Method Article
Recording of Inward Rectifying K+ Currents in Freshly Isolated Basilar Artery Smooth Muscle Cells by Patch Clamp Technique
In This Article
Summary
This protocol describes a rapid and efficient method for isolating smooth muscle cells from the rat basilar artery and recording inward rectifying potassium channel currents in these cells using the whole-cell patch clamp technique. It offers a novel approach for researchers studying the basilar artery and ion channels.
Abstract
Cerebrovascular disease is a prevalent condition among the elderly, with its incidence steadily rising. The basilar artery is a critical cerebral vessel that supplies the pons, cerebellum, posterior brain regions, and inner ear. Potassium (K+) channel activity plays a significant role in determining vascular tone by regulating the cell membrane potential. Activation of inward rectifying K+ (Kir) channels, like other K+ channels, leads to cell membrane hyperpolarization and vasodilation. In this study, freshly isolated smooth muscle cells from the basilar artery were used to record Kir currents via the whole-cell patch clamp technique. The effects of 100 µmol/L BaCl2, a Kir channel inhibitor, and 10 µmol/L sodium nitroprusside (SNP), a nitro vasodilator, on Kir channel currents were investigated. The results demonstrated that BaCl2 inhibited Kir channel currents in basilar artery smooth muscle cells, whereas SNP enhanced these currents. This protocol provides a comprehensive guide for preparing freshly isolated arterial smooth muscle cells and recording Kir channel currents using the patch clamp technique, offering a valuable resource for researchers seeking to master this method.
Introduction
Cerebrovascular disease is a prevalent condition in the elderly population. With improvements in living standards, increased life expectancy, and the aging population, the incidence of cerebrovascular disease is steadily rising1. The basilar artery, an unpaired vessel formed by the fusion of the bilateral vertebral arteries, runs beneath the pons within the skull and divides into two posterior cerebral arteries. It supplies the pons, cerebellum, posterior regions of the brain, and the inner ear. Insufficient blood supply to the basilar artery can lead to episodic vertigo, often accompanied by nausea and vomiting. Patients may also experience symptoms such as tinnitus, hearing loss, and other related issues. These symptoms are frequently associated with conditions such as cervical spondylosis, cerebral atherosclerosis, and abnormal blood pressure. Cerebrovascular disease, particularly prevalent among middle-aged and elderly individuals, is often linked to these underlying conditions2,3,4.
Resistance arteries play a vital role in cardiovascular function and maintaining bodily homeostasis. As the primary site of vascular resistance, they regulate blood pressure and cardiac output, ensuring sufficient blood flow to meet the metabolic and physiological demands of tissues and organs5. The basilar artery, classified as a resistance artery, primarily regulates blood flow to the brainstem6. Smooth muscle cells, which form the walls of resistance arteries, are key mediators of vascular resistance through the regulation of steady-state contraction or vascular tension. These cells harbor numerous ion channels, including K+ channels, Ca2+ channels, and Cl- channels, which are critical for the modulation of vascular tone5,7.
K+ channels are critical in establishing the membrane potential and regulating the contractile tone of arterial smooth muscle cells8. There are four types of K+ channels in arterial smooth muscle: voltage-dependent K+ (Kᴠ), Ca2+-dependent K+ (KCa), ATP-dependent K+ (KATP), and inward rectifier K+ (Kir) channels9,10,11. Kir channels are categorized into seven subtypes, with Kir2.x being classical Kir channels. Among these, the Kir2.x subfamilies are the most relevant in the vasculature. Kir currents exhibit inward rectification at negative voltages, indicating a net influx of K+ into the cell, whereas at positive voltages, there is minimal to no net K+ current flow5. In the cardiovascular system, Kir channels are essential for stabilizing the membrane potential. Their activation induces cell membrane hyperpolarization and vasodilation12,13,14.
Patch-clamp experiments on freshly isolated smooth muscle cells have been conducted in various arteries, including coronary, cerebral, renal, and mesenteric arteries15,16. While some methods utilize the same type of collagenase for cell isolation, the precise procedures vary. Few studies have comprehensively summarized the methods for isolating vascular smooth muscle cells. Therefore, this study focuses on the fresh isolation of primary vascular smooth muscle cells from the rat basilar artery and the recording of Kir channel currents in these cells using the whole-cell patch clamp technique, providing a detailed and complete protocol for researchers in related fields.
Protocol
The animal protocol was approved by the Chengdu University of Traditional Chinese Medicine Laboratory Animal Welfare Ethics Committee (Record No. 2024035). Male Sprague-Dawley (SD) rats, weighing 260-300 g and aged 8-10 weeks, were used in this study. The animals were provided with water and food (SPF experimental animal feed) ad libitum. Details of the reagents and equipment used in this study are listed in the Table of Materials.
1. Rat basilar artery dissection
- Solution Preparation
- Prepare solutions as described in Table 1.
- Anesthetize the rat by inhalation of 2% isoflurane. Confirm deep anesthesia using a toe pinch. If required, administer additional anesthetics. Immediately proceed to open the skull and expose the brain on the portable operating table.
- Quickly transfer the brain to a Petri dish containing PSS saturated with 95% O2 and 5% CO2 at 4 °C. Ensure the solution has a pH of 7.40.
- Position the brain ventrally upward in a Petri dish and secure it with needles. Under a light microscope, locate the basilar artery and carefully remove the surrounding tissue using autoclaved tweezers and scissors (see Figure 1A).
- Insert a 2 cm-long wire with a diameter of 25 µm into the isolated basilar artery. Gently rub the inner wall with the wire to effectively remove the vascular endothelium.
NOTE: The basilar artery in rats is situated within the basal sulci of the brainstem at the base of the brain and is formed by the confluence of the left and right vertebral arteries2. During the isolation process, handle the artery with care to avoid excessive stretching or compression that could result in damage.
2. Isolation of smooth muscle cells
- Preheat 1 mL of cell separation solution, 1 mL of enzymatic hydrolysate I, and 1 mL of enzymatic hydrolysate II to 37 °C in test tubes 1, 2, and 3, respectively (see Figure 1B).
- Transfer the isolated basilar artery to test tube 2. Continuously inject a mixture of 95% O2 and 5% CO2 into the tube, and maintain enzymatic treatment for 30 min (see Figure 1C).
- Transfer the basilar artery from test tube 2 to test tube 3. Continue injecting 95% O2 and 5% CO2 into the tube, and maintain enzymatic treatment for 5 min (see Figure 1D).
- Add 1 mL of 37 °C cell separation solution from test tube 1 into test tube 3. Continue injecting 95% O2 and 5% CO2 while maintaining enzymatic treatment for 3 min. Triturate the basilar artery preparation to release cells (see Figure 1E).
- Add 4 °C separation solution to test tube 3 to terminate the enzymatic process (see Figure 1F). Centrifuge the mixture at 59 × g for 6 min. Discard the supernatant using a pipette, add 4 °C separation solution again (see Figure 1G), and repeat centrifugation twice to remove residual enzymes.
- Remove the supernatant using a pipette, and store 1 mL of the cell suspension at 4 °C for up to 6-8 h.
- Take 100 µL of the cell suspension and place it in the bath (see Figure 1H). Add 1 mL of extracellular fluid to the bath and allow the cells to settle for 40 min to attach to the bottom (see Figure 2).
NOTE: For cells in the treatment group (e.g., BaCl2 or sodium nitroprusside), pre-incubate with the respective substances at room temperature (22-26°C) for 40 min during the attachment period.
3. Recording Kir current using whole-cell patch clamp
- Fabrication of micropipettes
- Turn on the micropipette puller (refer to the Table of Materials).
- Place a glass tube (outer diameter: 1.5 mm, inner diameter: 1.10 mm, length: 10 cm) in the puller. Select Program 1, click on Enter, and access Program 1. Perform a ramp test by clicking on Ramp on the control panel to measure the heat value of the glass tube.
NOTE: Edit Program 1 with the following parameters: Heat: Ramp, Pull: 0, Velocity: 25, Delay: 1, Pressure: 500, Mode: Delay, Safe heat enabled. The ramp test must be performed when changing filaments or glass pipette types. Micropipettes should have a tip diameter of ~1-2 µm and a cone length of approximately 5 mm. Smaller tip diameters and longer cones result in higher pipette resistance. - Insert a new glass tube and select Program 1. Click on Enter to fabricate the micropipette.
- Recording Kir Current
- Turn on the hardware devices sequentially: digital-to-analog converter, signal amplifier, micromanipulator, microscope, camera, and computer.
- Launch the software in the following order: camera, signal amplifier, and data acquisition software.
NOTE: If a microscope is not equipped with a camera, only the instrument software needs activation. Ensure software is opened after hardware initialization to enable proper functionality. - In the data acquisition software, select File > Set Data File Names to establish a data storage path.
- Edit the protocol for recording Kir currents as follows:
- Waveform interface: Epoch A & D: Type = step; First level = −60 mV; Delta level = 0 mV; First duration = 100 ms; Delta duration = 0 ms. Epoch B: Type = step; First level = −160 mV; Delta level = 0 mV; First duration = 1 ms; Delta duration = 0 ms. Epoch C: Type = ramp; First level = 40 mV; Delta level = 20 mV; First duration = 500 ms; Delta duration = 0 ms.
- Mode/Rate interface: Trial delay = 0 s; Runs = 1; Sweeps = 1; Sweep duration = 0.8 s.
- Outputs interface: Channel #0; Holding level = −60 mV. Save and name the protocol as "Kir protocol."
NOTE: Protocol setup is tailored for specific current recordings and can be reused after saving. Ensure that the output interface matches the instrument's wiring (e.g., Channel #0).
- Enable the Membrane Test function in the Tools menu of the data acquisition software.
- Position the cell for measurement in the center of the microscope's camera view.
- Immerse the reference wire tip into the bath solution. Fill 20% of the micropipette (fabricated in step 3.1) with the intracellular solution and secure it on the recording electrode holder.
- Apply positive pressure using a syringe, move the pipette tip into the bath solution, and adjust the pipette offset on the signal amplifier software to set the current baseline to 0 pA (see Figure 3A).
NOTE: Use Ag/AgCl reference wires. Pipette resistance should be 4-6 MΩ.
- Apply positive pressure using a syringe, move the pipette tip into the bath solution, and adjust the pipette offset on the signal amplifier software to set the current baseline to 0 pA (see Figure 3A).
- Gently press the pipette against the cell membrane. Observe an increase in seal resistance (Rt) to at least 1 MΩ (Figure 3B). Remove positive pressure and apply negative pressure to establish a high resistance with Rt ≥ 1 GΩ (Figure 3C).
- Set the holding voltage to −60 mV and compensate electrode capacitance using Cp Fast and Cp Slow (Figure 3D).
NOTE: If Rt remains above 1 GΩ, proceed to the next step; otherwise, replace the cell or pipette and repeat steps 3.2.6-3.2.8.
- Set the holding voltage to −60 mV and compensate electrode capacitance using Cp Fast and Cp Slow (Figure 3D).
- Apply brief negative pressure to rupture the cell membrane, forming a whole-cell configuration (Figure 3E). Select Whole Cell in the signal amplifier software, click on Auto for membrane capacitance compensation (Figure 3F), load the Kir protocol, and begin data recording. Repeat steps 3.2.6-3.2.9 for treated cells.
NOTE: If the series resistance (Ra) is >30 MΩ, select a new cell. If 30 MΩ > Ra > 10 MΩ, apply series resistance compensation before recording. This protocol requires Ra < 10 MΩ.
- Analyzing data
- Open the data analysis software and load the recorded data. Use cursors to analyze current traces and export data for I-V curve plotting.
- Create a stimulus waveform signal by selecting Edit > Create Stimulus Waveform Signal and confirm.
- Export the Kir protocol trace by selecting Edit > Transfer Traces, specifying the full trace region and signal (A0 #0), and copying data for further analysis.
- Export representative Kir current traces by selecting Edit > Transfer Traces, specifying the full trace region and signal (IN 0), and copying data to generate current trace plots.
NOTE: Normalize current data using current density (pA/pF) for accurate comparison between cells of varying sizes. Ensure membrane capacitance (Cm) is recorded during the experiment17.
Results
Isolation of arterial smooth muscle cells
The first section of the procedure details the process of isolating smooth muscle cells from the rat's cerebral basilar artery. This process is illustrated in Figure 1. The procedure involves enzymatic digestion and cell separation steps to release smooth muscle cells from the artery.
Representative images of isolated smooth muscle cells
The second section presents a representative diagram of the isolated smooth muscle cells. Figure 2 includes bright-field images of spindle-shaped smooth muscle cells, confirming their identity as smooth muscle cells based on their characteristic shape.
Whole-cell patch clamp technique
The third section outlines the flowchart of the whole-cell patch-clamp technique used to record Kir currents in freshly isolated smooth muscle cells. Figure 3 provides a detailed illustration of the patch clamp setup and procedures for establishing a whole-cell configuration.
Recording Kir current
The fourth part presents the representative Kir current recorded from freshly isolated smooth muscle cells using the whole-cell patch-clamp technique. Figure 4 shows the recorded currents and the effects of different interventions on Kir channel activity. Figure 4A displays the typical Kir current, demonstrating inward rectification at negative voltages, indicating the net influx of K+ into the cell. At positive voltages, there is minimal to no net K+ current flow. Figure 4B illustrates the inhibition of Kir currents by the specific inhibitor BaCl2. The results show that Ba2+ at concentrations of 100-300 µmol/L effectively blocks Kir channels, confirming the current as Kir. Figure 4C demonstrates the effect of sodium nitroprusside (SNP), a nitrovasodilator, on Kir currents. The data show that SNP increases the Kir current, suggesting the involvement of Kir channels in SNP-induced vasodilation. Figure 4F presents the capacitance (Cm) values in the control, BaCl2, and SNP groups. The results indicate no statistical significance in Cm values across these groups.
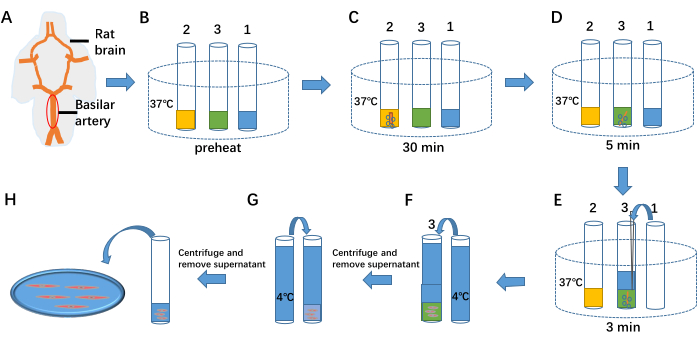
Figure 1: Isolation of arterial smooth muscle cells. (A) The ventral side of the rat brain and basilar artery. (B) Preheating 1 mL of cell separation solution, 1 mL of cell enzymatic hydrolysate I, and 1 mL of cell enzymatic hydrolysate II to 37 °C in test tubes 1, 2, and 3, respectively. (C-E) The three steps of separating cells from the vascular tissue by enzymatic treatment. (F,G) Termination of enzymatic treatment by adding 4 °C separation solution. (H) Cell fluid containing arterial smooth muscle cells. Please click here to view a larger version of this figure.

Figure 2: Arterial smooth muscle cells. (A) Bright-field view of freshly isolated vascular smooth muscle cells, exhibiting spindle morphology. Scale bar: 10 µm. (B) Healthy cells suitable for patch-clamp experiments. Scale bar: 30 µm. Please click here to view a larger version of this figure.
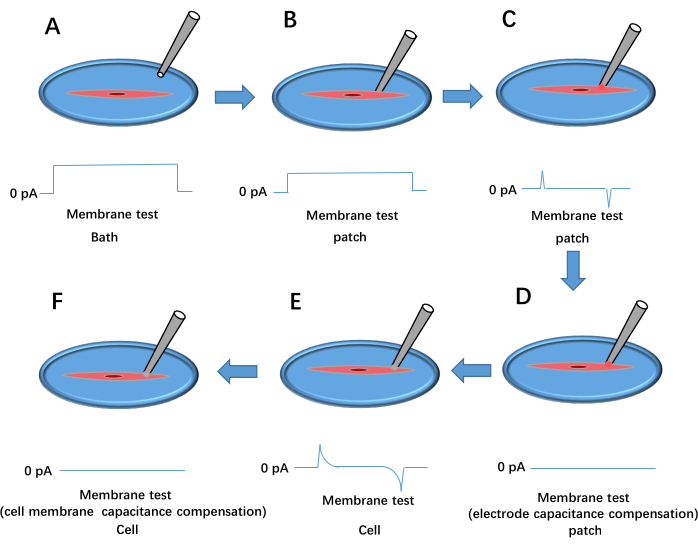
Figure 3: Whole cell patch-clamp formation process. This shows the outcome of the voltage pulse membrane test. (A) The pipette is dropped into the bath. (B) The recording electrode is moved to contact the cell and pressed against the membrane. (C) Formation of a high-resistance seal between the pipette and the cell. (D) Electrode capacitance compensation. (E) Rupture of the cell membrane. (F) Cell membrane capacitance compensation. Please click here to view a larger version of this figure.
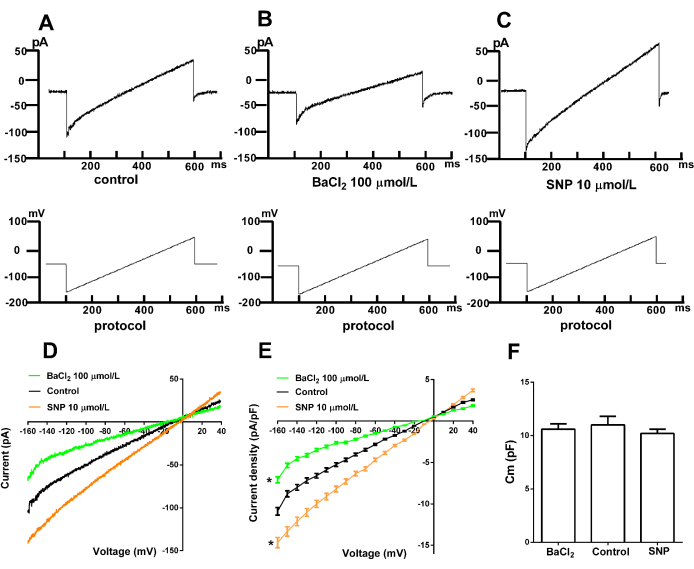
Figure 4. Representative Kir current in arterial smooth muscle cells. (A) Normal Kir current. (B) Inhibition by the specific inhibitor BaCl2. (C) Effect of sodium nitroprusside (SNP) on the Kir current. (D) I-V curve of Kir currents. (E) Statistical results. p < 0.05, BaCl2 (n = 6) and SNP (n = 6) versus the control group (n = 6); differences were analyzed by two-tailed two-way ANOVA. (F) Cm (pF), p > 0.05, BaCl2 (n = 6), and SNP (n = 6) versus the control group (n = 6); differences were analyzed by t-test. Stimulation protocol: Holding at -60 mV with ramp stimulation from -160 mV to +40 mV for 500 ms. Control, BaCl2, and SNP were conducted separately in different groups of cells. Compounds were added 40 min prior to recordings and could remain in the bath for up to 20 min during the recording. SNP: sodium nitroprusside. Please click here to view a larger version of this figure.
| Solution type | Solution composition | Key points | ||
| Physiological salt solution (PSS, mmol/L) | 118 NaCl, 4.7 KCl, 2.5 CaCl2, 1.2 KH2PO4, 1.2 MgCl2, 25 NaHCO3, 11 D-glucose, 5 HEPES | Saturate the solution and bubble it with a mixed gas consisting of 95% O2 and 5% CO2 before use. Then, adjust the solution's pH to 7.4 using NaOH. Temperature for making and storing it at room temperature and 4°C, respectively | ||
| Cell separation solution (mmol/L) | 118 NaCl, 4.7 KCl, 2.5 CaCl2, 1.2 KH2PO4, 1.2 MgCl2, 25 NaHCO3, 11 D-glucose, 5 HEPES | Same as the Physiological salt solution | ||
| Cell enzymatic hydrolysate I | Bovine serum albumin (BSA): 1 mg/mL, Dithiothreitol(DTT): 1 mg/mL and papain: 0.5 mg/mL were added to cell separation solution | Prepare the solution immediately for use. Temperature for making it at room temperature | ||
| Cell enzymatic hydrolysate II | BSA: 1 mg/mL, collagenase H: 0.3 mg/mL and collagenase F: 0.7 mg/mL were added into the cell separation solution | Same as the Cell enzymatic hydrolysate I | ||
| Kir current bath solution (mmol/L) | 12 NaCl, 130 KCl, 0.4 KH2PO4 , 0.3 NaH2PO4, 2 NaHCO3 , 1 MgCl2 , 1.8 CaCl2 , 10 HEPES, 5.5 glucose | Adjusted to a pH of 7.4 using NaOH and an osmolarity of 310 mOsm. Temperature for making and storing it at room temperature and 4°C, respectively | ||
| Kir current pipette-filling solution(mmol/L) | 100 potassium-D-gluconate, 30 KCl, 1 MgCl2 , 1 EGTA, 15 HEPES, 1 Na2-ATP | Adjusted to a pH of 7.2 using KOH and an osmolarity of 300 mOsm. Temperature for making and storing it at room temperature and -20°C, respectively | ||
Table 1: Composition and preparation precautions for various solutions.
Discussion
Whole-cell recording using freshly isolated cells dates back to the early 1980s18, and the recording of channel currents from rodent basilar smooth muscle cells became widely practiced in the 1990s19. With technological advancements, researchers are increasingly focused on the results achieved through these technologies. However, the attention given to updating and summarizing technical methods has gradually diminished. This paper introduces a detailed method for the fresh separation of vascular smooth muscle cells and the subsequent use of these cells to record Kir currents, aiming to assist researchers in understanding the standard methodological process.
Vascular smooth muscle plays a crucial role in regulating arterial tension. Numerous methods for separating vascular smooth muscle cells have been reported20,21,22,23,24,25, most of which involve enzymatic hydrolysis. However, the types and steps of enzymes vary across studies, and different experimental conditions can influence enzymatic efficiency. This article presents an enzymatic hydrolysis method, with slight modifications to enzyme types and procedures compared to previous studies. It provides a detailed protocol for the acute separation of primary vascular smooth muscle cells.
The key steps of this protocol are steps 2.2 to 2.4, as the successful completion of these steps directly impacts the quality of the isolated cells. The isolated vascular smooth muscle cells are suitable for various applications, including patch-clamp experiments to study ion channels, RNA and protein extraction for molecular studies and immunofluorescence to investigate protein expression on cells. Consequently, this method offers a versatile approach for relevant researchers, providing more experimental options.
Potassium (K+) conductance on the membrane of arterial smooth muscle cells plays a crucial role in regulating membrane potential, vascular tension, and, ultimately, local blood flow26,27. The Kir channel, a type of K+ channel found in arterial smooth muscle cells, helps stabilize membrane potential and acts as an electrical amplifier for other K+ channels. When activated, the Kir channel induces membrane hyperpolarization and promotes vascular dilation28. Patch clamp technology has long been considered the gold standard for ion channel research29,30, with whole-cell patch clamp being the most commonly used method31. While this technique demands a high level of operator skill, proficiency ultimately depends on following standardized protocols.
This study presents a detailed, standardized procedure for recording Kir currents from freshly isolated vascular smooth muscle cells using a whole-cell patch clamp, providing a valuable reference for researchers. Critical steps in the protocol are steps 3.2.7 to 3.2.9, as the successful completion of these steps determines whether subsequent Kir current recordings will be successful. Additionally, the selection of intracellular and bath fluids significantly impacts the recorded current. For instance, the use of extracellular high potassium in this study was intended to fully activate the inward rectifier potassium channel. Therefore, the success of the experiment depends on careful attention to every operational detail.
While freshly isolated cells offer more physiological relevance than cell lines, there are inherent limitations. These include the influence of environmental temperature on enzymatic separation, varying digestion times for blood vessels of different diameters, and the instability of the cells' native environment. As a result, enzymatic digestion times often need to be adjusted. Patch clamp technology also poses technical challenges, particularly in the sealing and membrane rupture steps, which require high operator skill. The state of the cell directly impacts the success of the entire patch-clamp procedure. Consequently, careful attention to these limitations is essential for ensuring the success of the experiment.
Ba2+ has been widely used as a specific blocker to study the role of Kir channels in cells and tissues. At concentrations below 100 µmol/L, Ba2+ selectively blocks Kir channels, while concentrations as high as 300 µmol/L can completely block them32. In this study, BaCl2 was used to verify the Kir channel current.
Kir channels are regulated by both vasoconstrictors and vasodilators. SNP (sodium nitroprusside), a nitrovasodilator, was used in this study to explore this. SNP is metabolized in vascular smooth muscle to produce nitric oxide, which reduces systemic vascular resistance by acting on both venous and arterial smooth muscle, potentially enhancing cardiac output. Due to its rapid onset and short half-life, SNP is commonly used as a first-line agent for preventing and treating hypertension33. The study observed that SNP augmented the Kir current in basilar artery smooth muscle cells, suggesting the activation of Kir channels during SNP-induced vasodilation.
A limitation of the present recordings is that they were obtained from different groups of cells, and comparisons were made by analyzing current densities between the groups. A more refined approach would involve recording the responses of the same cells before and after the application of the compounds, which would minimize the impact of cell-to-cell variability on data interpretation.
In conclusion, this article provides a detailed protocol for the acute isolation of rat basilar artery smooth muscle cells and the use of whole-cell patch clamp to study Kir currents. This protocol is versatile and can be applied to smooth muscle cells from other arteries, such as the coronary, mesenteric, and renal arteries. However, the enzymatic separation time may need to be adjusted depending on the type of artery. Additionally, isolated arterial smooth muscle cells can be employed in a variety of other experimental techniques.
Disclosures
The authors have no conflicts of interest to disclose.
Acknowledgements
This work was supported by the Special Talent Program of Chengdu University of Traditional Chinese Medicine for "Xinglin Scholars and Discipline Talents Research Promotion Plan" (33002324) and Key Research and Development Project for Introducing High-level Scientific and Technological Talents in Luliang City (2022RC28).
Materials
| Name | Company | Catalog Number | Comments |
| Bovine serum albumin | Sigma, USA | B2064 | |
| Barium chloride | Macklin Biochemical Co.,Ltd.,Shanghai, China | B861682 | |
| CaCl2 | Sangon Biotech Co., Ltd., Shanghai, China | A501330 | |
| Camera | Hamamatsu, Japan | C11440 | |
| Camera software | Image J, USA | Micro-manager 2.0.0-gammal | |
| Collagenase F | Sigma, USA | C7926 | |
| Collagenase H | Sigma, USA | C8051 | |
| Computer | Lenovo, China | ~ | |
| Data acquisition software | Molecular Devices, USA | Clampex 10.4 | |
| Data analysis software | Axon, USA | clampfit 10.4 | |
| D-glucose | Sangon Biotech Co., Ltd., Shanghai, China | A610219 | |
| Digital-analog converter | Molecular Devices, USA | Axon digidata 1550B | |
| Dithiothreitol | Sigma, USA | D0632 | |
| Drawing software | San Diego, California, USA | GraphPad | |
| EGTA | Sangon Biotech Co., Ltd., Shanghai, China | A600077 | |
| Glass tube | DL Naturegene Life Sciences.USA | B150-86-10 | |
| HEPES | Xiya Reagent Co., Ltd., Shandong, China | S3872 | |
| KCl | Sangon Biotech Co., Ltd., Shanghai, China | A100395 | |
| KH2PO4 | Sangon Biotech Co., Ltd., Shanghai, China | A100781 | |
| MgCl2·6H2O | Sangon Biotech Co., Ltd., Shanghai, China | A100288 | |
| Micromanipulator | sutter, USA | MP285A | |
| Micropipette puller | sutter, USA | P1000 | |
| Microscope | Olympus, Japan | IX73 | |
| Na2-ATP | Sigma, USA | A26209 | |
| Na2HPO4 | Sangon Biotech Co., Ltd., Shanghai, China | A610404 | |
| NaCl | Sangon Biotech Co., Ltd., Shanghai, China | A100241 | |
| NaH2PO4 | Sangon Biotech Co., Ltd., Shanghai, China | A600878 | |
| NaHCO3 | Sangon Biotech Co., Ltd., Shanghai, China | A100865 | |
| NaOH | Sangon Biotech Co., Ltd., Shanghai, China | A100173 | |
| Papain | Sigma, USA | P4762 | |
| Potassium-D-gluconate | Sangon Biotech Co., Ltd., Shanghai, China | A507810 | |
| Signal amplifier | Molecular Devices, USA | Axon MutiClamp 700B | |
| Signal amplifier software | Molecular Devices, USA | MultiClamp Commander software | |
| Sodium nitroprusside | Sangon Biotech Co., Ltd., Shanghai, China | A600867 | |
| Statistical analysis software | San Diego, California, USA | GraphPad |
References
- Goins, R. T., et al. Lower body functioning and correlates among older american indians: The cerebrovascular disease and its consequences in american indians study. BMC Geriatrics. 18 (1), 1-9 (2018).
- Mattle, H. P., Arnold, M., Lindsberg, P. J., Schonewille, W. J., Schroth, G. Basilar artery occlusion. Lancet Neurol. 10 (11), 1002-1014 (2011).
- Morales, A., Parry, P. V., Jadhav, A., Jovin, T. A novel route of revascularization in basilar artery occlusion and review of the literature. BMJ Case Reports. , 1-6 (2015).
- Ghantous, C. M., Azrak, Z., Rahman, F. A., Itani, H. A., Zeidan, A. Assessment of basilar artery reactivity in stroke and subarachnoid hemorrhage using wire myograph. Methods Mol Biol. 34, 625-643 (2016).
- Tykocki, N. R., Boerman, E. M., Jackson, W. F. Smooth muscle ion channels and regulation of vascular tone in resistance arteries and arterioles. Compr Physiol. 7 (2), 485-581 (2017).
- Wu, B. -. N., et al. Hyposmotic challenge inhibits inward rectifying K+ channels in cerebral arterial smooth muscle cells. Am J Physiol Heart Circ Physiol. 292 (2), H1085-H1094 (2007).
- Jackson, W. F. Ion channels and vascular. Hypertension. 35 (2), 173-178 (2000).
- Jackson, W. F. Potassium channels in the peripheral microcirculation. Microcirculation. 12 (1), 113-127 (2005).
- Dogan, M. F., Yildiz, O., Arslan, S. O., Ulusoy, K. G. Potassium channels in vascular smooth muscle: A pathophysiological and pharmacological perspective. Fundam Clin Pharmacol. 33 (5), 504-523 (2019).
- Daghbouche-Rubio, N., López-López, J. R., Pérez-García, M. T., Cidad, P. Vascular smooth muscle ion channels in essential hypertension. Front Physiol. 13, 1-9 (2022).
- Sahranavard, T., et al. The role of potassium in atherosclerosis. Eur J Clin Invest. 51 (3), 1-19 (2020).
- Crecelius, A. R., Dinenno, F. A. Vascular regulation via kir channels and Na+/K+-ATPase. Channels. 9 (4), 171-172 (2015).
- Liu, Y., et al. Prostanoids contribute to regulation of inwardly rectifying K+ channels in intrarenal arterial smooth muscle cells. Life Sci. 250, 1-9 (2020).
- Li, W., et al. Luteolin-induced coronary arterial relaxation involves activation of the myocyte voltage-gated K+ channels and inward rectifier K+ channels. Life Sci. 221, 233-240 (2019).
- Guo, P., et al. Coronary hypercontractility to acidosis owes to the greater activity of tmem16a/ano1 in the arterial smooth muscle cells. Biomed Pharmacother. 139, 1-14 (2021).
- Jing, Y., et al. Apigenin relaxes rat intrarenal arteries, depresses Ca2+-activated Cl− currents and augments voltage-dependent K+ currents of the arterial smooth muscle cells. Biomed Pharmacother. 115, 1-9 (2019).
- Manz, K. M., Siemann, J. K., Mcmahon, D. G., Grueter, B. A. Patch-clamp and multi-electrode array electrophysiological analysis in acute mouse brain slices. STAR Protocols. 2 (2), 1-30 (2021).
- Hamill, O. P., Marty, A., Neher, E., Sakmann, B., Sigworth, F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 391 (2), 85-100 (1981).
- Langton, P. D., Standen, N. B. Calcium currents elicited by voltage steps and steady voltages in myocytes isolated from the rat basilar artery. J Physiol. 469, 535-548 (1993).
- Kittiwoot, T. -. O., et al. Isolation of intrapulmonary artery and smooth muscle cells to investigate vascular responses. J Vis Exp. (184), e63686 (2022).
- Trask, A. J., Lucchesi, P. A., Mccallinhart, P. E., Zhang, X., Husarek, K. E. Isolation of murine coronary vascular smooth muscle cells. J Vis Exp. 111, e53983 (2016).
- Ribeiro, M. P., Relvas, R., Chiquita, S., Correia, I. J. Isolation of human umbilical arterial smooth muscle cells (HUASMC). J Vis Exp. (41), e1940 (2010).
- Kim, H. J., et al. Increased inward rectifier K+ current of coronary artery smooth muscle cells in spontaneously hypertensive rats; partial compensation of the attenuated endothelium-dependent relaxation via Ca2+-activated K+ channels. Clin Exp Pharmacol Physiol. 47 (1), 38-48 (2019).
- Qiao, Y., et al. Kir2.1 regulates rat smooth muscle cell proliferation, migration, and post-injury carotid neointimal formation. Biochem Biophys Res Commun. 477 (4), 774-780 (2016).
- Tykocki, N. R., Bonev, A. D., Longden, T. A., Heppner, T. J., Nelson, M. T. Inhibition of vascular smooth muscle inward-rectifier K+channels restores myogenic tone in mouse urinary bladder arterioles. Am J Physiol Renal Physiol. 312 (5), F836-F847 (2017).
- Ko, E. A., Han, J., Jung, I. D., Park, W. S. Physiological roles of K+ channels in vascular smooth muscle cells. Smooth Muscle Res. 44 (2), 65-81 (2008).
- Standen, N. B., Quayle, J. M. K+ channel modulation in arterial smooth muscle. Acta Physiol Scand. 164, 549-557 (1998).
- Smith, P. D., et al. Kir channels function as electrical amplifiers in rat vascular smooth muscle. J Physiol. 586 (4), 1147-1160 (2008).
- Kanda, H., Tonomura, S., Dai, Y., Gu, J. G. Protocol for pressure-clamped patch-clamp recording at the node of Ranvier of rat myelinated nerves. STAR Protocols. 2 (1), 1-12 (2021).
- Witchel, H. J., Milnes, J. T., Mitcheson, J. S., Hancox, J. C. Troubleshooting problems with in vitro screening of drugs for qt interval prolongation using HERG K+ channels expressed in mammalian cell lines and Xenopus oocytes. J Pharmacol Toxicol Methods. 48 (2), 65-80 (2002).
- Kodandaramaiah, S. B., Franzesi, G. T., Chow, B. Y., Boyden, E. S., Forest, C. R. Automated whole-cell patch-clamp electrophysiology of neurons in vivo. Nat Methods. 9 (6), 585-587 (2012).
- Park, W. S., Han, J., Earm, Y. E. Physiological role of inward rectifier K+ channels in vascular smooth muscle cells. Pflugers Arch. 457 (1), 137-147 (2008).
- Thomas, C., Svehla, L., Moffett, B. S. Sodium nitroprusside induced cyanide toxicity in pediatric patients. Expert Opin Drug Saf. 8 (5), 599-602 (2009).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved