Figure 10: The Glisson system responsible for the tumor's blood supply. This system was exposed, clamped, and severed. Please click here to view a larger version of this figure.

Figure 11: Exposed main trunk of the right hepatic vein during tumor dissection. The red circle marks its location on the 3D reconstruction image. Abbreviations: RHV: right hepatic vein. Please click here to view a larger version of this figure.

Figure 12: Thoroughly irrigated and cleaned surgical field after hemostasis. Abbreviations: MHV: middle hepatic vein. RHV: right hepatic vein. IVC: inferior vena cava. Please click here to view a larger version of this figure.
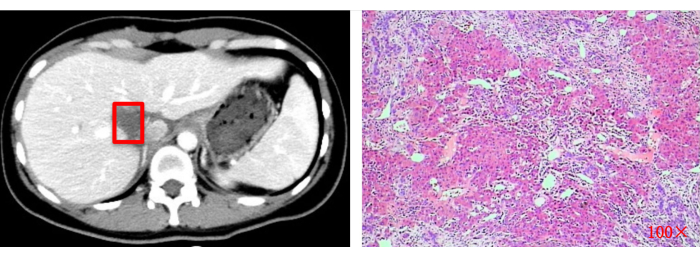
Figure 13: Postoperative imaging assessment (CT) and histopathological examination (100x). Red squares indicate the original tumor resection site. Please click here to view a larger version of this figure.
| Items | Results |
| Operation time (min) | 372 |
| Intraoperative bleeding volume (mL) | 300 |
| Blood transfusion volume (mL) | 0 |
| Number of hepatic portal occlusion | 5 |
| Hepatic portal occlusion time | 70 |
| Postoperative complication | None |
| Postoperative hospital stay(day) | 8 |
| Drain bilirubin levels on POD3 (U/L) | 30.1 |
| Drain bilirubin levels on POD5 (U/L) | 21.4 |
Table 1: Relevant outcomes of the patient. Abbreviations: POD = postoperative day.
Video 1: Laparoscopic left approach resection of the caudate lobe (surgical technique). Abbreviations and definitions: SHV: short hepatic vein. G1L: the first branch of the portal vein to the left caudate lobe. MHV: middle hepatic vein. G1C: the first branch of the portal vein to the right caudate lobe. RHV: right hepatic vein. IVC: inferior vena cava. Please click here to download this Video.
Discussion
The liver is a crucial organ involved in metabolism, immune function, and detoxification14. Hepatocytes, the liver's primary cells, are typically stable but can become unstable when triggered, initiating the regeneration of liver tissue. Following liver resection, insufficient residual liver volume may lead to severe postoperative complications such as acute liver failure. Thus, rigorous evaluation of liver function post-hepatectomy is of paramount importance15,16. Research indicates that the caudate lobe constitutes about 2%-3% of the liver's volume in a typical human body17. Given the preoperative assessment of liver function reserves, the resection of the caudate lobe alone generally does not significantly diminish the remaining liver volume, thus mitigating the risk of postoperative complications such as acute liver failure.
Kumon et al.'s study18 elucidates the vascular anatomy of the liver's caudate lobe, identifying two primary hepatic pedicles: the left and right. The left pedicle is more consistently positioned, entering the caudate lobe around the mid to lower third of the Spiegel lobe. Conversely, the right pedicle is more variable, potentially extending into the paracaval or caudate process areas, and may even be absent. The portal venous branches in the caudate lobe exhibit complexity, often manifesting as either independent branches or a shared trunk. The Spiegel lobe's portal vein is more stable, predominantly arising from a robust dorsal branch (G1L) of the left portal vein. In contrast, the portal veins serving the right caudate process and paracaval region primarily originate from branches of the right main portal vein, termed the first branch to the right caudate lobe (G1C)19. Cho et al's20 descriptions detail the caudate lobe arteries into three main types: independent branches, common trunks, and those arising from hepatic arterial anastomoses. Biliary drainage in the caudate lobe typically involves two main ducts, with 2 to 3 branches in the Spiegel lobe converging into the left hepatic duct. The paracaval region generally has 2 to 3 ducts merging into the right posterior sectoral duct, with a significant occurrence of interductal anastomoses18. Venous drainage from the caudate lobe is primarily through the short hepatic veins into the inferior vena cava. The caudate process vein traverses between the caudate process and the right posterior lobe, draining mainly the caudate process and joining the lower right edge of the posterior inferior vena cava, delineating the boundary between these regions. The main caudate lobe hepatic vein runs between the Spiegel lobe and the paracaval area, ultimately entering the middle left edge of the posterior inferior vena cava, serving as an anatomical demarcation between the Spiegel lobe and the paracaval region. We have meticulously clamped and transected key vessels and their branches, including the short hepatic veins, G1L, G1C, and the hepatic pedicle feeding the tumor. This precision ensures the safety and effectiveness of the surgical interventions.
The caudate lobe's distinctive anatomy, nestled deep within the liver and adjacent to key vasculature, including the inferior vena cava, middle hepatic vein, and right hepatic vein, presents a surgical challenge. Its encasement by the hepatic hila elevates the risk of hemorrhage, making resection technically demanding. Prior research21 indicated that an extended left hepatectomy coupled with caudate lobe resection is preferable for malignancies in the caudate lobe adjacent to the hepatic veins. However, advancements in our understanding of these tumors have led to an increased adoption of straightforward caudate lobe resections and partial resections. These approaches facilitate radical tumor removal while conserving liver function22. For both primary and secondary hepatic malignancies in the caudate lobe, surgery is indicated when technically feasible. Benign tumors of the caudate lobe, such as cavernous hemangiomas, focal nodular hyperplasia (FNH), intrahepatic bile duct stones, and hepatic adenomas, are also amenable to total or partial resection, offering effective treatment options.
Laparoscopic liver resection has emerged as a prevalent technique in hepatobiliary surgery23,24,25. The procedure's tunnel vision and magnification capabilities provide distinct advantages for the excision of the caudate lobe. Within this visual framework, the dissection around the inferior vena cava is facilitated, even in tight spaces, and the visualization of finer hepatic short veins is enhanced. However, the technique is not without its challenges, such as the absence of haptic feedback, limited retraction capabilities, and the constraints of the dissection planes. Overcoming these requires a surgeon with considerable expertise, particularly in partial resections of the caudate lobe. Common approaches for caudate lobe resection include the left, right, combined left-right, and anterior approaches. The left approach is typically used for the resection of the Spiegel lobe or in conjunction with the resection of the left lateral segment or left hemiliver. The right approach is indicated for the resection of the caudate process or for cases where a right hemiliver resection is considered. In instances where a tumor is extensive or involves the entire caudate lobe, necessitating difficult exposure, a combined left-right approach may be employed. The anterior approach is often utilized for the resection of the paracaval region, which involves dividing normal liver tissue. This method can lead to a larger surface area of liver trauma, heightening the risk of bile duct and vascular injuries, as well as increasing the likelihood of postoperative bleeding and bile leakage.
Traditionally, paracaval tumors have been addressed using laparoscopic anterior or right-sided approaches, with scant literature on left-sided procedures8,9,10. Feng et al., in their published case report26, successfully performed open left-sided approach resections on two cases of benign hemangiomas in the caudate lobe. This study focused on the en bloc resection of the caudate lobe rather than segmental resections of the three parts of the caudate lobe. For benign lesions, this method did not more effectively reduce unnecessary hepatic parenchymal resection, thereby decreasing the risk of bile leakage. Currently, considering the complex vascular structure of the caudate lobe, laparoscopic surgery can more clearly display the subtle vascular structures, thereby reducing the risk of bleeding. Furthermore, Zheng et al. conducted a study in which they introduced the "Huaxi dissection method" for the first time27. They innovatively used a laparoscopic left-sided approach to successfully resect complex tumors in the caudate lobe. During the surgery, they employed a counterclockwise dissection and layering method, progressing from simple to complex in a fixed sequence. This approach simplifies the surgical process using the learning curve, which is more conducive to scholars' learning and mastering, further confirming the feasibility of the left-sided approach for caudate lobe resection.
This dearth of left-sided cases may be attributed to the deep-seated nature of paracaval tumors, which poses difficulties in achieving full exposure from a left-sided view due to the intricate surrounding vascular anatomy. However, utilizing preoperative imaging, we conducted a differential diagnosis of focal nodular hyperplasia and hepatic adenomas in paracaval tumors, mitigating concerns over tumor margin adequacy. Unlike the "Huaxi dissection method," our center employs a laparoscopic left-sided approach resection technique specifically for tumors adjacent to the caval vein in the caudate lobe. Preoperatively, we use 3D reconstruction for assessment to ensure surgical safety. Moreover, the surgical method described here avoids exposure of the left hepatic vein, reducing the risk of tearing and bleeding from the left hepatic vein. This method also minimizes unnecessary damage to the hepatic parenchyma, thereby reducing the likelihood of postoperative bile leakage. With these refined surgical strategies, we aim to achieve safer and more effective resection of caudate lobe tumors. This technique avoids the need to divide liver tissue, as in the anterior approach. It offers advantages such as a more spacious operative field and shorter operative duration compared to the right-sided approach. Additionally, the use of preoperative three-dimensional reconstruction technology significantly minimized the risk of major vascular injury and enhanced the feasibility of tumor resection. The operation was efficiently completed in 372 min with a blood loss of only 300 mL. The patient had an uncomplicated postoperative course, was discharged smoothly on postoperative day eight, and made a swift recovery.
We must clearly recognize that the necessity of resecting benign tumors hinges on their potential for malignant degeneration and the presence of compressive symptoms due to large tumor size, which are critical in determining surgical indications. For malignant tumors, the appropriateness of a left-sided surgical approach and the assurance of adequate exposure and sufficient resection margins are issues that necessitate further investigation. Nonetheless, based on our research and clinical experience, we have reached the conclusion that the laparoscopic left-sided approach for the resection of benign paracaval tumors is entirely viable, especially with a solid grasp of anatomical knowledge and extensive surgical expertise. This surgical technique not only provides patients with a minimally invasive treatment option but also ensures the safety of tumor excision while minimizing surgical trauma and shortening postoperative recovery periods.
Disclosures
The authors have no conflicts or financial ties to disclose.
Acknowledgements
This study was supported by the Guangdong Basic and Applied Basic Research Foundation of China (2021B1515230011), Science and Technology Projects in Guangzhou of China (2023B03J1247) and the Key-Area Research and Development Program of Guangdong Province(2023B1111020008).
Materials
| Name | Company | Catalog Number | Comments |
| Absorbable hemostat | Ethicon, LLC | W1913T | |
| Disposable spiral negative pressure drainage pipeline | Jiangsu Aiyuan Medical Technology Corp | 424280 | Drainage of abdominal residual fluid |
| Disposable trocar | Kangji Medica | 10004, 10006 | |
| Electrocardiographic monitor | Philips Goldway (SHENZHEN) Industrial, Inc | UT4000B | Postoperative ECG monitoring |
| Laparoscopic system | Olympus | WM-NP2 | |
| Non-absorbable polymer ligation clips (Hem-o-lok) | Teleflex Medical | 544230 | |
| Ultrasound scalpel | Johnson | GEN11 | Tools for liver resection |
| Vicryl rapide | Ethicon, LLC | 3-0, VCP345H 90010 | Suture incision and Trocar hole |
| Video system | Lenovo | GK309 |
References
- Kumon, M., Kumon, T., Sakamoto, Y. Demonstration of the right-side boundary of the caudate lobe in a liver cast. Glob Health Med. 4 (1), 52-56 (2022).
- Chen, K. H., Jeng, K. S., Huang, S. H., Chu, S. H. Laparoscopic caudate hepatectomy for cancer--an innovative approach to the no-man's land. J Gastrointest Surg. 17 (3), 522-526 (2013).
- Chen, L., et al. Laparoscopic extended right hepatectomy for posterior and completely caudate massive liver tumor (with videos). Hepatobiliary Pancreat Dis Int. 22 (3), 326-330 (2023).
- Dulucq, J. L., Wintringer, P., Stabilini, C., Mahajna, A. Isolated laparoscopic resection of the hepatic caudate lobe: Surgical technique and a report of 2 cases. Surg Laparosc Endosc Percutan Tech. 16 (1), 32-35 (2006).
- Huang, J., Xu, D., Li, X. Laparoscopic resection of the spiegel lobe using a modified caudate lobe-first approach. Asian J Surg. 46 (11), 5351-5352 (2023).
- Parikh, M., Han, H. -. S., Cho, J. Y., D'silva, M. Laparoscopic isolated caudate lobe resection. Scientific Reports. 11 (1), 4328 (2021).
- Xu, J., Wang, J., Liu, Z. 3d-laparoscopic total caudate lobectomy for liver metastases from colorectal cancer: A video article. Asian J Surg. 46 (10), 4525-4526 (2023).
- Wang, Z. G., et al. Anterior hepatic parenchymal transection for complete caudate lobectomy to treat liver cancer situated in or involving the paracaval portion of the caudate lobe. J Gastrointest Surg. 19 (5), 880-886 (2015).
- Xu, G., et al. Laparoscopic caudate lobectomy: A multicenter, propensity score-matched report of safety, feasibility, and early outcomes. Surg Endosc. 35 (3), 1138-1147 (2021).
- Yamamoto, J., et al. Anterior transhepatic approach for isolated resection of the caudate lobe of the liver. World J Surg. 23 (1), 97-101 (1999).
- Nanashima, A., et al. Three-dimensional fusion images of hepatic vasculature and bile duct used for preoperative simulation before hepatic surgery. Hepatogastroenterology. 59 (118), 1748-1757 (2012).
- Costi, R., et al. Partial splenectomy: Who, when and how. A systematic review of the 2130 published cases. J Pediatr Surg. 54 (8), 1527-1538 (2019).
- Liao, K., et al. Laparoscopic anatomical versus non-anatomical hepatectomy in the treatment of hepatocellular carcinoma: A randomised controlled trial. Int J Surg. 102, 106652 (2022).
- Trefts, E., Gannon, M., Wasserman, D. H. The liver. Curr Biol. 27 (21), R1147-R1151 (2017).
- Black, D. M., Behrns, K. E. A scientist revisits the atrophy-hypertrophy complex: Hepatic apoptosis and regeneration. Surg Oncol Clin N Am. 11 (4), 849-864 (2002).
- Michalopoulos, G. K., Bhushan, B. Liver regeneration: Biological and pathological mechanisms and implications. Nat Rev Gastroenterol Hepatol. 18 (1), 40-55 (2021).
- Zhou, X. P., Lu, T., Wei, Y. G., Chen, X. Z. Liver volume variation in patients with virus-induced cirrhosis: Findings on MDCT. AJR Am J Roentgenol. 189 (3), W153-W159 (2007).
- Kumon, M. Anatomical study of the caudate lobe with special reference to portal venous and biliary branches using corrosion liver casts and clinical application. Liver Cancer. 6 (2), 161-170 (2017).
- Kumon, M., et al. Definition of the caudate lobe of the liver based on portal segmentation. Glob Health Med. 2 (5), 328-336 (2020).
- Cho, A., et al. Relationship between right portal and biliary systems based on reclassification of the liver. Am J Surg. 193 (1), 1-4 (2007).
- Li, H., Wei, Y. Laparoscopic extended left hemi-hepatectomy plus caudate lobectomy for caudate lobe hepatocellular carcinoma. J Gastrointest Surg. 23 (3), 617 (2019).
- Fernandes, E. S. M., et al. Anterior transhepatic approach for total caudate lobectomy including spigelian lobe, paracaval portion and caudate process: A brazilian experience. Hepatobiliary Pancreat Dis Int. 17 (4), 371-373 (2018).
- Li, H. J., et al. Laparoscopic versus open hepatectomy for intrahepatic cholangiocarcinoma: Systematic review and meta-analysis of propensity score-matched studies. Eur J Surg Oncol. 49 (4), 700-708 (2023).
- Yang, S. Y., et al. Perioperative and long-term survival outcomes of laparoscopic versus laparotomic hepatectomy for BCLC stages 0-a hepatocellular carcinoma patients associated with or without microvascular invasion: A multicenter, propensity score matching analysis. Hepatol Int. 16 (4), 892-905 (2022).
- Zhang, X. P., et al. Short-term and long-term outcomes after robotic versus open hepatectomy in patients with large hepatocellular carcinoma: A multicenter study. Int J Surg. 110 (2), 660-667 (2024).
- Feng, X., et al. A left-sided approach for resection of hepatic caudate lobe hemangioma: Two case reports and a literature review. Int Surg. 100 (6), 1054-1059 (2015).
- Zheng, K., et al. A laparoscopic left-sided approach combined with the counterclockwise dissection method (huaxi dissection method) for complex tumors located in caudate lobe: A pilot study. J Gastrointest Surg. 28 (5), 754-756 (2024).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved