Method Article
Two-Dimensional Super-Resolution Visualization of Rat Brain Microvasculature Using Ultrasound Localization Microscopy
In This Article
Summary
Here, we describe a protocol for ultrasound localization microscopy (ULM), which achieves 12.5 µm spatial resolution to image the brain microvasculature in rats. It enables detailed visualization of blood flow direction and velocity, offering a powerful tool for advancing studies of cerebral circulation and vascular disorders.
Abstract
The cerebral microvasculature forms a complex network of vessels essential for maintaining brain function. Diseases such as stroke, Alzheimer's disease, gliomas, and vascular dementia can profoundly disrupt the microvascular system. Unfortunately, current medical imaging modalities offer only indirect observations at this scale. Inspired by optical microscopy, ultrasound localization microscopy (ULM) overcomes the classical trade-off between penetration depth and spatial resolution. By localizing and tracking individual injected microbubbles (MBs) with sub-wavelength precision, vascular and velocity maps can be generated at the micrometer scale. Here, we present a robust protocol for super-resolution imaging of the brain microvasculature in vivo in rats using a commercial ultrasound platform. This method achieves 12.5 µm spatial resolution, reconstructing the microvascular architecture and providing detailed information on blood flow direction and velocity, greatly enhancing our understanding of cerebral microcirculation. The protocol can be extended to rat disease models, offering a powerful tool for the early diagnosis and treatment of neurovascular diseases.
Introduction
The cerebral microvasculature, comprising capillaries, arterioles, and venules, is essential for maintaining brain function by facilitating nutrient delivery, oxygen exchange, and waste removal1,2. Disruptions in this network are implicated in neurological disorders such as stroke3, Alzheimer's disease4, gliomas5, and vascular dementia6, leading to impairments in brain physiology. Microvascular changes frequently precede the onset of clinical symptoms, making them a critical target for diagnostic and therapeutic interventions7,8. A comprehensive understanding of vascular alterations at both structural and functional levels is key to advancing research and treatment strategies.
However, imaging the cerebral microvasculature is particularly challenging due to the small size and partly deep location within the brain. Conventional imaging modalities like magnetic resonance imaging (MRI)9 and computed tomography (CT)10, while adequate for capturing large-scale vascular changes, offer a spatial resolution (~100 µm) that is far too coarse for visualizing small vessels. Optical methods like two-photon microscopy11 provide excellent spatial resolution (down to 1 µm) to image individual capillaries but are hindered by limited field of view and penetration depth (less than 1 mm), restricting their ability to image deep brain regions. As an ultrasound-based technique, Doppler12, while offering real-time blood flow assessment, remains constrained by a resolution of 50-200 µm, insufficient for microvascular detail. Overall, no single method currently meets the dual requirement of high spatial resolution and sufficient brain penetration necessary for cerebral microvasculature imaging.
Inspired by optical microscopy13,14, ultrasonic localization microscopy (ULM) allows visualization of fine structures on the micron scale by locating individual injected microbubbles (MBs) and tracking their displacement with subwavelength resolution15. It bypasses the classic compromise between penetration and resolution in ultrasound imaging16. This study details a robust protocol for implementing ULM in a living rat model and thereby enabling super-resolution imaging of the brain microvasculature through the commercially available ultrasound platform. The protocol not only provides a comprehensive reconstruction of the microvascular structure but also provides detailed information about the direction and velocity of blood flow, which is not possible with conventional imaging techniques. Although the protocol was validated in normal rats, it is extendable to rat disease models, offering possibilities for customized studies in different pathological conditions.
Protocol
All animal experiments performed in this work are approved by the Ethics Committee of Fudan University (Approval Number: 2022JS-004). The protocol strictly follows the animal care guidelines of Fudan University to ensure the humane treatment of animals. Prior to experimental initiation, rats must be allowed a 1-week period for environmental acclimatization, during which they are provided with sufficient feed and water. The photoperiod is carefully regulated in accordance with their biological rhythms to ensure the maintenance of normal physiological states. At the end of the experiment, euthanasia is performed using an overdose of inhaled isoflurane.
NOTE: The experimental setup is shown in Figure 1A-H.
1. Animal preparation for ULM imaging
- Anesthesia
- Induce anesthesia in the rat by administering a mixture of 3.5% isoflurane and 100% oxygen at a flow rate of 3 L/min. After approximately 4 min, remove the animal from the chamber and place it prone on an imaging platform pre-warmed to 37 °C. Confirm the depth of anesthesia by the absence of a withdrawal reflex upon the firm toe pinch of the rat's hind limb.
- Place the rat's nose in a breathing mask connected to the anesthesia system. Maintain a stable sedation by administering a mixture of 1.5% isoflurane and 100% oxygen at a flow rate of 1.5 L/min.
NOTE: Anesthesia methods and dosages strictly follow the manufacturer's guidelines.
- Pre-surgical animal preparation
- Secure the rat's upper incisors in the notch of the incisor bar, positioning the lower jaw beneath the bar. Use ear bars to stabilize the rat's head by positioning them in the bony indentation slightly anterior and superior to the ear canal (palpable by hand), ensuring the whole cranial surface remains horizontal (Figure 1A).
- Gently probe the stability of the fixation by applying a small amount of pressure to different parts of the head. Ensure that there is no movement or slipping from the original position.
- Fine-tune the height of the imaging platform and meticulously adjust the angle of the rat's head by raising or lowering the incisor bar notch to ensure unobstructed breathing.
- Apply erythromycin ointment or 30% glycerin solution to the rat's eyes to prevent damage from prolonged exposure to surgical lights and to keep the eyes moist under anesthesia. Additionally, use round-tip tweezers to gently pull the rat's tongue to one side of the mouth to prevent asphyxiation during the surgery.
- Use a handheld electric clipper and shave the rat's head against the direction of hair growth (from back to front), typically between the ears, from the eyes to the beginning of the neck (Figure 1B). Clean the shaved area with 1% iodine solution to prepare for the surgical incision.
- Rat craniotomy
- Make an incision along the sagittal suture of the rat's skull, starting from just behind the occipital bone and extending approximately 4 cm anteriorly. Use hemostats to retract the skin on both sides (Figure 1C). Optionally, entirely excise the skin over the skull to provide greater surgical access, but this may increase the risk of infection.
- Use small scissors to remove the periosteum from the skull, fully exposing the hard bone layer.
- Perform the craniotomy using a handheld mini cranial drill equipped with a spherical drill bit (Figure 1D). Start with a coarse drill bit (2.5 mm diameter) and gently tap to abrade the bone, applying the drill for 2-3 s at a time. Begin in the central area and progress towards the thicker areas on the sides.
NOTE: Excessive bleeding during craniotomy impacts blood circulation, resulting in a lack of vascular reconstruction in cortical areas (Figure 1H). - Pause every 2-3 s to prevent overheating, and every 2 min, inject approximately 1 mL of 0.9% NaCl into the drilling area using a syringe to cool and rinse away debris.
- Once the white bone tissue no longer appears consistently connected, switch to a finer drill bit (1 mm diameter). Continue drilling until the central major blood vessels are clearly visible as dark brown, and the surrounding area appears pink, with microvessels visible as slightly reddish (Figure 1F).
NOTE: The final craniotomy range is from Bregma +3 mm to Lambda anterior-posteriorly and 7 mm on each side along the midline of the skull (Figure 1E). After completing the surgery, allow the rat to rest for approximately 10 min to ensure relatively stable physiological conditions before beginning data collection.
2. Setup before data collection
- Contrast agent preparation and injection
- Dissolve the contrast agent (one vial containing 59 mg of SF6 gas and 25 mg of lyophilized powder), per the official guidelines, in 5 mL of 0.9% NaCl. Vigorously shake the mixture to form an MB suspension. The final concentration of SF6 in the suspension is 8 µL/mL.
- Draw 0.8 mL of the MB suspension into a 1 mL syringe and secure it to a microinjection pump programmed to infuse at 100 µL/min.
- Insert a 26 G indwelling needle with a catheter attached into the tail vein of the rat (Figure 1G).
- Selecting the imaging plane
- Mount a probe with a central frequency of 15.625 MHz on the manipulator arm of the brain stereotaxic instrument, equipped with a clamp (movement range: vertical, horizontal, and anterior-posterior up to 80 mm; reading precision: 0.1 mm).
- Position the ultrasound probe directly above the exposed rat brain. Apply coupling gel to the exposed brain surface to ensure optimal signal transmission.
- Open the software's main interface, which integrates a rat brain atlas with motor motion control programs.
- Set the Bregma point as the origin. The software interface provides a real-time display of the probe's trajectory and the corresponding rat brain slice location. Select the target imaging plane; for example, Bregma -1 mm.
3. Data collection (Timing ~ 20 min)
NOTE: Verasonics (ultrasound system) provides the original MATLAB scripts for use with the Vantage system and has not been modified.
- Software startup
- Launch MATLAB 2021a software.
- Enter the data acquisition script in MATLAB 2021a.
- In the root directory, type activate in the command line window to activate the runtime environment.
- Parameter configuration and RF signals acquisition
- Set the data collection start and end depths at 5 and 120 wavelengths, respectively, to effectively capture the region of interest.
NOTE: Adjustments should be made based on the imaging plane and the specific animal being studied. - Set the plane wave transmission steering angles from -5° to 5° in 2.5° increments to enhance image resolution and contrast.
NOTE: Adjust these settings based on specific imaging requirements and target characteristics. - Set the transmit voltage to 20 V to ensure adequate penetration and optimal signal-to-noise ratio for the region of interest.
- Click the Run button to start data collection and save the radio frequency (RF) signals in .mat file format.
NOTE: Begin data collection 30 s after starting the microinjection pump to ensure uniform and stable MB distribution within the rat's body.
- Set the data collection start and end depths at 5 and 120 wavelengths, respectively, to effectively capture the region of interest.
4. Data processing and analysis (Timing ~ 8 h)
- Data processing
- Import the RF data into MATLAB 2021a and demodulate it to generate In-phase and Quadrature (I/Q) data (see Table of Materials).
- Click the Run button to utilize the Delay-and-Sum (DAS) algorithm for beamforming on the I/Q data (see Table of Materials).
NOTE: Post-compounding frame rate of 800 Hz, with a total of 120,000 frames.
- ULM imaging
- Apply the Singular Value Decomposition (SVD) spatiotemporal filtering algorithm17 to IQ data to remove background noise and clutter. Each 800 frames is stored as a data block, and the SVD threshold is set to [15, 800].
- Locate the center of each MB using the Gaussian fitting algorithm18.
- Utilize the Kuhn-Munkres algorithm to track MB trajectories across consecutive frames based on their positions (see Table of Materials).
- Map MB trajectories onto an 8x upsampled grid, using a hot colormap to display the number of MB trajectories and a jet colormap to encode flow speed by mapping the calculated velocity at each position within the microvasculature.
- Apply a custom color scheme to distinguish upward and downward flow directions, resulting in high-resolution images for enhanced visualization.
- Randomly split the original MB trajectories into two groups to create two sub-images, perform a Fourier transform on each, and compute the Fourier Ring Correlation (FRC) as the normalized correlation of their spectra. Define the resolution as the inverse of the spatial frequency where the FRC falls below the threshold19.
Results
Figure 1 illustrates the detailed setup for in vivo brain microvascular ULM imaging in rats, with each element carefully designed to minimize experimental variability and ensure accurate data acquisition for reliable super-resolution imaging results.
Figure 2A displays the ULM-reconstructed structure of the microvasculature in the rat brain, positioned at -1 mm from the Bregma point, with an imaging depth nearing 12 mm. The effective slice thickness across the imaging plane ranges from 0.1 mm to 0.3 mm. Both shallower and deeper microvessels are clearly visible, and the image quality does not deteriorate with increased depth (Figure 2B). By calculating the full width at half maximum (FWHM) of the intensity distribution along the dashed line in the regions of interest (ROI), vessels of various diameters can be detected, with the smallest being 13 µm (Figure 2C). Applying Fourier ring correlation (FRC) for resolution assessment, the spatial resolution of the rat brain microvasculature imaging is quantified as 12.5 µm (Figure 2D).
Blood flow information is crucial for reflecting physiological responses and diagnosing diseases. Figure 3A illustrates the blood flow directions in a cross-sectional slice of the rat brain, where blue indicates flow towards the probe, and red indicates flow away from the probe. Based on this, specific brain regions such as small arteries in the cortex flowing downward and small veins flowing upward can be differentiated20 (Figure 3B). Figure 3C shows a brain blood flow velocity map coded in different colors, with larger vessels displaying noticeably higher flow rates. The distribution of velocities ranges from 1 to 80 mm/s, predominantly concentrated within the 10-25 mm/s range (Figure 3D), which represents 81.57% of the entire velocity range. This proportion is calculated by determining the number of velocity data points within the 5-25 mm/s range relative to the total number of data points in the velocity matrix.
Figure 4 presents the imaging results of a glioblastoma rat model using the proposed protocol. The C6 glioblastoma cells were implanted in the rat brain. Figure 4A shows the microvascular structure in the brain of a glioblastoma rat model, with abnormal vascular dilation and structural irregularities observed around the tumor. The vessels in the tumor region exhibit greater tortuosity compared to the normal area on the left side. Figure 4B provides information on blood flow direction, allowing for an understanding of flow patterns within the tumor region. Figure 4C displays a blood flow velocity map, revealing heterogeneity in vascular flow within and surrounding the tumor.
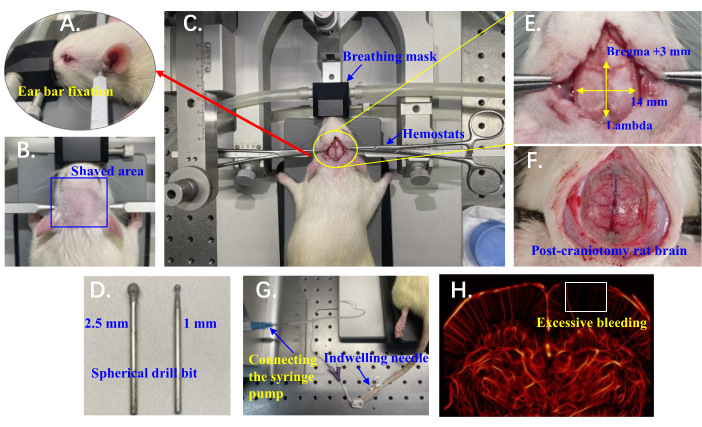
Figure 1: Experimental setup details for in vivo ULM imaging of rat brain microvasculature. (A) Position of ear bars used to stabilize the rat's head. (B) The shaved area on the rat's head prepared for surgical access. (C) Rat positioned on the stereotaxic instrument with a breathing mask; hemostats are used to retract the skin on both sides. (D) Spherical drill bits (2.5 mm and 1 mm) mounted on the cranial drill for craniotomy. (E) Craniotomy area marked relative to Bregma and Lambda. (F) Exposed rat brain following craniotomy. (G) An indwelling needle inserted into the rat's tail vein and connected to a syringe pump for microinjection. (H) Example of rat brain vasculature with excessive bleeding; the white box highlights a region lacking vascular reconstruction. Please click here to view a larger version of this figure.
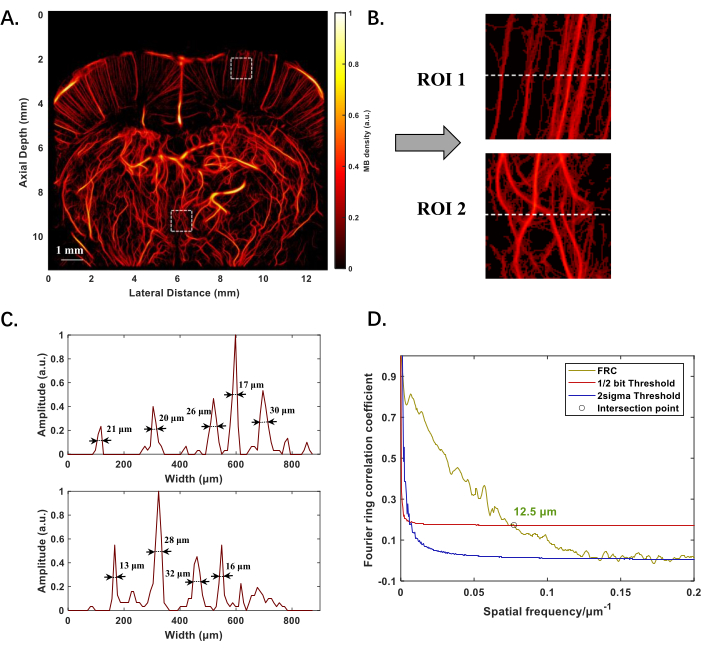
Figure 2: Experimental results of ULM reconstruction of the microvascular structure of the rat brain in vivo. (A) Microvascular structure of the rat brain at -1 mm from the Bregma point. (B) Magnified images of two regions of interest (ROI) at shallower and deeper locations in A, to highlight the morphology of small vessels. (C) Intensity distribution along the dashed line in B, with numerical values indicating vessel diameters measured using full width at half maximum (FWHM). (D) Evaluation of ULM reconstruction performance using the Fourier ring technique, the 1/2-bit was chosen to standardize the resolution without sacrificing image quality, which defines the highest spatial frequency where the correlation still indicates statistically meaningful information. The "○" marks the intersection point of the correlation curve and the 1/2-bit threshold, indicating the resolution limit. Please click here to view a larger version of this figure.

Figure 3: Experimental results of ULM reconstruction of the microvascular blood flow direction and velocity in the rat brain in vivo. (A) Blood flow direction in the rat brain. Blue indicates upward flow towards the probe, and red indicates downward flow away from the probe. (B) Small arteries and veins in the rat brain cortical regions delineated based on the direction of blood flow. (C) Blood flow velocity in the rat brain. (D) Velocity histogram of blood flow in the rat brain, showing the distribution from 1 mm/s to 80 mm/s. The light blue bars represent the 5-25 mm/s velocity range. The red line represents the fitted curve to the data, illustrating the typical velocity distribution profile. Please click here to view a larger version of this figure.

Figure 4: Experimental results of ULM reconstruction of the microvascular structure in the rat brain glioblastoma model in vivo. (A) Microvascular structure of the rat brain. (B) Blood flow direction in the rat brain. (C) Blood flow velocity in the rat brain. Please click here to view a larger version of this figure.
Discussion
This protocol successfully utilized ULM to perform super-resolution imaging of in vivo rat brain microvasculature. Compared to other imaging modalities, ULM simultaneously accommodates both spatial resolution and penetration depth. The exposed rat brain was imaged rather than through the skull, avoiding attenuation and distortion caused by the presence of bone. Under a transducer with a central frequency of 15.625 MHz, vascular structures at a depth of approximately 12 mm were captured, with a spatial resolution of up to 12.5 µm. The direction of blood flow facilitated the differentiation of specific regions of small arteries and veins. Additionally, the technique supports a wide range of flow velocity measurements (1-80 mm/s).
The craniotomy performed on rats is critical in this protocol. During the surgery, it is essential to minimize excessive bleeding. On one hand, excessive bleeding can impact blood circulation, a typical manifestation being the absence of vascular reconstruction in cortical areas. On the other hand, it can also cause physiological changes or even death in the experimental animals. The stereotactic device used in the procedure features an automated craniotomy program that can reduce trauma and increase the success rate but relies heavily on the experience of the practitioner. This is because precise determination of the drill site's location and depth is necessary to avoid causing damage or secondary injuries to the rat brain. While craniotomy is not always required, transcranial ULM imaging shows promise but requires robust compensation or distortion correction algorithms. Additionally, a microinjection pump is employed to administer MBs steadily, ensuring that they remain sparse and stable within the rat's vascular system. This approach is friendly for conventional localization techniques, as it facilitates high-quality ULM reconstructions. However, it extends both data acquisition and imaging duration, rendering ULM imaging a protracted process. An alternative strategy involves the use of high-density MB injections, which necessitates the implementation of advanced algorithms, such as deep learning21,22, to maintain image resolution without degradation.
Isoflurane anesthesia was used following the dosage recommended by the equipment manufacturer to maintain physiological stability in the rat. However, as documented in previous studies, isoflurane anesthesia has known effects on the circulatory system, potentially influencing cardiovascular parameters such as blood pressure and heart rate23,24. These changes may introduce variability in blood flow dynamics, potentially affecting the accuracy of blood flow measurements.
In conclusion, this protocol demonstrates the extensive application potential of ULM, providing a reference for prospective brain disease research based on small animal models. It holds significant value for understanding pathophysiological changes at the microvascular level and for assessing the response of disease progression to treatments.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported in part by the National Key Research and Development Program of China under Grant 2023YFC2410903, the National Natural Science Foundation of China (Grants 12274092, 12034005), the Explorer Program of Shanghai (Grant 21TS1400200), the International Science and Technology Cooperation Program of Shanghai (Grant 24490710400), and the AI for Science Foundation of Fudan University (Grant FudanX24AI016).
Materials
| Name | Company | Catalog Number | Comments |
| Alcohol | DICHANG | https://www.dehsm.com/goods-17187.html | 75% |
| Beamforming program | Institute of Biomedical Engineering at the University of Montreal | Matlab Ultrasound Toolbox 3.4 version | |
| Body temperature maintenance device | RWD Life Science Co., Ltd. | 69026 | |
| Brain stereotaxic instrument | RWD Life Science Co., Ltd. | 71000-R | Adaptable to breathing mask |
| Cranial Microinjection Surgical Instrument Kit | RWD Life Science Co., Ltd. | SP0005-R | |
| Digital microscope | RWD Life Science Co., Ltd. | DOM-1001 | |
| Drug delivery catheter | RWD Life Science Co., Ltd. | https://www.rwdls.com/product-solutions/life-sciences/administration/draw-blood | |
| Erythromycin ointment | Renhe Pharma | H36020018 | 1% x 15 g |
| Gas anesthesia machine | RWD Life Science Co., Ltd. | R500IE | Includes breathing mask |
| Handheld electric clipper | GUAZHOUMU | MJD-DTJ02 | |
| Handheld mini cranial drill | RWD Life Science Co., Ltd. | 78001 | |
| Indwelling needle | Kindly EnterpriseDevelopment Group Co., LTD | Positive Pressure Model | 26 G |
| Iodine solution | HYNAUT | https://www.hainuocn.com/index/detail/524.html | 4.5–5.5 g/L |
| IQ demodulation program | Institute of Biomedical Engineering at the University of Montreal | Matlab Ultrasound Toolbox 3.4 version | |
| Isoflurane | RWD Life Science Co., Ltd. | R510-22-10 | |
| MATLAB software | MathWorks | Version R2021a | |
| Microinjection pump | RWD Life Science Co., Ltd. | R462 | |
| Sodium chloride injection | SHENG'AO animals pharmaceutical Co., Ltd. | 270071460 | 0.90% |
| SonoVue | Bracco | https://www.bracco.com/en-se/product/sonovue | |
| Spherical drill bit | RWD Life Science Co., Ltd. | HM1027/HM1010 | |
| Supporting Positioning Software | RWD Life Science Co., Ltd. | V2.0.0.30400 | |
| Syringe | Kindly EnterpriseDevelopment Group Co., Ltd. | RWLB | 1 mL |
| Tracking program | Jean-Yves Tinevez | 2016 version | |
| Ultrasound gel | Junkang Medical Equipment Co., Ltd. | Model DS-1 | |
| Ultrasound probe | VERASONICS, INC. | L22-14vX LF | |
| Verasonics Ultrasound System | VERASONICS, INC. | Vantage-256 | ultrasound platform |
References
- Sweeney, M. D., Ayyadurai, S., Zlokovic, B. V. Pericytes of the neurovascular unit: Key functions and signaling pathways. Nat Neurosci. 19 (6), 771-783 (2016).
- Wardlaw, J. M., Smith, C., Dichgans, M. Mechanisms of sporadic cerebral small vessel disease: Insights from neuroimaging. Lancet Neurol. 12 (5), 483-497 (2013).
- Fang, J., Wang, Z., Miao, C. -. Y. Angiogenesis after ischemic stroke. Acta Pharmacol Sin. 44 (7), 1305-1321 (2023).
- Zlokovic, B. V. Neurovascular pathways to neurodegeneration in Alzheimer's disease and other disorders. Nat Rev Neurosci. 12 (12), 723-738 (2011).
- Kane, J. R. The role of brain vasculature in glioblastoma. Mol Neurobiol. 56 (9), 6645-6653 (2019).
- Iadecola, C. The pathobiology of vascular dementia. Neuron. 80 (4), 844-866 (2013).
- Ren, B., et al. Cerebral small vessel disease: Neuroimaging features, biochemical markers, influencing factors, pathological mechanism and treatment. Front Neurol. 13, 843953 (2022).
- Bradley, C. P., Berry, C. Microvascular arterial disease of the brain and the heart: A shared pathogenesis. QJM. 116 (10), 829-834 (2023).
- Baltes, C., Radzwill, N., Bosshard, S., Marek, D., Rudin, M. Micro MRI of the mouse brain using a novel 400 MHz cryogenic quadrature RF probe. NMR Biomed. 22 (8), 834-842 (2009).
- Brancaccio, R., Bettuzzi, M., Morigi, M. P., Casali, F., Ragazzini, L. Image quality and dose assessment in inner ear computed tomography imaging with a flat panel-based system. J Comput Assist Tomogr. 39 (2), 232-239 (2015).
- Svoboda, K., Yasuda, R. Principles of two-photon excitation microscopy and its applications to neuroscience. Neuron. 50 (6), 823-839 (2006).
- Osmanski, B. -. F., et al. Ultrafast Doppler imaging of blood flow dynamics in the myocardium. IEEE T Med Imaging. 31 (8), 1661-1668 (2012).
- Rust, M. J., Bates, M., Zhuang, X. Sub-diffraction-limit imaging by stochastic optical reconstruction microscopy (storm). Nat Methods. 3 (10), 793-796 (2006).
- Hess, S. T., Girirajan, T. P., Mason, M. D. Ultra-high resolution imaging by fluorescence photoactivation localization microscopy. Biophys J. 91 (11), 4258-4272 (2006).
- Christensen-Jeffries, K., et al. Super-resolution ultrasound imaging. Ultrasound Med Biol. 46 (4), 865-891 (2020).
- Couture, O., Hingot, V., Heiles, B., Muleki-Seya, P., Tanter, M. Ultrasound localization microscopy and super-resolution: A state of the art. IEEE Trans Ultrason Ferroelectr Freq Control. 65 (8), 1304-1320 (2018).
- Demené, C., et al. Spatiotemporal clutter filtering of ultrafast ultrasound data highly increases Doppler and fUltrasound sensitivity. IEEE Trans Med Imaging. 34 (11), 2271-2285 (2015).
- Errico, C., et al. Ultrafast ultrasound localization microscopy for deep super-resolution vascular imaging. Nature. 527 (7579), 499-502 (2015).
- Banterle, N., Bui, K. H., Lemke, E. A., Beck, M. Fourier ring correlation as a resolution criterion for super-resolution microscopy. J Struct Biol. 183 (3), 363-367 (2013).
- Lowerison, M. R., et al. Super-resolution ultrasound reveals cerebrovascular impairment in a mouse model of Alzheimer's disease. J Neurosci. 44 (9), e1251232024 (2024).
- Zhang, G., et al. ULM-MBCNRT: In vivo ultrafast ultrasound localization microscopy by combining multi-branch CNN and recursive transformer. IEEE Trans Ultrason Ferroelectr Freq Control. , (2024).
- Zhang, G., et al. In vivo ultrasound localization microscopy for high-density microbubbles. Ultrasonics. 143, 107410 (2024).
- Redfors, B., Shao, Y., Omerovic, E. Influence of anesthetic agent, depth of anesthesia and body temperature on cardiovascular functional parameters in the rat. Lab Anim. 48 (1), 6-14 (2014).
- Lenz, C., Rebel, A., Van Ackern, K., Kuschinsky, W., Waschke, K. F. Local cerebral blood flow, local cerebral glucose utilization, and flow-metabolism coupling during sevoflurane versus isoflurane anesthesia in rats. Anesthesiology. 89 (6), 1480-1488 (1998).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved