Method Article
Optimized Intravenous Injection in Adult Zebrafish
In This Article
Summary
Here, we present an optimized and effective protocol for the intravenous injection of cells or pharmacological agents into adult zebrafish, resulting in enhanced cell engraftment and increased survival rates of the treated zebrafish.
Abstract
Intravenous (IV) injection is widely recognized as the most effective and commonly utilized method for achieving systemic delivery of substances in mammalian research models. However, its application in adult zebrafish for drug delivery, stem cell transplantation, and regenerative and cancer studies has been limited due to the challenges posed by their small body size and intricate blood vessels. To overcome these limitations, alternative injection techniques such as intracardiac and retro-orbital (RO) injection have been explored in the past for stem cell transplantation in adult zebrafish. However, these techniques have their drawbacks, including the need for meticulous injection techniques or increased risk of mortality.
In this study, we have developed a refined and optimized IV injection procedure specifically tailored to adult zebrafish, addressing the challenges associated with their unique anatomy. To demonstrate the effectiveness of this technique, we performed successful IV injections of whole kidney marrow cells from Tg(mpo: EGFP) fish and FITC-dextran dye into adult Casper fish. The subsequent visualization of injected cells and dyes using a fluorescence microscope confirmed their successful delivery and engraftment within the zebrafish. Furthermore, we demonstrated that compared with the intracardiac and RO injections, the IV injection resulted in improved survival rates and engraftment efficiency in treated zebrafish. This approach enables precise delivery and localization of substances and holds great potential for large-scale drug and chemical screening using adult zebrafish. Additionally, the ability to visually track the injected cells and dyes provides invaluable insights into their engraftment, migration, and interactions with host tissues, enabling a more comprehensive evaluation of therapeutic effects and biological processes in zebrafish models.
Introduction
Zebrafish (Danio rerio) has emerged as a valuable model organism in biomedical research, primarily due to its genetic similarity to humans, with more than 70% of human genes having zebrafish counterparts1,2. This genetic resemblance, combined with zebrafish's compact size, rapid developmental cycle, and capacity for extensive genetic manipulation, makes it a powerful tool for scientific exploration. These attributes are particularly advantageous for experiments involving cell transplantation, drug delivery, and cell tracing. Furthermore, the optical clarity of transparent larvae and specific pigmentation mutants, such as the Casper zebrafish, allows for precise visualization of transplanted cells or substances, offering a more efficient and cost-effective alternative to traditional animal models.
Zebrafish are widely used to develop cancer transplantation models and to conduct drug screenings for severe diseases like glioma, melanoma, pancreatic tumors, and leukemia3,4,5,6,7,8,9,10. Typically, these models are initiated at the larval stage to take advantage of the larvae's immature immune system and inherent transparency, which simplifies the injection process and enhances the feasibility of short-term studies. However, using larvae limits the duration for assessing engraftment and therapeutic interventions7,11. Transitioning these experimental protocols to adult zebrafish introduces challenges such as more complex injection procedures, reduced engraftment efficiency, higher mortality rates, and increased variability in individual responses. These challenges highlight the critical need for improved injection and substance delivery techniques, particularly for studies involving adult zebrafish that require extended observation periods.
Historically, intracardiac12 and retro-orbital (RO) injections13 have been the primary methods for drug delivery and cell transplantation in adult zebrafish. Intracardiac injection, which involves injecting substances directly into the heart, ensures immediate systemic circulation of drugs but is associated with significant risks, including potential cardiac injury and high mortality. On the other hand, RO injection, which delivers materials to the venous sinus behind the eye, also promotes rapid systemic distribution but can be stressful for the fish and demands high precision in execution14. IV injections, well-established for systemic substance delivery in mice and rats, are crucial for pharmacokinetics studies, drug efficacy assessments, and therapeutic interventions in these models15,16,17,18. Recently, IV injections have gained prominence in zebrafish research, particularly in studies of innate immune responses19,20 and acute kidney injury21 in larval zebrafish. However, their application in adult zebrafish remains limited.
In this study, we developed and optimized an IV injection procedure tailored specifically for adult zebrafish, which significantly improves survival rates, precision, and delivery efficiency. We visually demonstrate this method and provide a detailed protocol using this refined technique. We successfully administered IV injections of whole kidney marrow (WKM) cells from Tg(mpo: EGFP) fish and FITC-dextran dye into adult Casper fish, with confirmation of delivery and engraftment assessed by fluorescent microscopy. Our findings show that the refined IV injection technique is more efficient and consistent compared to the traditional intra-cardiac and RO methods. As the use of zebrafish in scientific research continues to expand, the adoption of this improved IV injection technique is likely to enhance our understanding of disease pathogenesis and accelerate the development of new therapeutic approaches.
Protocol
All animal procedures were approved by the Committee of the Use of Laboratory and Research Animals (CULATR) at the University of Hong Kong (HKU).
1. Preparation of injection material
- Prepare cell suspension medium: 0.9x PBS + 5% fetal bovine serum (FBS) + 1% penicillin/streptomycin (Pen/Strep).
- Sterilize the Hamilton syringe and attached needle (34 G, 10 mm length, 10 µL capacity, see Table of Materials) under UV light with a 270-280 nm wavelength and expose the items for 2 h.
- Prepare sterilized cotton buds, foam pads, and tissue paper.
- Prepare the anesthesia mixture by diluting tricaine with sterilized fish water to a final concentration of 0.2 mg/mL.
- For the transplantation of WKM cells in Casper fish, 2 days before the transplantation, administer a sublethal irradiation dose of 25 Gy, divided into two sessions of 12.5 Gy each, given on the same day with a 7-8 h interval between doses. For the irradiation process, place 5-10 adult fish in a 100 mm Petri dish containing fish water and expose them in a gamma irradiator.
NOTE: The dose was determined to be sublethal based on survival analysis after exposing Casper fish to various irradiation levels up to 35 Gy, monitored over 30 days (Figure 1A). The exact duration of exposure should be calibrated according to the settings of the irradiator. No irradiation is needed for procedures involving only drug delivery. - Use a laboratory autoclave steam sterilizer to sterilize the fish water intended for housing treated fish (see Table of Materials) to prevent infections and ensure a safe recovery environment for the fish post-injection.
NOTE: The sterilization process is tailored to the specific autoclave unit used, and we adhere to the manufacturer's guidelines for autoclaving liquid solutions to prepare the fish water. - Prepare needle washing buffer: several tubes of 75% ethanol and ultrapure water in 1.5 mL microcentrifuge tubes.
- Prepare transplantation buffer by dissolving FITC-Dextran (Molecular Weight: 10,000) in the previously prepared cell suspension medium (0.9x PBS with 5% FBS and 1% Pen/Strep). Adjust the concentration to 100 µg/mL.
NOTE: This buffer will be used for the injections, with a planned volume of 2 µL per fish.
2. Preparation of whole kidney marrow for transplantation
- Euthanize the necessary number of Tg(mpo: EGFP) fish by submerging them in a 0.4 mg/mL tricaine solution, ensuring they remain in the solution for at least 10 min following the cessation of opercular movement.
- Dissect the Tg(mpo: EGFP) fish to isolate and transfer the kidney marrow (KM) into the cell suspension medium within a 1.5 mL microcentrifuge tube as described previously22.
- Disaggregate the KM cells by pipetting and filtering the suspension through a 40 µm nylon cell strainer.
- Centrifuge the suspension at 500 × g for 8 min.
- Discard the supernatant and resuspend the pellet in 500 µL of cell suspension medium.
- Count the cells using an automated counter with a 10 µL aliquot of the cell suspension.
- Centrifuge again at 500 × g for 8 min.
- Adjust the cell concentration to 150,000 cells/µL using the transplantation buffer.
3. Injection procedure
- Thoroughly wash the Hamilton syringe 3-4x with 75% ethanol before the injection. Rinse the syringe 3-4x with ultrapure water to remove any ethanol residue.
- Administer anesthesia to the zebrafish by immersing them in the anesthesia mixture until they are fully anesthetized, evidenced by diminished movement and loss of reflex responses. Once the fish are sedated, position them with their dorsal side facing upward and their head oriented to the left (Figure 1B). Carefully lay the fish on clean, damp tissue paper, which will help stabilize them for the injection. Make sure the tissue is sufficiently moist to prevent the fish from drying out, yet not so wet as to cause slipping.
- Grasp the Hamilton syringe firmly and angle the needle at ~45° to the primary vessel (the caudal vein) to target it effectively while minimizing damage to surrounding tissues. For Capser zebrafish, aim for a section of the vessel that is visible through its translucent body for accurate needle placement (Figure 1B). For wild-type zebrafish (Tubingen, TU) with pigmented skin, aim for primary vessels which are along the body axis and posterior to the anus in the region of the primary vessels (Figure 1B). Gently and smoothly insert the needle into the designated vessel, administer the injection (2 µL) slowly to minimize stress, ensure optimal uptake of the substance, and apply gentle pressure to the site with a cotton bud for 10 s to control any bleeding after the injection.
- Observe under a microscope to confirm the presence of GFP signals, ensuring the effectiveness of the injection. Confirm that the cells or dyes appear as green signals within the vessel (Figure 2A).
- Allow the fish to recover in freshly sterilized fish water post injection for 10 min till transfer to a static tank.
- Clean the needle as previously described between injections of different reagents.
- To prevent cell clumping, flick the cell suspension every 10 min to resuspend the cells.
- Maintain a maximum of 15 transplanted fish in a 4 L tank under static water conditions for the treatment duration. Alternatively, house the transplanted fish individually in 1 L static tanks. Equip the tank with an oxygen pump and perform daily water changes to minimize the risk of infection.
- Monitor and assess GFP expression by flow cytometry in the fish approximately 14 days post-transplantation to evaluate the engraftment rate. Record the daily mortality of fish to accurately calculate survival rates. Perform one-way ANOVA for engraftment analysis and the Log-rank test for the survival analysis.
Results
To evaluate the effectiveness and precision of different injection methods in adult Casper fish, a comparative analysis was conducted using intracardiac, RO, and IV techniques (Figure 2A). Varying quantities of WKM cells from Tg(mpo: EGFP) fish were injected, each mixed with FITC-dextran dye at concentrations of 1 × 105 and 3 × 105 cells. The success of each method was determined through immediate microscopic assessment of GFP signal presence post-injection. The results revealed significant variations in delivery success across the methods. The IV injection method demonstrated the highest effectiveness, with GFP signals detected in 41 out of 43 fish. In contrast, the RO method showed limited success, with successful injections observed in only 3 out of 37 fish. The intracardiac method achieved successful delivery in 11 out of 40 fish (Figure 2A). The survival of the transplanted fish was also monitored. Fish injected via the RO and intracardiac methods had significantly lower survival rates compared to those injected intravenously (Figure 2B). Notably, the number of cells injected influenced the survival rates among the IV-injected fish, with recipients receiving 3 × 105 cells showing better survival. These findings highlight the superior efficacy and reliability of the IV injection method in delivering cells and substances into adult zebrafish.
The engraftment efficiency of WKM cells in recipient Casper fish was thoroughly assessed by monitoring the GFP signal from the transplanted cells. In fish that received IV injections, GFP signals were detectable as early as day three post-transplantation (Figure 2C), indicating a rapid and effective engraftment process. In contrast, fish that underwent RO and intracardiac injections showed a delayed GFP signal emergence, indicating a slower engraftment rate. By day 17 post-transplantation, the transplanted cells had predominantly repopulated the kidney marrow, with the most pronounced GFP signals observed in the IV-injected fish (Figure 2C). Flow cytometric analysis of KM from the recipients further underscored the efficacy of the IV injection method. In recipients initially injected with 3 × 105 cells intravenously, approximately 18% of the cells in the KM were GFP-positive, a proportion similar to that found in the kidney marrow of the donor Tg(mpo: EGFP) fish that had not undergone any transplantation (Figure 2D,E). In contrast, the intracardiac injection resulted in about 5% GFP-positive cells, while the RO method yielded only about 2% GFP-positive cells in the recipients' kidney marrow. Additionally, the rate of engraftment correlated positively with the number of cells injected, demonstrating that higher cell doses led to greater engraftment. These results confirm the robustness of the IV injection technique in facilitating cell engraftment and subsequent repopulation of the kidney marrow.
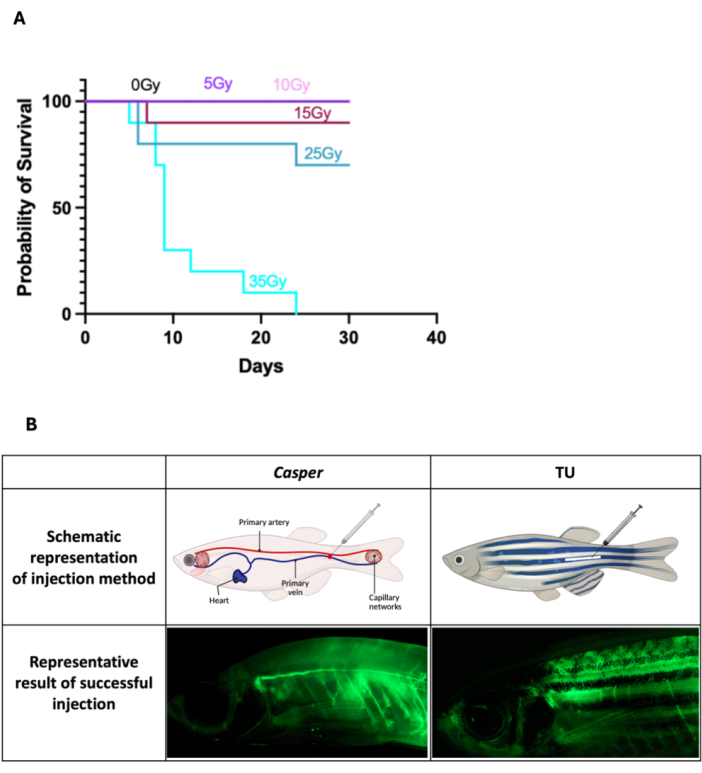
Figure 1: Survival outcomes post-irradiation and intravenous injection in adult zebrafish. (A) Casper fish were exposed to up to 35 Gy irradiation and monitored survival over 30 days, confirming 25 Gy as a sublethal dosage (n = 10 for all groups). (B) Schematic diagrams that illustrate the injection sites for both Casper and TU zebrafish, along with examples of successfully injected fish. Please click here to view a larger version of this figure.

Figure 2: Comparison of retro-orbital, intracardiac, and intravenous injections in adult zebrafish. (A) Schematic representations and representative outcomes of RO, intracardiac, and IV injection methods in adult zebrafish. The numbers at the bottom right-hand corner indicate the count of successfully injected or mis-injected fish relative to the total number attempted for each method, the GFP signal was verified under the fluorescent microscope immediately after injection. (B) Overall survival of the recipients after transplanted with different numbers of donor kidney marrow cells by different injection methods (IV-100K: n = 24; Intracardiac-100K: n = 10; RO-100K: n = 12; IV-300K: n = 20; Intracardiac-300K: n = 6; RO-300K: n = 8). (C) Observation of the engraftment efficiency in recipients post transplantation via RO, intracardiac, and IV injection methods (D) Representative flow cytometry plot for the GFP-positive cells in KM of the recipients at 17 days post-transplantation. (E) The percentage of GFP+ cells in the KM of the recipients 17 days post-transplantation, as well as in the KM of donor Tg(mpo:EGFP) fish without irradiation (IV-100K: n = 30; Intracardiac-100K: n = 28; RO-100K: n = 22; IV-300K: n = 22; Intracardiac-300K: n = 26; RO-300K: n = 18). Data are mean ± s.e.m. One-way ANOVA was performed for D. Log-Rank test was performed for B. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. Scale bars = 1 mm (A, C). Abbreviations: RO = retro-orbital; IV = intravenous; GFP = green fluorescent protein; KM = kidney marrow; SSC-A = side scatter-peak area; FITC-A = fluorescein isothiocyanate-peak area. Please click here to view a larger version of this figure.
Discussion
In this study, we developed an IV injection protocol tailored for adult zebrafish to enhance the precision and consistency of delivering cells or drugs. A critical element of this method is the ability to localize and visualize the primary vein, which is crucial for the accurate administration of substances. While the translucent Casper zebrafish strain is recommended for optimal vessel visibility, our protocol is adaptable for use with various adult zebrafish strains, provided suitable adjustments are made for visibility and accuracy. The selection of an appropriate needle gauge was essential to minimize vascular damage and ensure effective substance transport. Moreover, comprehensive postinjection care was prioritized, including maintaining clean and well-oxygenated water. These practices not only streamlined the injection process but also significantly improved the health and survival of the zebrafish, thereby leading to more reliable and reproducible experimental outcomes.
Furthermore, we conducted a comparative analysis of intracardiac, RO, and IV injection techniques in adult zebrafish, demonstrating the distinct advantages of the IV method in enhancing survival rates and promoting cell engraftment. Despite their traditional use, intracardiac injections are linked to high mortality rates13, and the precision required for effective RO injections is challenging to consistently achieve14. The minimally invasive nature of the IV technique significantly mitigates stress and physical trauma to the fish, leading to substantially improved survival and engraftment outcomes post-injection. Additionally, the rapid detectability of GFP signals by day 3 post-transplantation in IV-injected fish further underscores the method's efficiency in facilitating effective cell engraftment. In contrast, the slower emergence of GFP signals in intracardiac-injected fish and the near absence of signals in RO-injected fish underline the limitations of these alternative methods, suggesting the IV approach as a more effective approach.
While the IV injection method offers significant advantages, it has potential limitations, such as the need for specialized equipment and training to achieve high precision. Furthermore, while the Casper strain's translucency facilitates vein visualization, adapting this method to less translucent strains may require additional visibility and technological enhancements. Additionally, further investigation is needed to assess the long-term effects of IV injections and to evaluate the stability of engrafted cells over extended periods in zebrafish, which will be crucial for validating the sustained success and safety of this technique.
The effectiveness of the IV injection technique in delivering substances into the bloodstream and achieving high engraftment levels in adult zebrafish has several implications for future research. This method enables more effective use of adult zebrafish in long-term studies, addressing some limitations associated with larval models. The reliability of the IV method enhances the accuracy of models for studying complex diseases and conducting drug screenings, potentially leading to significant advancements in therapeutic strategies. Additionally, the success of IV injections in achieving robust engraftment lays a strong foundation for further exploration in genetic manipulation and transplantation, which is essential for investigating genetic disorders and immune responses. Advancements in this IV injection protocol are poised to make substantial contributions to zebrafish research, refining tools for biomedical research and accelerating progress in understanding and treating human diseases.
Disclosures
The authors have no conflicts of interest to declare.
Acknowledgements
We thank the Zebrafish Facility from the Centre for Comparative Medicine Research (CCMR) at The University of Hong Kong. We thank Ms Jo Yiu Ling Wong for animal assistance. The works were supported by the Theme-based Research Scheme (T12-702/20-N), Health and Medical Research Fund Projects No.08192066 and No. 08193106, National Natural Science Foundation of China (NSFC)/Research Grants Council (RGC) Joint Research Scheme 2021/22 N_HKU745/21, the National Key R & D program of China (2023YFA1800100) and the Centre for Oncology and Immunology under the Health@InnoHK Initiative funded by the Innovation and Technology Commission, the Government of Hong Kong SAR, China (A.Y.H.L.).
Materials
| Name | Company | Catalog Number | Comments |
| Autoclaves Steam Sterilizer | HIRAYAMA | HRG-140 | |
| Centrifuge 5424 | Eppendorf | ||
| Countess 3 Automated Cell Counter | Thermofisher | ||
| Countess II FL | Invitrogen | ||
| Ethyl 3-aminobenzoate methanesulfonate salt (Tricaine) | Sigma-aldrich | MKCL9483 | |
| Falcon 40 µm Cell Strainer | CORNING | Blue, Sterile, Individually Packaged, 50/Case | |
| FBS, Qualified | Gibco | 26140079 | |
| FITC-Dextran (MW 10000) | MedChemExpress | 60842-46-8 | |
| Injection needle | Hamilton | HAMI207434 | 34 G, 10 mm length |
| Micro syringe | Hamilton | 7635-01 | 10 µL capacity, Model 701 RN |
| Nikon SMZ18 | |||
| PBS pH 7.4 (1x) | Gibco | 10010023 | |
| Penicillin-Streptomycin (5,000 U/mL) | Gibco™ | 15070063 | 100x |
| Pipette Tips | Eppendorf | epTIPS | |
| Single Channel Pipette | Eppendorf | 05-403-151 | |
| UltraPure Distilled Water | Invitrogen | 10977015 |
References
- Choi, T. Y., Choi, T. I., Lee, Y. R., Choe, S. K., Kim, C. H. Zebrafish as an animal model for biomedical research. Exp Mol Med. 53 (3), 310-317 (2021).
- Lieschke, G. J., Currie, P. D. Animal models of human disease: zebrafish swim into view. Nat Rev Genet. 8 (5), 353-367 (2007).
- Wu, J. Q., et al. Patient-derived xenograft in zebrafish embryos: a new platform for translational research in gastric cancer. J Exp Clin Cancer Res. 36 (1), 160 (2017).
- Welker, A. M., et al. Correction: Standardized orthotopic xenografts in zebrafish reveal glioma cell-line-specific characteristics and tumor cell heterogeneity. Dis Model Mech. 9 (9), 1063-1065 (2016).
- Stoletov, K., Montel, V., Lester, R. D., Gonias, S. L., Klemke, R. High-resolution imaging of the dynamic tumor cell vascular interface in transparent zebrafish. Proc Natl Acad Sci U S A. 104 (44), 17406-17411 (2007).
- Etchin, J. P. K. J., Look, A. T. Zebrafish as a model for the study of human cancer. Methods Cell Biol. 105, 309-337 (2011).
- Khan, N., Mahajan, N. K., Sinha, P., Jayandharan, G. R. An efficient method to generate xenograft tumor models of acute myeloid leukemia and hepatocellular carcinoma in adult zebrafish. Blood Cells Mol Dis. 75, 48-55 (2019).
- Corkery, D. P., Dellaire, G., Berman, J. N. Leukaemia xenotransplantation in zebrafish--chemotherapy response assay in vivo. Br J Haematol. 153 (6), 786-789 (2011).
- He, B. L., et al. Functions of flt3 in zebrafish hematopoiesis and its relevance to human acute myeloid leukemia. Blood. 123 (16), 2518-2529 (2014).
- Wang, D., et al. Transgenic IDH2(R172K) and IDH2(R140Q) zebrafish models recapitulated features of human acute myeloid leukemia. Oncogene. 42 (16), 1331 (2023).
- Fan, R. Y., et al. Zebrafish xenograft model for studying mechanism and treatment of non-small cell lung cancer brain metastasis. J Exp Clin Cancer Res. 40 (1), 371 (2021).
- LeBlanc, J., Bowman, T. V., Zon, L. Transplantation of whole kidney marrow in adult zebrafish. J Vis Exp. (2), e159 (2007).
- Pugach, E. K., Li, P., White, R., Zon, L. Retro-orbital injection in adult zebrafish. J Vis Exp. (34), e1645 (2009).
- Benjamin, D. C., Hynes, R. O. Intravital imaging of metastasis in adult zebrafish. BMC Cancer. 17 (1), 660 (2017).
- Hilligan, K. L., et al. Intravenous administration of BCG protects mice against lethal SARS-CoV-2 challenge. J Exp Med. 219 (2), e20211862 (2022).
- Moreo, E., et al. Intravenous administration of BCG in mice promotes natural killer and T cell-mediated antitumor immunity in the lung. Nat Commun. 14 (1), 6090 (2023).
- Wang, Z., et al. Intravenous administration of IL-12 encoding self-replicating RNA-lipid nanoparticle complex leads to safe and effective antitumor responses. Sci Rep. 14 (1), 7366 (2024).
- Saleem, M., et al. A new best practice for validating tail vein injections in rat with near-infrared-labeled agents. J Vis Exp. (146), e59295 (2019).
- Rojas, A. M., Shiau, C. E. Brain-localized and Intravenous Microinjections in the Larval Zebrafish to Assess Innate Immune Response. Bio Protoc. 11 (7), e3978 (2021).
- van Soest, J. J., et al. Comparison of static immersion and intravenous injection systems for exposure of zebrafish embryos to the natural pathogen Edwardsiella tarda. BMC Immunol. 12, 58 (2011).
- Cianciolo Cosentino, C., Roman, B. L., Drummond, I. A., Hukriede, N. A. Intravenous microinjections of zebrafish larvae to study acute kidney injury. J Vis Exp. (42), e2079 (2010).
- Gerlach, G. F., Schrader, L. N., Wingert, R. A. Dissection of the adult zebrafish kidney. J Vis Exp. (54), e2839 (2011).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved