Hydrogel Synthesis
Overview
Source: Amber N. Barron, Ashlea Patterson, and Taylor D. Sparks, Department of Materials Science and Engineering, The University of Utah, Salt Lake City, UT
Hydrogels are a versatile class of cross-linked polymers produced through relatively simple procedures and with generally inexpensive materials. They can be formed from solution and involve a polymer backbone formed from monomer reagents, an initiator which makes the polymer reactive and a crosslinking species which binds the polymer chains together. An important aspect of these materials is that they swell in the presence of water, but this response can be tuned further to enhance swelling as a function of salinity, pH, or other signals. As a final product, hydrogels can be used in aqueous or dry environments, with a range of useful properties such as flexibility, high absorbance, transparency and thermal insulation. They are commonly used for liquid absorbance, sensors, consumer products, and drug delivery.
Principles
Hydrogels are a class of cross-linked polymers capable of absorbing hundreds of times their weight in water. Water enters the network and solubilizes hydrophilic and/or ionic species on the polymer backbone. The water molecules are larger than the solubilized groups and their presence inside the network causes the hydrogel to swell (Figure 1). The cross-links connecting the polymer backbone prevent the hydrogel from dissolving or breaking.

Figure 1: Hydration of a hydrogel.
In this example, the hydrogel is synthesized via free radical polymerization. A free radical is an unpaired, highly reactive electron created from a free radical initiator, such as 2,2-Dimethoxy-2-phenylacetophenone (DMPAP). UV light cleaves the carbon-carbon bond in DMPAP to form a free radical on each carbon atom (Figure 2).

Figure 2: 2,2-Dimethoxy-2-phenylacetophenone fragmenting into two free radical-carrying molecules.
The radical species reacts with double and/or triple bonds found in the polymer backbone and cross-linker. For free radical polymerization, the polymer backbone contains one double bond that propagates the chain. The free radicals react with the carbon-carbon double bond in 2-hydroxyethyl methacrylate (Figure 3) to form a propagating chain with a free radical on the end (propagation step in Figure 4). The hydroxyl group coming off the backbone is soluble in water, causing the crosslinked-network to swell.
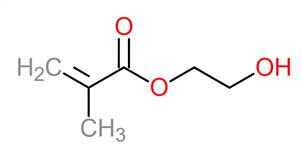
Figure 3: 2-hydroxyethyl methacrylate.

Figure 4: UV initiated free radical polymerization steps.
The radicals also react with the two carbon-carbon double bonds in tetraethylene glycol dimethacrylate (TEGDMA) (Figure 5), the chemical cross-linker, to link the backbone chains together. Hydrogel synthesis is complete when the free radicals have been consumed or have completely reacted.

Figure 5: Tetraethylene glycol dimethacrylate.
Procedure
The pre-gel solution was created in a 1000µl test tube; materials, role in polymerization and amounts added are listed in Tables 1.
| Material | Purpose | Structure | Mole percent |
| 2,2-Dimethoxy-2-phenyl-acetophenone (DMPAP) | Free readical initiator (photoinitiator) |  |
0.0012 |
| 2-Hydroxethyl methacrylate
(HEMA) |
Polymer backbone |  |
21.2121 |
| Tetraethylene glycol dimethacrylate (TEGDMA) | Crosslinker |  |
3.0303 |
| Ethylene glycol
(EG) |
Solvent | 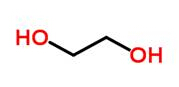 |
75.7576 |
Table 1. Hydrogel pre-gel constituents, their roles in hydrogel free radical polymerization, chemical 2D polymeric structure and the amounts added to the pre-gel solution.
Synthesis
- Prior to beginning hydrogel synthesis, a synthesis mold was assembled from two glass slides and three, 520-micron thick polyolefin-sheet spacers; this configuration was held together by binder clips as shown in Figure 6. The large glass slides were offset by a few millimeters to create a channel for pipetting the pre-gel solution into the mold.
- Before beginning hydrogel synthesis, obtain a 1000µl test tube, the chemicals described in Table 1, a micropipette with clean tips, and setting mold (Figure 6). All work should be performed in a fume hood with proper Personal Protective Equipment (PPE). PPE includes safety glasses or goggles, a lab coat and protective gloves.
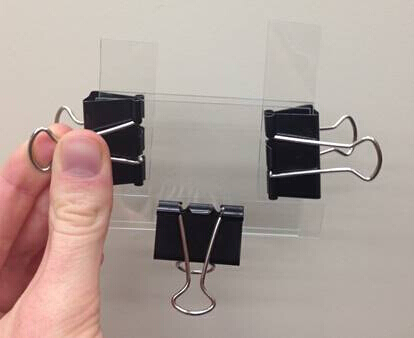
Figure 6: Hydrogel synthesis mold, created from two glass slides, three strips of 520-micron thick polyolefin-sheet as spacers, and large binder clips.
- Add 0.0012 mole percent 2,2-Dimethoxy-2-phenyl-acetophenone (DMPAP), the solid photoinitator (free radical initiator that is initiated by light), to the test tube first.
- Add 21.2121 mole percent 2-hydroxethyl methacrylate (HEMA), the backbone molecule, and 3.0303 mole percent tetraethylene glycol dimethacrylate (TEGDMA), the crosslinking molecule to the test tube, using new pipette tips each time. TEGDMA will chemically crosslink the HEMA chains in the presence of free radicals by connecting the polymer chains into a network polymer.
- Mix the solution using a vortex machine until a homogenous solution is achieved.
- Measure 0.25 grams of Bromocresol Purple and rinse it into the solution using 75.7576 mole percent ethylene glycol (EG), the solvent. The pigment is only for viewing purposes (the hydrogel is transparent otherwise), and EG serves as a solvent to dissolve the initiating free radical initiator and keeps the hydrogel flexible.
- Mix the solution using the vortex machine until the pigment completely dissolves and the solution is homogenous.
- Using a micropipette, deposit the solution into the mold by aligning the tip of the micropipette with the offset edge of the large glass slides and uniformly injecting the pre-gel solution into the center of the mold.
- Place mold 5 centimeters below a UV emitting flashlight (Warson SK66) and irradiate the mold for one minute. UV light cleaves the bonds in the initiator species, turning them into free radicals which can then attack the polymer and crosslinker molecules. When fully networked, the hydrogel should be a rubber solid with a jello-like consistency.
- Remove mold from the light and disassemble the mold configuration. Remove hydrogel from the glass slides.
- Rinse both sides of the hydrogel with deionized water to remove any unreacted chemical species and oligomers from the product.
- To characterize how various UV-light exposure times affect degree of crosslinking and swelling ability, this procedure can be repeated while varying step nine. For characterization, the solution was exposed to UV light for 1 minute, 1.5 minutes and 5 minutes, producing a total of three hydrogels.
Characterization
The swelling degree of the hydrogel can be calculated by drying, hydrating and then re-drying the polymer.
- Place the finished hydrogels in a container with an alcohol such as Isopropyl Alcohol so that they are totally submerged. Leave in the alchohol for 4-8 hours, when the alcohol has replaced all the ethylene glycol in the hydrogel.
- Remove the hydrogels from the alcohol and leave to dry in the open, about 30 minutes. The alcohol evaporates more quickly than water or the solvent, allowing the hydrogel to maintain its structure.
- Weigh the dried hydogels.
- Submerge the hydrogels in DI water for at least 30 minutes, until they're completely swollen. Remove the gels from water, gently wipe dry and weigh.
- Calculate the swelling degree using the equation:
 , where
, where  is the weight of the swollen polymer and
is the weight of the swollen polymer and 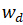 is the weight of the dried polymer.
is the weight of the dried polymer.
Results
The final hydrogel monomer is shown in Figure 7, and the synthesized hydrogels are shown in Figure 8. The degree of swelling was found to be approximately 136% for the 1 min sample, 387% for the 1.5 min sample and 81% for the 5 min sample. These results demonstrate the relationship between degree of crosslinking, or the extent to which the network is connected, and swelling ability. More links between the polymer molecules mean more elastic restraining forces on those polymer chains, which inhibit them from expanding to the same degree as a less-crosslinked hydrogel.
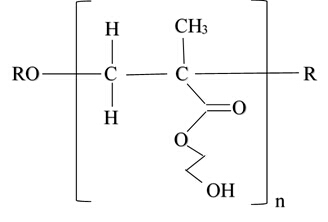
Figure 7: Monomer created from photoinitiator DMPAP, HEMA backbone, TEGDMA crosslinker, EG solvent and photochromic pigment after free radical polymerization.

Figure 8: Hydrogels after polymerization. From left to right: 1 minute under UV light during polymerization, 1.5 minutes under UV light during polymerization, 5 minutes under UV light during polymerization. The 1-minute sample appears more transparent and gel-like then the 1.5 minute and 5 minutes samples, which had increasing degrees of polymerization
Application and Summary
Hydrogel synthesis is a technique for producing crosslinked polymeric materials which can swell in response to liquid, UV light, pH, or a range of other stimulants. Synthesis by combination of liquid solutions is advantageous for the simplicity of mixing and forming hydrogels, though the final product is generally impure and tends to contain polymers with low molecular weights. This specific procedure, while simple, involves chemicals that are both toxic and flammable, and therefore requires extreme care and preventative measures. The hydrogels produced by this method are useful in applications ranging from drug delivery to sensors to absorbent hygiene products.
Hydrogels are used in a variety of consumer products, medical devices, and sensors. Consumer products such as hospital pads, feminine hygiene pads, and diapers contain sodium polyacrylate, one of the most common superabsorbent polymers. The hydrogel swells in the presence of the fluid between 300-800 times its weight. This allows manufacturers to use less material and create products that are slim and comfortable for the user to wear.
Additionally, soft contact lenses are made of silicone hydrogels, which allow oxygen to easily pass to the cornea and are more comfortable than hard contact lenses. Hydrogels are also commonly used in drug delivery because the cross-linked network allows for drugs to be stored in the three-dimensional network and slowly released into the body.
Hydrogels can also be tuned to swell as a function of salinity, pH, or other signals, making them suitable in sensor applications. The hydrogel synthesized in this video is used as a sensor in a sprinkler lawn sensor. The hydrogel is in contact with the soil and while the lawn is being watered, it swells until it triggers the sprinkler shut-off.
Tags
Skip to...
Videos from this collection:

Now Playing
Hydrogel Synthesis
Materials Engineering
23.9K Views

Optical Materialography Part 1: Sample Preparation
Materials Engineering
15.6K Views

Optical Materialography Part 2: Image Analysis
Materials Engineering
11.2K Views

X-ray Photoelectron Spectroscopy
Materials Engineering
21.9K Views

X-ray Diffraction
Materials Engineering
89.8K Views

Focused Ion Beams
Materials Engineering
9.0K Views

Directional Solidification and Phase Stabilization
Materials Engineering
6.7K Views

Differential Scanning Calorimetry
Materials Engineering
38.7K Views

Thermal Diffusivity and the Laser Flash Method
Materials Engineering
13.4K Views

Electroplating of Thin Films
Materials Engineering
20.2K Views

Analysis of Thermal Expansion via Dilatometry
Materials Engineering
16.0K Views

Electrochemical Impedance Spectroscopy
Materials Engineering
23.4K Views

Ceramic-matrix Composite Materials and Their Bending Properties
Materials Engineering
8.4K Views

Nanocrystalline Alloys and Nano-grain Size Stability
Materials Engineering
5.2K Views
Copyright © 2025 MyJoVE Corporation. All rights reserved