Method Article
A Cognitive Fusion-guided Prostate Biopsy Using Multiparametric Magnetic Resonance Imaging and Transrectal Ultrasound
* These authors contributed equally
In This Article
Summary
Prostate biopsy is the gold standard diagnostic method for prostate cancer. Cognitive fusion-guided prostate biopsy, which combines transrectal ultrasound with pre-measured MRI parameters, improves biopsy accuracy and enhances the detection rate of clinically significant prostate cancer.
Abstract
Traditional transrectal ultrasound (TRUS)-guided prostate biopsy has limited sensitivity and specificity, particularly for detecting early-stage prostate cancer, due to a lack of precise lesion targeting. An improved cognitive fusion-guided prostate biopsy method has been developed to enhance lesion targeting by integrating three parameters of prostate multiparametric MR (mpMRI) images into TRUS images. Prostate mpMRI measurement is initially performed to obtain three key parameters: the rotation angle (α), the distance from the rectal wall (X), and the distance from the prostate apex (Y). These parameters are then cognitively applied in real-time, TRUS-guided prostate needle biopsy to detect target lesions. This improved transperineal cognitive fusion biopsy method enhances diagnostic accuracy, improves reproducibility, and reduces reliance on operator experience. Clinical application in 423 patients demonstrated a prostate cancer detection rate of 73.5%, with 62.9% classified as clinically significant cancers. Compared with equipment-intensive methods such as MRI-ultrasound fusion biopsy, this approach is cost-effective, practical, and well-suited for broader clinical adoption. Additionally, the method's flexibility supports integration with other imaging techniques, such as 68Ga-PSMA PET/CT, further improving detection rates for patients with high-risk prostate cancer.
Introduction
Prostate cancer is a major global health concern, with an estimated 1,466,680 new cases and 396,792 deaths reported worldwide in 2022. Prostate cancer is the second most common cancer and the fifth leading cause of cancer death among men1. By 2040, the number of new prostate cancer cases is projected to rise to 2.9 million, with deaths expected to reach 700,0002. Early diagnosis and standardized treatment are crucial for improving survival rates in patients with prostate cancer, and prostate biopsy remains the gold standard for early diagnosis.
Since 1968, transrectal ultrasound (TRUS) has been an important tool for guiding prostate biopsies. However, the sensitivity and specificity of TRUS-guided prostate biopsies are limited by 65-74% and 40-57%3, respectively, particularly in detecting early-stage or small-volume lesions4. To overcome these limitations, multiparametric MRI (mpMRI) has emerged as a superior imaging technique, providing more detailed evaluations of prostate tissue and improved localization of clinically significant prostate cancer. Compared with traditional TRUS-guided biopsy, mpMRI can more accurately identify suspicious lesions within the prostate and improve the precision of targeted biopsies5,6.
Several MRI-guided prostate biopsy techniques have been developed, leveraging the enhanced diagnostic capabilities of prostate mpMRI. These techniques include MRI-targeted prostate biopsy, MRI-transrectal ultrasound fusion prostate biopsy, and cognitive fusion-guided prostate biopsy7,8. MRI-targeted prostate biopsy is performed directly inside the MRI scanner, allowing real-time image guidance during biopsy. This technique offers excellent lesion localization; however, it is costly and time-consuming due to the prolonged imaging and procedural requirements. MRI-transrectal ultrasound fusion prostate biopsy combines MR images and real-time TRUS images via specialized software, making it complex and costly.
In contrast, cognitive fusion-guided prostate biopsy involves clinicians memorizing lesion locations from MR images and mentally integrating this information with real-time TRUS during biopsy. This technique requires no additional equipment, making it simple, cost-effective, and highly suitable for clinical adoption. However, cognitive fusion-guided prostate biopsy is highly dependent on the clinician's experience, and the process of lesion localization relies entirely on memory and judgment, which results in reduced reproducibility and limits its broader application. To address these challenges, an improved transperineal cognitive fusion biopsy method was developed by integrating three key parameters from prostate mpMR images with TRUS. This method is highly reproducible, easy to perform, and well suited for widespread clinical implementation, offering significant support for the accurate diagnosis of prostate cancer. This paper details the protocol and clinical utility of this standardized approach, highlighting its potential to improve prostate cancer detection in routine practice.
Protocol
This study involving human participants was conducted in accordance with the principles outlined in the Declaration of Helsinki. Written informed consent was obtained from all participants prior to their inclusion in the study. The inclusion and exclusion criteria were carefully defined to ensure participant safety and the suitability of the procedure.
1. Patient selection
- Set the following inclusion criteria for patient selection: prostate-specific antigen (PSA) > 10 ng/mL; presence of a suspicious prostate nodule detected by digital rectal examination (DRE), irrespective of PSA level; suspicious lesions identified via transrectal ultrasound (TRUS), MRI, or PSMA PET/CT, regardless of the PSA level; PSA levels between 4 and 10 ng/mL accompanied by f/tPSA < 0.16, and/or PSA density (PSAD) > 0.15 ng/mL2, and/or PSA velocity (PSAV) > 0.75 ng/ml annually; abnormal results from other prostate-related tests, such as elevated prostate health index (PHI) or positive urinary prostate cancer antigen 3 (PCA3) results.
- Exclude participants from the study based on the following criteria: acute infection or fever during the study period; hypertensive crisis; decompensated heart failure; severe bleeding disorders; poorly controlled or unstable comorbidities such as hypertension or diabetes; severe anal or rectal conditions, including advanced internal/external hemorrhoids or significant rectal/anal pathology; severe immunosuppressive state; severe psychological disorders or participants unwilling or unable to cooperate with the procedure.
2. Determining the three-dimensional coordinates of the lesion on mpMRI
- Thoroughly review the T2-weighted imaging (T2WI, Figure 1A), diffusion-weighted imaging (DWI, Figure 1B), and apparent diffusion coefficient (ADC, Figure 1C) maps of multiparametric MRI (mpMRI) scans to identify the prostate lesions.
NOTE: Consultation with a radiologist may be necessary to confirm the location of the lesion prior to biopsy. - Use a point approximately 7 mm from the anterior rectal wall as the vertex. Draw one line through the body's midline, which can be determined by the pubic symphysis or the bulbous urethra. Draw another line through the center of the lesion. The angle between these two lines is the angular displacement, recorded as α (Figure 2A).
NOTE: This angle α is used to determine how much the ultrasound probe is rotated to align with the lesion during biopsy. - Measure the distance from the center of the lesion to the rectal serosal surface on the T2WI sequence and record it as X (Figure 2A).
NOTE: Distance X is used to guide the needle insertion point during biopsy and determine the location where the biopsy needle is inserted relative to the rectum. - Measuring the Distance from the Apex of the Prostate (Y) Create a plane that passes through both the center of the vertex of the α angle and the center of the lesion via a DICOM viewer with MPR (multiplanar reconstruction) or another slice function. Measure the distance from the lesion to the apex of the prostate in this plane and record it as Y (Figure 2B).
NOTE: The distance Y is critical for determining the depth of needle penetration; it corresponds to the depth the biopsy needle needs to reach during biopsy.
3. Patient preparation and imaging
- Place the patient in the lithotomy position. Position the buttocks at the exact center edge of the examination table. Both legs are symmetrically supported in the leg rests.
- Retract the scrotum upward and fully expose the perineal region.
- Disinfect the perineal area with povidone-iodine (iodophor) and drape the area, leaving the procedural site accessible.
- Subcutaneously inject 1% lidocaine for local anesthesia at the projection of the prostate's largest transverse plane on the perineal skin.
- Gently insert the biplane transrectal ultrasound (TRUS) probe into the rectum. Position the probe at a 45° upward angle relative to the anus.
NOTE: The anesthesia insertion point is typically 1.5 cm from the anus (Figure 3). - Inject 1% lidocaine into the levator ani muscle, prostatic capsule, and apex of the prostate via ultrasound guidance in the sagittal plane to ensure adequate anesthesia during the biopsy.
4. TRUS and cognitive fusion
- Align the ultrasound probe with the midline by locating a plane on the transverse ultrasound image that closely matches the lesion's position from the T2WI transverse image on the mpMRI. Once identified, hold the probe steady, and freeze the ultrasound image at this cross-sectional level.
NOTE: Ensure that the sagittal plane of the ultrasound probe is aligned with the body's midline. In the standard lithotomy position, the sagittal plane of the probe is typically positioned directly overhead. - Freeze the image and use the center of the probe as the vertex to measure the α angle on ultrasound. Align one edge of the α angle with the central guide line on the transverse ultrasound image (Figure 4A).
NOTE: The location of the other edge of the α angle, which corresponds to the position of the lesion on the ultrasound image. - Unfreeze the image and rotate the probe until the central guide line of the transverse ultrasound image aligns with the position of the lesion identified on MRI. Hold the probe steady without further rotation once the rotation to the α angle is achieved.
- Position the ultrasound probe in the lesion plane by advancing the probe horizontally along the rectum until the linear array ultrasound probe displays the prostate image, after rotating the probe to the correct α angle.
NOTE: The current image displayed corresponds to the plane of the lesion as determined by the previous MRI measurements (Figure 4B). - Measure the distance from the rectal serosal surface to the location corresponding to the previously measured X value on MRI, confirming the insertion point for the biopsy needle.
- Measure the distance from the apex of the prostate to the location corresponding to the previously measured Y value on MRI in the direction parallel to the rectum, ensuring the correct depth for the biopsy needle insertion (Figure 4B).
NOTE: These X and Y measurements confirm that the probe is correctly aligned with the lesion on the ultrasound image, allowing for precise targeting during biopsy.
5. Targeted biopsy
- Insert the biopsy needle along the needle guide line corresponding to the distance from the rectum (measured as X on the ultrasound image) under sagittal plane ultrasound guidance using the linear array probe.
- Adjust the needle depth according to the previously measured Y value, which represents the distance from the apex of the prostate. Perform 2-3 targeted biopsy cores in the lesion area once the correct depth is reached (Figure 4B).
- Repeat the above steps for each target lesion if multiple lesions are identified.
NOTE: It is crucial to maintain the stability of the ultrasound probe, and the X and Y coordinates must be applied accurately for each biopsy site throughout the procedure. To ensure precision and reduce hand movement, it is recommended to use an ultrasound probe support arm for assistance.
6. Systematic biopsy
- Take one biopsy core from the apex, midline, and base in both the peripheral and central zones of the left lobe of the prostate.
- Adjust the probe position and take one core each from the apex, midline, and base in both zones; then, repeat the process on the right lobe of the prostate.
NOTE: It is advised to collect a total of 12 cores during the systematic biopsy.
7. Completion
- Gently remove the TRUS probe from the rectum to avoid any discomfort after biopsy.
- Clean the perineal and rectal areas with sterile wipes to remove any residual gel or blood.
- Place each biopsy core into prelabeled containers with appropriate preservatives. Mark each container with the patient's information and biopsy site.
- Transport the labeled containers with biopsy cores to the Pathology Lab for histopathological analysis.
Results
In this case, the cognitive fusion-guided prostate biopsy accurately identified a clinically significant prostate cancer lesion. This lesion was indicated by MRI in the left apex of the prostate with a maximum diameter of approximately 6 mm and a PI-RADS score of 4, suggesting a high likelihood of clinically significant prostate cancer.
The pathologic diagnosis of this biopsy lesion was prostatic acinar adenocarcinoma with the following details (Figure 5):
Gleason score: 4 + 4 = 8, with 60% major Gleason pattern 4 and 40% minor Gleason pattern 3
WHO/ISUP Grade Group: Classified as Grade Group 4, indicating a moderate risk for cancer progression
Tumor burden: The tumor occupied 30% of the biopsy cores.
Perineural invasion: Negative (no evidence of nerve involvement)
Vascular invasion: Negative (no evidence of spread into blood vessels)
The other systematic biopsy cores of the prostate were reported as benign.
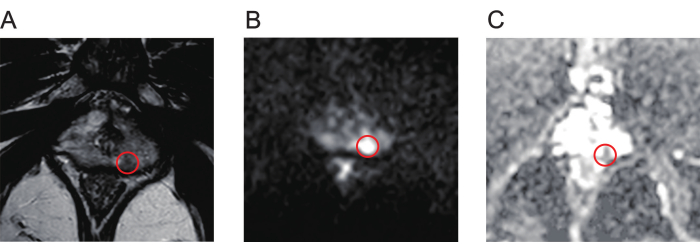
Figure 1: Typical mpMR images of prostate cancer lesions. (A) The red circle indicates the lesion with low signal intensity, as shown by T2-weighted imaging. (B) The red circle indicates the lesion with high signal intensity, as shown by diffusion-weighted imaging. (C) The red circle indicates the lesion, showing low signal intensity on the apparent diffusion coefficient map. Abbreviation: mpMR = multiparametric magnetic resonance. Please click here to view a larger version of this figure.
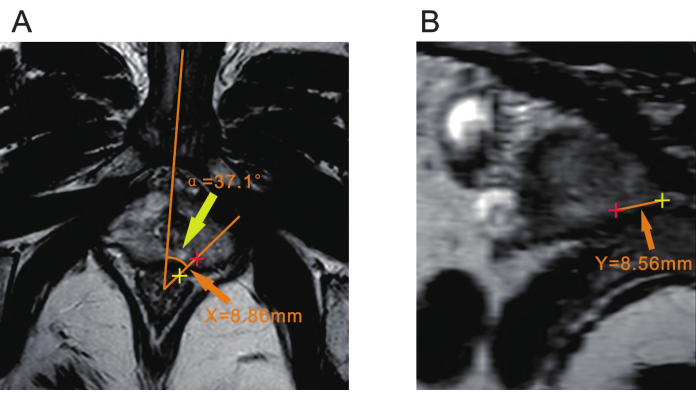
Figure 2: Three parameters of prostate mpMRI. (A) Yellow arrowhead indicates the angle (α) between the plane of the lesion and the body's midline. The orange arrowhead indicates the distance (X) from the center of the lesion (red plus) to the rectal serosal surface (yellow plus). (B) The orange arrowhead indicates the distance (Y) from the center of the lesion (red plus) to the apex of the prostate (yellow plus). Abbreviation: mpMRI = multiparametric magnetic resonance imaging. Please click here to view a larger version of this figure.
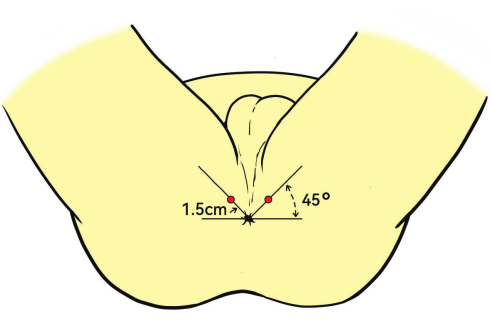
Figure 3: Patient position and anesthesia site. The patients are placed in the lithotomy position. The injection site shown as the red dots for deep infiltration anesthesia is 1.5 cm from the anus, angled 45°. Please click here to view a larger version of this figure.
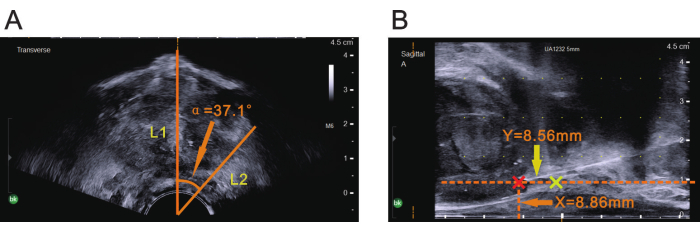
Figure 4: Three parameters of prostate mpMRI shown on ultrasound. (A) With the ultrasound image frozen, the center of the probe was used as the vertex, and the central guide line was used as one edge (L1) to measure the α angle indicated by the orange arrowhead, as shown on mpMRI. Once the α angle indicated by the orange arrowhead was measured, the other edge (L2) of the angle was determined on the transverse ultrasound image. (B) X value on mpMRI indicated by the orange arrowhead was used as the vertical distance for drawing a parallel line with the rectal serosal surface, and the Y value on mpMRI indicated by the yellow arrowhead was used as the horizontal distance for drawing a vertical line from the apex of the prostate (the yellow plus). The red plus at the intersection site indicates the lesion area (biopsy site). Please click here to view a larger version of this figure.

Figure 5: Pathologic staining of the biopsy target lesion from the prostate. (A) HE staining showing prostatic acinar adenocarcinoma with a Gleason score of 4 + 4 = 8 (200x). Scale Bar=10 µm. (B) Immunohistochemical staining for positive expression of NKX3.1 indicates poor differentiation in prostate cancer (200x). Scale Bar=10 µm. Abbreviation: HE = Hematoxylin-eosin. Please click here to view a larger version of this figure.
Discussion
MRI-guided biopsy (MRI-GB) is a cornerstone of targeted prostate biopsy and includes MRI-targeted biopsy (MRI-TB), MRI-transrectal ultrasound fusion biopsy (FUS-TB), and cognitive fusion biopsy (COG-TB). MRI-TB achieves high diagnostic accuracy through real-time MR imaging, with an overall cancer detection rate of 80% and a clinically significant cancer detection rate of 55%9. However, its high cost and operational complexity limit its widespread use. FUS-TB combines MRI precision with real-time ultrasound guidance using specialized software, offering precise localization; however, it requires additional equipment and complex workflows. In contrast, COG-TB relies solely on the operator's ability to mentally integrate MR and ultrasound images during the procedure, eliminating the need for specialized equipment. This approach is cost-effective, simple to perform, and holds significant potential for widespread clinical application.
Studies have shown that the diagnostic accuracy of COG-TB in detecting clinically significant prostate cancer (csPCa) is comparable to that of advanced MRI-guided biopsy techniques, such as MRI-TB and FUS-TB7,10. This comparable efficacy underscores COG-TB as a viable alternative, especially in settings with limited access to specialized equipment. Despite its simplicity, however, COG-TB remains dependent on the operator's experience. Traditional cognitive fusion methods often rely on anatomical landmarks, such as cysts, benign prostatic hyperplasia nodules, or calcifications, to position lesions8,11,12,13,14,15. However, research indicates that approximately 50% of prostate cancer lesions do not exhibit typical hypoechoic characteristics on TRUS, with some patients lacking distinct anatomical markers16. This variability, coupled with the operator's reliance on spatial awareness, limits the reproducibility and scalability of COG-TB.
In this improved transperineal cognitive fusion biopsy method, lesion localization is parameterized using three key metrics of mpMR images: rotation angle (α), distance from the rectal serosa (X), and distance from the prostate apex (Y). Using T2WI sequences from MR images, the vertex of the α angle is defined as the axis of rotation, and the prostate is segmented into planes to determine the lesion's location and measure the α, X, and Y values. These parameters are then applied under TRUS guidance for precise lesion targeting. The application of these three parameters is similar to rotating the TRUS probe in the rectum. Using T2WI, the prostate is divided into fan-shaped sectors, allowing for the identification of the lesion's plane. Both MRI and TRUS use the body's midline as a reference for the α angle, which corrects for any positional discrepancies between the two imaging modalities.
The parameters X and Yare crucial for determining the location and depth of needle insertion. The X value measured on the T2WI plane represents the distance from the lesion center to the rectal serosal surface. The distance from the lesion center to the prostate base was not measured, as the ultrasound screen's guideline effectively guides the biopsy needle. The rectal serosal surface is close to the zero marker on the guideline, which provides an easy and accurate reference for determining the X and Y values. Unlike traditional cognitive fusion, this approach does not position lesions based on the internal anatomical landmarks of the prostate. In most cases, the ultrasound images do not need to be frozen to perform these measurements. Positioning lesions by mpMRI parameters ensures that this method can be applied to a broad range of patients with high reproducibilityand ashort learning curve, enabling novice operators to achieve high accuracy after training on approximately 10 cases.
Despite the method's advantages, discrepancies between MR and ultrasound imaging modalities, as well as variations in patient positioning and tissue compression, may introduce errors. The key optimizations include the following:
X Value (Distance from Rectal Serosa): X is typically measured with an error range of 2-3 mm. Thin-slice MR images can enhance the resolution and contrast, whereas multiple measurements by radiologists can improve the accuracy.
Y Value (Distance from Prostate Apex): Y relies on reconstructed T2WI planes, which may have limited resolution. However, as a biopsy needle sample is 2 cm in length, approximating the lesion's location is often sufficient. Thin-slice scanning and clear boundary marking can reduce measurement errors.
α Angle (Rotation Angle): Ensuring consistent patient positioning between MRI and TRUS, using the body midline as a reference, and stabilizing the ultrasound probe can minimize angle-related deviations.
This improved method was recently applied in a cohort of 423 patients, with an average age of 70.8 years and a median PSA value of 59.2 ng/mL. Prostate cancer was detected in 311 patients (73.5%), of whom 62.9% had clinically significant cancer. These results validate the reliability and effectiveness of this approach. Prostate biopsy following MRI-ultrasound fusion can be performed using either the transrectal or transperineal approach. Pepe et al. analyzed clinical data from 8,500 cases of transperineal prostate biopsy, reporting a prostate cancer detection rate of 37.1% and a complication rate of 35.9%17. These findings highlight the high safety profile of the transperineal approach. Additionally, the transperineal method offers a higher cancer detection rate and significantly lower risk of infection than the transrectal approach does. Based on these advantages, the European Association of Urology (EAU) guidelines recommend the transperineal route as the preferred approach for prostate biopsy18.
This method can be effectively integrated with other imaging modalities, such as 68Ga-PSMA PET/CT, to enhance the detection of high-risk prostate cancer. Studies have shown that with a standardized uptake value (SUVmax) cutoff of 8, 68Ga-PSMA PET/CT achieves 100% diagnostic accuracy for clinically significant prostate cancer (csPCa) in patients with an ISUP grade ≥319. Targeted biopsies of regions with an SUVmax ≥ 8 can further improve the detection rate of csPCa. In conclusion, this improved transperineal cognitive fusion biopsy method addresses key limitations of traditional COG-TB, offers enhanced reproducibility, reduces operator dependency, and broadens its clinical applicability.
Disclosures
The authors have no conflicts of interest to declare.
Acknowledgements
This work was supported by the Joint Project of Chongqing Health Commission and Science and Technology Bureau (2025MSXM046 to JY. D.), and the National Natural Science Foundation of China (82470420 to J.L.), and the Program for Outstanding Medical Academic Leader of Chongqing (YXLJ202406 to J.L.).
Materials
| Name | Company | Catalog Number | Comments |
| 5% Povidone-Iodine Solution | Chengdu Yong'an Pharmaceutical Co., Ltd. | H51022885 | For disinfection of the surgical area |
| 10% Neutral Buffered Formalin Fixative | Guangzhou Vigrass Biotechnology Co., Ltd. | 24010506 | For fixing biopsy tissue |
| AccuCARE Transperineal Solutions | CIVCO Medical Instruments Co., Inc | 620-119 | For supporting the probe |
| Injection syringe (20 mL) | Shandong weigao group medical polymer Co., LTD | 20211001 | For local anesthesia |
| Lidocaine | Hubei Tiansheng Pharmaceutical Co., Ltd. | H42021839 | Diluted with saline to 1% for local anesthesia |
| MRI 3.0T | Philips | Ingenia | For prostate examination |
| RadiAnt DICOM Viewer | Medixant | V2024.1 | For reading prostate MRI, outlining lesions, measuring distances, and angles |
| Single-use Biopsy Needle MC1820 | Bard Peripheral Vascular, Inc. | REHU3231 | For needle biopsy sampling |
| Single-use Sterile Needle 0.7 x 80 TWLB | Zhejiang Kangdeli Medical Devices Co., Ltd. | C20230923 | For local anesthesia |
| Sodium chloride injection | Southwest pharmaceutical Co., LTD | H50021610 | For diluting lidocaine |
| Ultrasound | BK Medical | bk3000-01 | For guiding prostate biopsy |
References
- Bray, F., et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 74 (3), 229-263 (2024).
- James, N. D., et al. The Lancet Commission on prostate cancer: planning for the surge in cases. Lancet. 403 (10437), 1683-1722 (2024).
- Jansen, H., Gallee, M. P., Schröder, F. H. Analysis of sonographic pattern in prostatic cancer: comparison of longitudinal and transversal transrectal ultrasound with subsequent radical prostatectomy specimens. Eur Urol. 18 (3), 174-178 (1990).
- Heijmink, S. W. T. P. J., et al. A comparison of the diagnostic performance of systematic versus ultrasound-guided biopsies of prostate cancer. Eur Radiol. 16 (4), 927-938 (2006).
- Verma, S., et al. The current state of MR imaging-targeted biopsy techniques for detection of prostate cancer. Radiology. 285 (2), 343-356 (2017).
- Moore, C. M., et al. Image-guided prostate biopsy using magnetic resonance imaging-derived targets: A systematic review. Eur Urol. 63 (1), 125-140 (2013).
- Wegelin, O., et al. The FUTURE trial: A multicenter randomised controlled trial on target biopsy techniques based on magnetic resonance imaging in the diagnosis of prostate cancer in patients with prior negative biopsies. Eur Urol. 75 (4), 582-590 (2019).
- Falagario, U. G., et al. Prostate cancer detection and complications of MRI-targeted prostate biopsy using cognitive registration, software-assisted image fusion or in-bore guidance: a systematic review and meta-analysis of comparative studies. Prostate Cancer Prostatic Dis. , (2024).
- Pokorny, M., et al. MRI-guided in-bore biopsy for prostate cancer: what does the evidence say? A case series of 554 patients and a review of the current literature. World J Urol. 37 (7), 1263-1279 (2019).
- Wegelin, O., et al. Comparing three different techniques for magnetic resonance imaging-targeted prostate biopsies: A systematic review of in-bore versus magnetic resonance imaging-transrectal ultrasound fusion versus cognitive registration. Is there a preferred technique. Eur Urol. 71 (4), 517-531 (2017).
- Puech, P., et al. Multiparametric MRI-targeted TRUS prostate biopsies using visual registration. BioMed Res Int. 2014, 819360 (2014).
- Ito, M., et al. Superior detection of significant prostate cancer by transperineal prostate biopsy using MRI-transrectal ultrasound fusion image guidance over cognitive registration. Int J Clin Oncol. 28 (11), 1545-1553 (2023).
- Oberlin, D. T., et al. Diagnostic value of guided biopsies: Fusion and cognitive-registration magnetic resonance imaging versus conventional ultrasound biopsy of the prostate. Urology. 92, 75-79 (2016).
- Khoo, C. C., et al. A comparison of prostate cancer detection between visual estimation (cognitive registration) and image fusion (software registration) targeted transperineal prostate biopsy. J Urol. 205 (4), 1075-1081 (2021).
- Fleville, S., et al. Diagnostic pathway outcomes for biparametric magnetic resonance imaging-targeted lesions using cognitive registration and freehand transperineal prostate biopsy in biopsy-naïve men (CRAFT single-center study). J Urol. 212 (6), 821-831 (2024).
- Won, S. Y., Cho, N. H., Choi, Y. D., Park, S. Y. Transrectal ultrasound-guided targeted biopsy of transition zone prostate cancer under cognitive registration with prebiopsy MRI and sonographic findings. Clin Radiol. 75 (2), 157.e21-157.e27 (2020).
- Pepe, P., Pennisi, M. Morbidity following transperineal prostate biopsy: Our experience in 8.500 men. Arch Ital Urol Androl. 94 (2), 155-159 (2022).
- Cornford, P., et al. EAU-EANM-ESTRO-ESUR-ISUP-SIOG guidelines on prostate cancer-2024 update. Part I: Screening, diagnosis, and local treatment with curative intent. Eur Urol. 86 (2), 148-163 (2024).
- Pepe, P., et al. 68Ga-PSMA PET/CT and prostate cancer diagnosis: Which SUVmax value. In Vivo. 37 (3), 1318-1322 (2023).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved