Method Article
Cryopreservation of Oocytes Retrieved from Ovarian Tissue to Optimize Fertility Preservation in Prepubertal Girls and Women
In questo articolo
Riepilogo
We propose a protocol for fertility preservation in prepubertal girls and women at risk of premature ovarian insufficiency. It combines ovarian tissue freezing and cryopreservation of oocytes retrieved from ovarian tissue. This strategy improves the safety and optimizes the reproductive potential of fertility preservation, maximizing the chance of childbirth.
Abstract
Human ovarian tissue cryopreservation (OTC) is increasingly used worldwide to preserve female fertility in prepubertal girls and women at risk of premature ovarian insufficiency (POI) in the context of urgent gonadotoxic treatments or ovarian surgery. Fertility preservation is challenging because there is no consensus regarding patient management, preservation fertility strategies, or even technical laboratory protocols, which implies that each procedure must be adapted to the characteristics of the patient profile and its own risk-benefit ratio. During OTC, mature/immature oocytes can be aspirated directly from large/small antral follicles within ovarian tissue samples and/or be released into culture media from growing follicles during ovarian tissue dissection in prepubertal girls and women. In this manuscript, we present a protocol that combines ovarian tissue freezing with the cryopreservation of mature/immature oocytes retrieved from ovarian tissue samples, improving the reproductive potential of fertility preservation. Appropriate collection, handling, and storage of ovarian tissue and oocytes before, during, and after the cryopreservation will be described. The subsequent use and safety of cryopreserved/thawed ovarian tissue samples and oocytes will also be discussed, as well as the optimal timing for in vitro maturation of immature oocytes. We recommend the systematic use of this protocol in fertility preservation of prepubertal girls and women as it increases the whole reproductive potential of fertility preservation (i.e., oocyte vitrification in addition of OTC) and also improves the safety and use of fertility preservation (i.e., thawing of oocytes versus ovarian graft), maximizing the chance of successful childbirth for the patients at risk of POI.
Introduzione
The field of fertility preservation has grown over the last two decades due to the increasing number of patients at risk of premature ovarian insufficiency (POI)1,2,3. The current available medical options to preserve fertility are ovarian tissue cryopreservation (OTC)4, oocyte/embryo freezing after ovarian stimulation5, administration of GnRH analogues6, or ovarian transposition7. OTC is a major advance for fertility preservation, particularly in prepubertal girls, where it is the only option currently available to preserve fertility and also in women who cannot delay the onset of their gonadotoxic treatment2,4.
OTC allows the preservation of a high number of primordial follicles, which are located in the outer 1 mm of the ovarian cortex2. Frozen/thawed ovarian tissue can be subsequently used by graft (orthotopic or heterotopic, autologous or donor) or cultured in vitro to obtain mature oocytes2. The graft of frozen-thawed prepubertal ovarian tissue samples has been shown to induce puberty8,9. In women, the reproductive outcomes after orthotopic autografting of frozen-thawed ovarian cortex are reassuring, with live birth rates reaching 57.5% after natural conceptions10 and between 30% to 70% after Assisted Reproductive Techniques (ART) conceptions11. Since the first live birth from orthotopic transplantation of frozen/thawed human ovarian tissue in 200412, this technique allowed the birth of at least 130 children worldwide2. The hormonal and reproductive functions of the graft tissue generally last for several years11,13,14, confirming its long-term functionality.
However, auto-transplantation of ovarian tissue samples carries a theoretical risk of reintroducing viable malignant cells in some patients15,16,17,18, particularly in leukemia survivors19. To date, no case of transmission of cancer via the graft of frozen/thawed ovarian cortex has been reported in healthy cancer survivors11, suggesting that the fibrous avascular nature of the ovarian cortex could represent an inhospitable microenvironment for the dissemination of malignant cells. Nevertheless, the graft of ovarian tissue still represents an experimental and challenging technique, indicating that the use of oocytes should be currently considered as an easier and safer approach than ovarian tissue graft to restore fertility. Interestingly, immature oocytes could be easily retrieved from ovarian tissue during OTC in both prepubertal girls and women16, suggesting that it could represent a reliable source to maximize the fertility-restoring potential in addition to ovarian cortical tissue freezing. These oocytes could be aspirated manually ex vivo in the ART laboratory from visible antral follicles or be isolated from spent media after ovarian tissue dissection. Retrieved oocytes could then be directly vitrified at an immature stage or be matured before the vitrification step using in vitro maturation (IVM)20,21.
In this manuscript, we propose a protocol that combines the cryopreservation of ovarian tissue with the isolation and cryopreservation of mature (MII-stage oocytes) and/or immature oocytes (i.e., Germinal Vesicle (GV) and Metaphase I (MI)-stage oocytes) retrieved from ovarian tissue. This protocol outlines all the specific steps required to maximize the fertility preservation potential in both prepubertal girls and women.
Protocollo
All women (over 18 years of age) as well as all minor girls and their parents signed an informed consent form to preserve the fertility of the patient at risk of POI. This protocol is considered as a routine ART procedure in our center for fertility preservation. It follows the guidelines of our institutions human research ethics committee.
1. Quality control
- Include female age ≤ 38 years, containing circulating anti-mullerian hormone concentration >1 ng/mL and showing the presence of an increased risk of POI due to gonadotoxic treatment. Exclude women eligible for oocyte vitrification for fertility preservation procedure, women with a condition that prevents giving a fully informed consent or women with a high risk of complications from anesthesia or surgery.
- Confirm the presence of a signed patient consent before initiating this procedure.
- Identify two vacant locations within two distinct cryopreservation storage tanks. Half of the cryotubes/straws will be kept in the first storage tank while the other half of the cryotubes/straws will be kept in the other tank to minimize the risk of a total loss of the samples in the case of a tank failure.
- Check the availability and functionality of all medical devices required in this protocol. Sterile surgical dissecting scissors with sharp straight blades and finely sharpened points as well as sterile atraumatic tissue forceps are recommended for the dissection step.
- Check that all quality-control steps have been respected for all medical devices (particularly, validation and routine control of sterilization processes, expiration/lot number verification and tracking).
- Maintain an aseptic/a sterile environment throughout the procedure and realize with caution all the safety precautions.
- Use different dishes, pipettes, and surgical instruments for each patient.
2. The day before the preservation
- Prepare one sterile 60 mm IVF Petri dish containing an IVF medium covered with mineral oil and incubate the dish overnight at 37 °C in an atmosphere of 5% O2 and 5% CO2. Use this dish to collect the cumulus-oocyte complexes (COCs) that will be potentially retrieved from the tissue (see section 7.2).
- Prepare one sterile 35 mm IVF Petri dish containing IVF medium and incubate the dish overnight at 37 °C in an atmosphere of 5% O2 and 5% CO2. Use this dish for the oocyte denudation step (see section 9.1).
- Prepare one sterile 35 mm IVF Petri dish containing IVF medium covered with mineral oil and incubate the dish overnight at 37 °C in an atmosphere of 5% O2 and 5% CO2. Use this dish to collect the oocytes after the denudation step (see section 9.4).
3. Ovarian tissue collection and transport
- Perform the laparoscopic surgery under general anesthesia using one 10 mm port positioned at the umbilicus and two 5 mm ports, one positioned at the left-lower quadrant and another one positioned at the right.
- Perform a partial or complete unilateral oophorectomy according to the consensus decision of the oncologist, the surgeon and the medical staff of the ART unit based on the ovarian reserve, the size, and the macroscopic aspect of both ovaries (Figure 1, A1–B1) and the planned gonadotoxic protocol. Perform oophorectomy with sharp scissors or a surgical staple. Do not use any dissection device that could induce collateral, electric, or thermal injury to the ovarian tissue that is to be preserved.
NOTE: A complete unilateral oophorectomy is generally performed in prepubertal girls16,22, whereas a partial unilateral oophorectomy could be commonly achieved in adult patients with large ovaries and/or a high ovarian reserve.
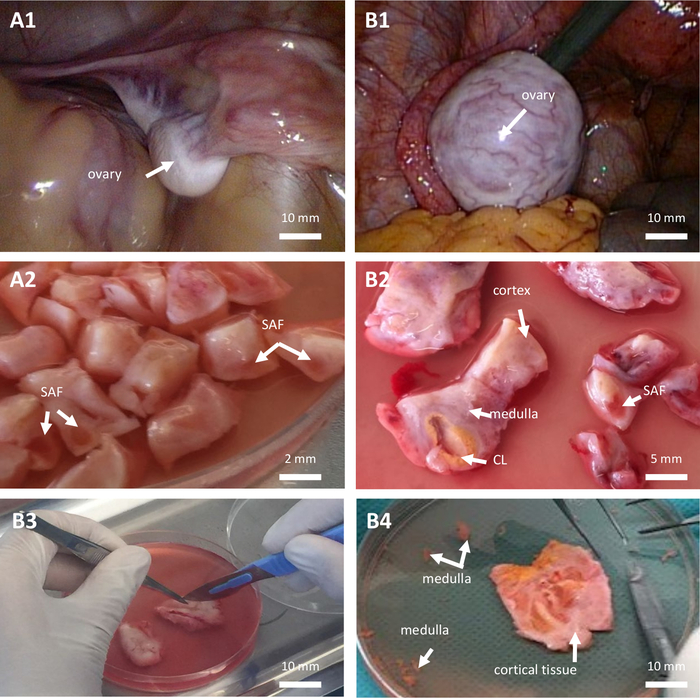
Figure 1: Ovarian tissue cryopreservation. Ovarian tissue cryopreservation in a prepubertal girl (A, 7-year-old patient) and in a woman (B, 28-year-old patient) both suffering from acute myeloid leukemia. (A1–B1) Laparoscopic view of the ovary. (A2–B2–B3) Dissection of ovarian tissue. (B4) Cortical ovarian tissue. CL: corpus luteum. SAF: small antral follicle. Please click here to view a larger version of this figure.
- Perform the evacuation of the ovarian tissue using a handmade or commercial laparoscopic specimen retrieval bag.
- Put the ovarian tissue in a sterile tube containing culture medium at 4 °C. The tissue must be completely immersed in the culture medium.
- Immediately transport the ovarian tissue in an insulated pouch at 4 °C to the ART laboratory for optimal results.
NOTE: If necessary, the ovarian tissue can be transport at cold temperatures (around 4 °C) to the laboratory for up to 26 h after the collection without threatening the quality of the tissue23.
4. Ovarian tissue preparation
- Transfer the ovarian tissue into a sterile 90 mm IVF Petri dish containing 20 mL of pre-cooled culture medium at 4 °C.
- Put the Petri dish containing the ovarian tissue on a cold plate (at 4 °C) placed on a vertical laminar flow clean bench to minimize the risk of microorganism contamination.
5. Manual follicle aspiration from ovarian tissue samples
- Aspirate visible antral follicles (if present on the surface of ovarian tissue) with a 21 G syringe needle connected to a 1 mL disposable syringe.
NOTE: This step could be tricky. An alternative may be to gently open each visible antral follicle with a scalpel and rinse the inside of each follicle with IVF media in order to softly release COCs within the dissection media. - Flush the collected follicular fluids into a sterile 60 mm IVF Petri dish containing 5 mL of IVF culture medium at room temperature.
- Rinse the syringe with 1 mL of IVF culture medium at room temperature and flush the liquid into the same IVF Petri dish. Repeat this step two times.
- Identify and isolate the COCs under an inverted microscope (50x–200x magnification).
- Transfer the healthy COCs with a pipette into a new sterile 60 mm IVF Petri dish containing pre-equilibrated IVF medium covered with oil at 37 °C in an atmosphere of 5% O2 and 5% CO2.
NOTE: Healthy COCs contain a translucent oocyte. Discard the atretic COCs presenting a brown coloured oocyte. - Store the IVF Petri dish in the incubator at 37 °C in an atmosphere of 5% O2 and 5% CO2 until the denudation step (see step 9).
6. Ovarian tissue dissection
- Prepare the fresh freezing solution in a 50 mL sterile tube. The freezing solution contains 1.5M dimethylsulfoxide (DMSO) and 10% of human serum albumin in IVF culture medium. Gently vortex.
NOTE: It should be prepared just before use. - Cut the ovarian tissue and remove medulla as much as possible (Figure 1, B4).
- Cut the ovarian cortex into slices measuring 10 mm x 10 mm x 1 mm or 0.5 mm x 0.5 mm x 1 mm in the case of complete or partial unilateral oophorectomy, respectively (Figure 1, A2–B2–B3). The cutting is performed with sterile surgical sharp scissors and atraumatic forceps.
NOTE: One slice of ovarian tissue is kept intact with both ovarian cortex and medulla for further histological analysis (see step 8). - After the dissection, perform two washing steps (or more, if required) for each ovarian cortical sample in 1 mL of IVF culture medium to remove blood. After the washing, transfer all the tissue samples into a new 60 mm Petri dish with fresh IVF culture medium.
- Place each ovarian cortical sample in a cryotube containing 1 mL of freezing solution.
NOTE: Mixing is unnecessary. The slice of ovarian tissue kept for further histological analysis is not cryopreserved (see step 8). - Keep the samples for 30 min at 4 °C for equilibration with the freezing solution.
- Cryopreserve all samples with a slow freezing technique. The cooling rate is -2 °C/min from +4 °C until -9 °C, -50 °C/min until -30 °C; hold during 1 min, +4 °C/min until -15 °C, -2 °C/min until -40 °C; and finally -25 °C/min until -150 °C using a programmable freezer.
- Remove the cryotubes immediately and plunge them into liquid nitrogen at -196 °C.
- Place half of the cryotubes in a first liquid nitrogen storage tank and the other half of the cryotubes in a second liquid nitrogen tank.
7. Manual COC isolation from the dissection spent media
- Identify COCs from the dissection spent media under a microscope (50x–200x magnification) (Figure 2).

Figure 2: Mature and immature COCs retrieved from ovarian tissue. Mature (A) and immature (B1–B3,C1–C2) COCs retrieved from ovarian tissue. (A) Mature COC containing a mature oocyte (presence of the first polar body, Metaphase II-stage oocyte). (B1) Healthy immature COC containing an immature oocyte (no polar body, Metaphase I-stage oocyte). (B2–B3) Healthy immature COCs containing immature Germinal Vesicle-stage oocytes (no polar body, Prophase I-stage oocytes). (C1–C2) Unhealthy COCs containing an atretic brown colored oocyte. Please click here to view a larger version of this figure.
- Transfer the healthy COCs with a pipette into a new sterile 60 mm IVF Petri Dish containing pre-equilibrated IVF medium covered with oil at 37°C in an atmosphere of 5% O2 and 5% CO2 (see step 2.1).
NOTE: Discard the atretic COCs. - Store the IVF Petri dish in the incubator at 37 °C in an atmosphere of 5% O2 and 5% CO2 until the denudation step (see step 9).
8. Ovarian tissue histological analysis
- Fix the remaining slice of ovarian tissue (containing both ovarian cortex and medulla) in a 3% formalin solution in a chemical clean bench for histological examination.
NOTE: It should be performed only once the cortical samples are successfully frozen and stored.
CAUTION: The formalin solution is irritating, corrosive, and toxic. This step should be carried out carefully and outside the IVF laboratory. - Prescribe a complete histological analysis of this ovarian tissue sample with an evaluation of the potential presence of malignant cells as well as a description of the number and type of ovarian follicles (i.e., primordial, primary, secondary, and antral follicles, respectively).
9. Oocyte denudation and selection
- Perform oocyte denudation. Briefly expose COCs to hyaluronidase solution (80 IU/mL or final concentration 0.1 mg/mL) with gentle pipetting (repeated aspirating and expelling the COCs into the denudation solution) using a large diameter pipette tip (volume 0.1–20 µL, length: 40.5 mm, diameter work cone: 6 mm, diameter opening: 0.36 mm) for 30 s, immediately followed by two washing steps in pre-equilibrated medium covered with mineral oil to remove the excess enzyme (see step 2.1).
- Optimize the removal of cumulus and corona radiata cells by gentle pipetting using a 150 µm pipette tip in pre-equilibrated IVF medium (see step 2.1).
- Perform a selection step of healthy oocytes using an inverted microscope (200x–400x magnification). The maturation stage of healthy oocytes could be GV, MI, or MII-stage oocytes (Figure 3). Healthy oocytes have the following morphological characteristics: an intact and round cytoplasm, a size between 100–150 µm, and a pale color.
NOTE: Discard atretic and unhealthy oocytes (Figure 3, D1–D3).

Figure 3: Mature and Immature oocytes retrieved from ovarian tissue. (A) Mature oocyte (Metaphase II-stage oocyte: presence of the first polar body (PB, arrow), no visible nucleus), (B1–B2) Immature oocytes (Metaphase I-stage oocyte: no polar body, no visible nucleus), (C1–C3) Immature oocytes (Germinal Vesicle-stage (VG) oocytes: no polar body, presence of a large halo with nucleoli within the cytoplasm (arrow)). In C1, Germinal Vesicle-stage oocytes display heterogenous size. (D1–D3) Atretic oocytes. PB: polar body. GV: Germinal Vesicle. Please click here to view a larger version of this figure.
- Incubate healthy oocytes inside the incubator at 37 °C, 5% O2 and 5% CO2 until the vitrification step (see step 2.1).
10. Oocyte vitrification
- Confirm patient/specimen identification.
- Perform a new selection step of healthy oocytes using an inverted microscope (200x–400x magnification).
NOTE: Discard atretic and unhealthy oocytes. - Take a picture of each healthy oocyte using an inverted microscope just before the vitrification step. Note the maturation stage of each oocyte.
NOTE: Attach all these data (image, size, maturation stage) in the file of the patient. - Use different identification numbers per straw to differentiate them if cryopreserving more than one straw per patient.
- Cryopreserve one or two oocyte(s) per straw.
NOTE: Mature and immature viable oocytes must be cryopreserved separately. When cryopreserving two oocytes together in the same straw, it is recommended to select oocytes with similar morphological characteristics. - Cryopreserve healthy oocytes using a vitrification kit according to the manufacturer’s instructions (see Table of Materials).
- Load the cryopreserve oocytes on an appropriate device for cryostorage in liquid nitrogen according to the manufacturer’s instructions (see Table of Materials).
- Place half of the straws in a first liquid nitrogen storage tank and the other half of the straws in a second liquid nitrogen tank.
Risultati
A total of 81 OTC from 81 female patients have been performed between 2007 and 2020, including 43 prepubertal girls and 38 women. The mean age of female patients (prepubertal girls and women) was 14.21 ± 9.61 years (mean ± standard error). The youngest patient was 5 months old and the oldest was 33.6 years old (Table 1). The mean age of prepubertal girls and women was 6.84 ± 4.81 years old (min–max: 0.5–15.1) and 22.76 ± 5.92 years old (min–max: 14.2–33.6), respectively (Mann-Whitney, p = 3.37 x 10-10).
The percentage of patients with a history of gonadotoxic treatment before OTC was 48.84% (21/43) in prepubertal girls and 13.16% (5/38) in women (Chi2, p = 0.0006). A complete oophorectomy was performed for the majority of patients (88.89% (72/81), 43 prepubertal girls and 29 women) whereas a partial oophorectomy was only performed in 9 women (Fisher's Exact Test, p = 0.0006). Figure 4 shows the negative impact of using a dissection device that could induce collateral electric or thermal injury to the ovarian tissue during oophorectomy. Figure 5 shows the negative impacts of two different treatments on the quality of the collected ovarian tissue. The percentage of patients with a positive retrieval of oocytes from ovarian tissue was 41.86% (18/43) in prepubertal girls and 71.05% (27/38) in women (Chi2, p = 0.0008). No oocytes could be isolated in 36 patients. The youngest patient from whom oocytes were retrieved was 3 years old whereas the oldest was 33.6 years old. The total number of mature and immature oocytes was 71 oocytes (min–max, 1–9 per patient) in prepubertal girls and 377 oocytes (min–max, 1–27 per patient) in women (Fisher's Exact Test, p = 1). The mean number of retrieved oocytes per oophorectomy was 1.65 ± 2.68 in prepubertal girls and 9.92 ± 9.51 in women (Mann Whitney, p = 9.15 x 10-5). In women, the mean number of retrieved oocytes was similar in partial oophorectomy compared to complete oophorectomy (13.11 ± 7.18 versus 8.93 ± 10.03, Mann-Whitney, p = 0.16). The mean number of retrieved oocytes per patient with a positive retrieval was significantly higher in women compared to prepubertal girls (13.98 ± 6.38 versus 3.94 ± 2.86, Mann-Whitney, p = 0.0003). The retrieval of mature oocytes (i.e., MII-stage oocyte) was extremely rare in women (i.e., only one mature oocyte from one woman) and inexistent in prepubertal girls. A total of 71 immature oocytes (i.e., 4 MI-stage oocytes and 67 GV-stage oocytes) were obtained from prepubertal girls and 376 immature oocytes (i.e., 28 MI-stage and 348 GV-stage oocytes) were retrieved from women (Fisher's Exact Test, p = 0.8). The percentage of atretic oocytes was significantly higher in prepubertal girls compared to women (Chi2, p = 1.43 x 10-7).
The percentage of patients with a cryopreservation of oocytes retrieved from ovarian tissue was 23.26% (10/43) in prepubertal girls and 65.79% (25/38) in women (Chi2, p = 0.0001) (Table 2). The total number of cryopreserved mature and immature oocytes was 38 oocytes (i.e., 2 MI-stage and 36 GV-stage oocytes) in prepubertal girls and 310 oocytes (i.e., 23 MI and 286 GV) in women (Fisher's Exact Test, p = 1 and p = 1, respectively). The mean number of cryopreserved oocytes per patient who had the chance to benefit from oocyte freezing was 3.80 ± 2.35 in prepubertal girls and 12.40 ± 6.40 in women (Mann-Whitney, p = 0.0008).
After fertility preservation, the majority of patients chose to continue the storage of ovarian tissue and oocyte samples (Supplementary Table 1). The main reason for fertility preservation destruction was patient death (9.30% (4/43) in prepubertal girls and 15.79% (6/38) in women). No request for use has so far been requested. The loss of follow up was 4.65% (2/43) in prepubertal girls and 10.53% (4/38) in women.
| Prepubertal girls | Women | Statistical analyses | P value | ||
| n=43 | n=38 | ||||
| Age (mean ± SE) (min-max) | 6.84 ± 4.81 (0.5-15.1) | 22.76 ± 5.92 (14.2-33.6) | Mann-Whitney | 3.37 x 10-10 | |
| % of patients with an history of gonadotoxic treatment | 48.84% (21/43) | 13.16% (5/38) | Chi2 | 0.0006 | |
| Complete/partial oophorectomy | 43/0 | 29/9 | Fisher's Exact Test | 0.0006 | |
| Number of retrieved oocytes per oophorectomy (mean +/- SE) | 1.65 ± 2.68 | 9.92 ± 9.51 | Mann-Whitney | 9.15 x 10-5 | |
| Number of retrieved oocytes per complete oophorectomy (mean +/- SE) | 1.65 ± 2.68 | 8.93 ± 10.03a | Mann-Whitney | 0.0068 | |
| Number of retrieved oocytes per partial oophorectomy (mean +/- SE) | - | 13.11 ± 7.18a | Mann-Whitney | 0.16a | |
| % of patients with a positive retrieval of oocytes from ovarian tissue | 41.86% (18/43) | 71.05% (27/38) | Chi2 | 0.008 | |
| Number of retrieved oocytes per patient with a positive retrieval (mean +/- SE) | 3.94 ± 2.86 | 13.98 ± 6.38 | Mann-Whitney | 0.0003 | |
| Total number of mature oocytes (min-max per patient with a positive retrieval) | 0 (0-0) | 1 (0-1) | Fisher's Exact Test | 1 | |
| Total number of immature oocytes (min-max per patient with a positive retrieval) | 71 (1-9) | 376 (1-26) | |||
| Maturation stage of immature oocytes | 4 M1 | 28 M1 | Fisher's Exact Test | 0.80 | |
| 67 GV | 348 GV | ||||
| % of atretic mature and immature oocytes | 46.48% (33/71) | 18.04% (68/377) | Chi2 | 1.43 x 10-7 | |
Table 1: Patient clinical characteristics and biological parameters of oocyte retrieval from ovarian tissue. SE = standard error. aStatistical analysis of the number of oocytes retrieved from ovarian tissue obtained after partial oophorectomy compared to complete oophorectomy in women.
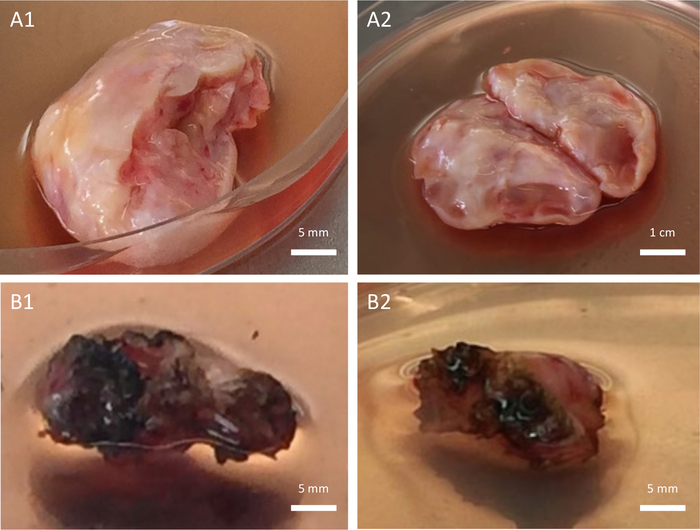
Figure 4: Ovarian tissue quality. Collected ovarian tissue of high quality (A1–A2) versus low quality (B1–B2). The low quality is due to the use of an inappropriate dissection device that had induced collateral injury to the ovarian tissue during oophorectomy. Please click here to view a larger version of this figure.
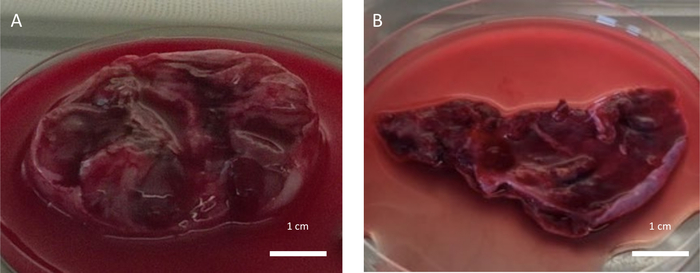
Figure 5: Low quality ovarian tissue. Collected ovarian tissue of low quality due to the negative impacts of (A) ovarian puncture after hormonal stimulation performed 2 days before OTC and (B) recent chemotherapy treatment administrated the week before OTC. Please click here to view a larger version of this figure.
| Prepubertal girls | Women | Statistical analyses | P value | ||
| n=43 | n=38 | ||||
| % of patients with a cryopreservation of oocytes retrieved from ovarian tissue | 23.26% (10/43) | 65.79% (25/38) | Chi2 | 0.0001 | |
| Number of cryopreserved oocytes per patient with a cryopreservation (mean +/- SE) | 3.80 ± 2.35 | 12.40 ± 6.40 | Mann-Whitney | 0.0008 | |
| Total number of healthy cryopreserved mature oocytes (min-max per patient) | 0 (0-0) | 1 (0-1) | Fisher's Exact Test | 1 | |
| Total number of healthy immature cryopreserved oocytes | 38 (1-9) | 309 (1-23) | |||
| Maturation stage of healthy immature cryopreserved oocytes | 2 M1 | 23 M1 | Fisher's Exact Test | 1 | |
| 36 GV | 286 GV | ||||
Table 2: Proportion of patients who beneficiated from oocytes cryopreservation retrieved from ovarian tissue and biological parameters of cryopreserved oocytes.
Supplementary Table 1: Storage/destruction of fertility preservation and follow-up of patients. Fertility preservation: cryopreserved ovarian tissue +/- retrieved oocytes from ovarian tissue. Please click here to download this table.
Discussione
The current manuscript provides a protocol combining ovarian tissue freezing and cryopreservation of oocytes retrieved from ovarian tissue, increasing the potential of fertility in prepubertal girls and women at risk of POI. We strongly recommend performing this protocol before the start of any gonadotoxic therapy in order to optimize the quantity (i.e., number of viable oocytes) as well as the quality (i.e., DNA integrity and cytoplasm competence) of the preserved oocytes, optimizing their safety for clinical use24. If it is not possible to perform this protocol before any form of gonadotoxic treatment, this protocol can also be conducted after the start of gonadotoxic therapy but an extreme caution is then advised when using these oocytes in ART, warranting specific precautions such as pre-implantation genetic testing (PGT), careful fetal monitoring, and amniocentesis16.
In the absence of a clear consensus on the amount of ovarian tissue that should be retrieved for fertility preservation, we recommend to perform complete oophorectomy in prepubertal girls as well as in patients associated with a moderate/high risk of POI or with a low ovarian reserve. Indeed, the volume of ovarian tissue collected during oophorectomy will be directly correlated to the number of oocytes that can be retrieved and then cryopreserved for the same patient. In our results, we observed that the numbers of retrieved oocytes were similar in partial oophorectomy compared to complete oophorectomy in women, but this result reflects the differences in ovarian reserve between the patients to whom we proposed partial oophorectomy (i.e., young patients associated with a high ovarian reserve and a low risk of POI) and those to whom we proposed total oophorectomy (i.e., older patients associated with a low ovarian reserve and a moderate/high risk of POI)16. To date, no significant harm of unilateral oophorectomy on long-term female fertility has been reported and the onset of menopause seems similar (or at worst perhaps 1 year earlier in human), probably due to a compensatory mechanism that lead to slower recruitment rate of primordial follicles11,25. Hence, there seems to be no significant negative effect of complete oophorectomy while it could allow the cryopreservation of a high number of oocytes, increasing the chances of having a live birth. Moreover, the use of cryopreserved oocytes by in vitro fertilization is much easier and safer than the graft of cryopreserved/thawed ovarian cortical tissue because of the risk of reseeding the disease of the patient is then avoided. Hence, this approach is particularly appropriate in blood or metastatic diseases associated with a risk of possible malignancy reseeding from transplanted ovarian tissue15,16,17,18, specifically in patients with leukemia19.
To date, slow freezing seems to give better results than vitrification for the cryopreservation of ovarian tissue samples but vitrification is clearly better for oocyte freezing26. These results explain why we choose to preserve the ovarian tissue with the slow freezing technique whereas retrieved oocytes were vitrified. We also choose to vitrify immature oocytes before any in vitro maturation (IVM) step because we hope that the performance of IVM will be significantly improved in future. At present, data is scarce on the optimal timing for oocyte vitrification (i.e., before or after IVM), preventing any conclusion on the most efficient strategy. Only one meta-analysis on this topic has been published in 201827, but it mainly concerns immature oocytes recovered after hormonal stimulation (and not oocytes retrieved from ovarian tissue) as well as it only evaluates oocyte maturation rate and no other clinical outcomes (such as fertilization rates, embryo development, or pregnancy/live birth rates). Moreover, the same IVM protocol was performed before and after the vitrification/thawing steps, excluding the impact of future advances in IVM protocols. As frozen oocytes for fertility preservation will not be used for several years or even decades in the case of prepubertal girls, progress will most certainly be made in IVM protocols in the coming years, giving hope for better results in terms of oocyte maturation rate as well as developmental/implantation potential.
Limited data is available on the developmental potential of oocytes derived from ovarian tissue after oophorectomy, specifically from prepubertal girls. Some studies reporting higher rates of atresia and abnormal morphology combined with a lower maturation potential16,28,29 in oocytes retrieved from prepubertal girls compared to women, whereas other publications describing similar characteristics are reference16,24,30,31,32,33,34,35. To the best of our knowledge, there is no report of pregnancy after IVM of oocytes frozen during childhood or adolescence. In women, clinical pregnancy rates and neonatal morbidity seem similar after IVM compared to standard IVF/ICSI36. Further reports are warranted on the fertility-restoring potential of oocytes retrieved from ovarian tissue, specifically in prepubertal patients.
Divulgazioni
None of the authors have competing interests.
Riconoscimenti
We thank all members of our centers involved in the activity of fertility preservation (Gynecologists, Biologists, Oncologists, and Anatomopathologists). The study was conducted as part of the routine procedures for fertility preservation. No funding was received.
Materiali
| Name | Company | Catalog Number | Comments |
| 1 ml disposable syringe | CDD | 1323101/7002655 | Other material and sizes may also be suitable |
| 21-gauge syringe needle | Merck | Z192481 | Other material and sizes may also be suitable |
| 35 mm IVF Petri Dish | Nunc | 150255 | Other material and sizes may also be suitable |
| 60 mm IVF Petri Dish | Nunc | 150270 | Other material and sizes may also be suitable |
| 90 mm IVF Petri Dish | Nunc | 150360 | Other material and sizes may also be suitable |
| Atraumatic forceps | Medlane | PI 299 04 | Other material and sizes may also be suitable |
| Continuous Single Culture Complete with HSA | Irvine Scientific | 90165 | IVF culture medium for follicular fluid collection, COCs incubation, oocyte denudation and oocyte incubation until the vitrification step. |
| Cryotube | Thermo Scientific | 368632 | Other products may also be suitable |
| Dimethylsulfoxide (DMSO) | MILTENYI BIOTEC SAS | 170-076-303 | CryoMACS DMSO 10 (EP) |
| GT40 | Air Liquide | 1,13,517 | Storage tank |
| HSV High Security Vitrification Straw | Irvine Scientific | 25251 | Vitrification straws |
| Human serum albumin | Vitrolife | 10064 | Other products may also be suitable |
| Leibovitz L15 medium | Eurobio | CM1L15000U | Culture medium for ovarian tissue collection, transport and tissue dissection |
| Leibovitz L15 medium | Eurobio | CM1L15000U | Culture medium for freezing solution |
| Mars-IVF Class II Workstation/L126 IVF Dual | CooperSurgical | WM1500/6-133-911-121 | Workstation |
| Programmable freezer | Planner KRYO 500 | Kryo 560-16 | Other equipements may also be suitable |
| Scissors with sharp straight blades and finely sharpened points | Medlane | CI 034 03 | Other material and sizes may also be suitable |
| Stripper | CooperSurgical | MXL3-STR-CGR | Other products may also be suitable |
| Tips (150µm) for Stripper | CooperSurgical | MXL3-150 | Other products may also be suitable |
| Vitrification Kit | Irvine Scientific | 90133 | Protocols are available at http://www.irvinesci.com/products/90133-so-vitrification-freeze-solutions. Other products may also be suitable |
Riferimenti
- Ataman, L. M., et al. Creating a global community of practice for oncofertility. Journal of Global Oncology. 2 (2), 83-96 (2016).
- Rivas Leonel, E. C., Lucci, C. M., Amorim, C. A. Cryopreservation of human ovarian tissue: a review. Transfusion Medicine and Hemotherapy. 46 (3), 173-181 (2019).
- Chae-Kim, J. J., Gavrilova-Jordan, L. Premature ovarian insufficiency: procreative management and preventive strategies. Biomedicines. 7 (1), (2018).
- Anderson, R. A., Wallace, W. H. B., Telfer, E. E. Ovarian tissue cryopreservation for fertility preservation: clinical and research perspectives. Human Reproduction Open. 2017 (1), (2017).
- Donnez, J., Dolmans, M. M. Fertility preservation in women. The New England Journal of Medicine. 377 (17), 1657-1665 (2017).
- Lambertini, M., et al. Ovarian suppression using luteinizing hormone-releasing hormone agonists during chemotherapy to preserve ovarian function and fertility of breast cancer patients: a meta-analysis of randomized studies. Annals of Oncology. 26 (12), 2408-2419 (2015).
- Gubbala, K., et al. Outcomes of ovarian transposition in gynaecological cancers; a systematic review and meta-analysis. Journal of Ovarian Research. 7, 69 (2014).
- Poirot, C., et al. Induction of puberty by autograft of cryopreserved ovarian tissue. Lancet. 379 (9815), 588 (2012).
- Ernst, E., Kjaersgaard, M., Birkebaek, N. H., Clausen, N., Andersen, C. Y. Case report: stimulation of puberty in a girl with chemo- and radiation therapy induced ovarian failure by transplantation of a small part of her frozen/thawed ovarian tissue. European Journal of Cancer. 49 (4), 911-914 (2013).
- Pacheco, F., Oktay, K. Current success and efficiency of autologous ovarian transplantation: a meta-analysis. Reproductive Sciences. 24 (8), 1111-1120 (2017).
- Silber, S., Kagawa, N., Kuwayama, M., Gosden, R. Duration of fertility after fresh and frozen ovary transplantation. Fertility and Sterility. 94 (6), 2191-2196 (2010).
- Donnez, J., et al. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet. 364 (9443), 1405-1410 (2004).
- Donnez, J., et al. Restoration of ovarian activity and pregnancy after transplantation of cryopreserved ovarian tissue: a review of 60 cases of reimplantation. Fertility and Sterility. 99 (6), 1503-1513 (2013).
- Jensen, A. K., et al. Outcomes of transplantations of cryopreserved ovarian tissue to 41 women in Denmark. Human Reproduction. 30 (12), 2838-2845 (2015).
- Abir, R., et al. Occasional involvement of the ovary in Ewing sarcoma. Human Reproduction. 25 (7), 1708-1712 (2010).
- Abir, R., et al. Cryopreservation of in vitro matured oocytes in addition to ovarian tissue freezing for fertility preservation in paediatric female cancer patients before and after cancer therapy. Human Reproduction. 31 (4), 750-762 (2016).
- Abir, R., et al. Ovarian minimal residual disease in chronic myeloid leukaemia. Reproductive BioMedicine Online. 28 (2), 255-260 (2014).
- Dolmans, M. M., Luyckx, V., Donnez, J., Andersen, C. Y., Greve, T. Risk of transferring malignant cells with transplanted frozen-thawed ovarian tissue. Fertility and Sterility. 99 (6), 1514-1522 (2013).
- Soares, M., et al. Eliminating malignant cells from cryopreserved ovarian tissue is possible in leukaemia patients. The British Journal of Haematology. 178 (2), 231-239 (2017).
- Son, W. Y., Henderson, S., Cohen, Y., Dahan, M., Buckett, W. Immature oocyte for fertility preservation. Frontiers in Endocrinology. 10, 464 (2019).
- Yang, Z. Y., Chian, R. C. Development of in vitro maturation techniques for clinical applications. Fertility and Sterility. 108 (4), 577-584 (2017).
- Imbert, R., et al. Safety and usefulness of cryopreservation of ovarian tissue to preserve fertility: a 12-year retrospective analysis. Human Reproduction. 29 (9), 1931-1940 (2014).
- Duncan, F. E., et al. Ovarian tissue transport to expand access to fertility preservation: from animals to clinical practice. Reproduction. 152 (6), 201-210 (2016).
- Abir, R., et al. Selection of patients before and after anticancer treatment for ovarian cryopreservation. Human Reproduction. 23 (4), 869-877 (2008).
- Yasui, T., et al. Factors associated with premature ovarian failure, early menopause and earlier onset of menopause in Japanese women. Maturitas. 72 (3), 249-255 (2012).
- Cobo, A., Diaz, C. Clinical application of oocyte vitrification: a systematic review and meta-analysis of randomized controlled trials. Fertility and Sterility. 96 (2), 277-285 (2011).
- Mohsenzadeh, M., Salehi-Abargouei, A., Tabibnejad, N., Karimi-Zarchi, M., Khalili, M. A. Effect of vitrification on human oocyte maturation rate during in vitro maturation procedure: A systematic review and meta-analysis. Cryobiology. 83, 84-89 (2018).
- Anderson, R. A., McLaughlin, M., Wallace, W. H., Albertini, D. F., Telfer, E. E. The immature human ovary shows loss of abnormal follicles and increasing follicle developmental competence through childhood and adolescence. Human Reproduction. 29 (1), 97-106 (2014).
- Revel, A., et al. At what age can human oocytes be obtained. Fertility and Sterility. 92 (2), 458-463 (2009).
- Margulis, S., et al. morphogenetic protein 15 expression in human ovaries from fetuses, girls, and women. Fertility and Sterility. 92 (5), 1666-1673 (2009).
- Kedem, A., et al. Alginate scaffold for organ culture of cryopreserved-thawed human ovarian cortical follicles. Journal of Assisted Reproduction and Genetics. 28 (9), 761-769 (2011).
- Lerer-Serfaty, G., et al. Attempted application of bioengineered/biosynthetic supporting matrices with phosphatidylinositol-trisphosphate-enhancing substances to organ culture of human primordial follicles. Journal of Assisted Reproduction and Genetics. 30 (10), 1279-1288 (2013).
- Ben-Haroush, A., Sapir, O., Fisch, B. Aspiration of immature oocytes during cesarean section for fertility preservation and future surrogacy. American Journal of Obstetrics and Gynecology. 203 (1), 12-14 (2010).
- Farhi, J., Sapir, O., Maman, M., Fisch, B., Ben-Haroush, A. Novel protocol for scheduling oocyte retrieval in IVM cycles in PCOS patients: a case series. Reproductive BioMedicine Online. 23 (6), 765-768 (2011).
- Segers, I., et al. In vitro maturation (IVM) of oocytes recovered from ovariectomy specimens in the laboratory: a promising "ex vivo" method of oocyte cryopreservation resulting in the first report of an ongoing pregnancy in Europe. Journal of Assisted Reproduction and Genetics. 32 (8), 1221-1231 (2015).
- Ellenbogen, A., Shavit, T., Shalom-Paz, E. IVM results are comparable and may have advantages over standard IVF. Facts, Views & Vision in ObGyn. 6 (2), 77-80 (2014).
Ristampe e Autorizzazioni
Richiedi autorizzazione per utilizzare il testo o le figure di questo articolo JoVE
Richiedi AutorizzazioneThis article has been published
Video Coming Soon