Thermal Diffusivity and the Laser Flash Method
Overview
Source: Elise S.D. Buki, Danielle N. Beatty, and Taylor D. Sparks, Department of Materials Science and Engineering, The University of Utah, Salt Lake City, UT
The laser flash method (LFA) is a technique used to measure thermal diffusivity, a material specific property. Thermal diffusivity (α) is the ratio of how much heat is conducted relative to how much heat is stored in a material. It is related to thermal conductivity ( ), how much heat is transferred through a material due to a temperature gradient, by the following relationship:
), how much heat is transferred through a material due to a temperature gradient, by the following relationship:
 (Equation 1)
(Equation 1)
where ⍴ is the density of the material and Cp is the specific heat capacity of the material at the given temperature of interest. Both thermal diffusivity and thermal conductivity are important material properties used to assess how materials transfer heat (thermal energy) and react to changes in temperature. Thermal diffusivity measurements are obtained most commonly by the thermal or laser flash method. In this technique a sample is heated by pulsing it with a laser or xenon flash on one side but not the other, thus inducing a temperature gradient. This temperature gradient results in heat propagating through the sample towards the opposite side, heating the sample as it goes. On the opposite side an infrared detector reads and reports the temperature change with respect to time in the form of a thermogram. An estimate of the thermal diffusivity is obtained after these results are compared and fit to theoretical predictions using a least squares model.
The laser flash method is the only method that is supported by multiple standards (ASTM, BS, JIS R) and is the most widely used method for determining thermal diffusivity.
Principles
In the laser flash method, a sample with flat, parallel top and bottom surfaces is placed in a controlled atmosphere (air, oxygen, argon, nitrogen etc) inside a sealed furnace. Samples are often thin discs with diameter of 6mm to 25.4mm and thicknesses between 1mm and 4mm. A laser with power around 15 J/pulse provides an instantaneous energy pulse to the bottom face of the sample. An infrared detector lies above the top face of the sample; this detector registers the change in temperature with time of the top face of the sample after each laser pulse. Laser pulses and resulting temperature change data are recorded for set temperature measurement points, within the range of -120℃ to 2800℃, depending on the instrument. Between each measurement taken, the temperature of the sample is allowed to equilibrate. LFA can be run on powder, liquid, bulk, composite, layered, porous, and semi-transparent samples (some modifications may be necessary depending on sample type).
The resulting data is presented in the form of a thermogram and is compared to analytical, 1-dimensional heat transport models, which assume sample opacity, homogeneity, and minimal radial heat loss. These models also assume thermal properties and sample density remain constant within the temperature ranges measured. Experimental deviations from model assumptions often require correction calculations.
There are several mathematical models used for obtaining thermal diffusivity from results of the laser flash method. The original model (Park's ideal model) involves solving a differential equation with boundary conditions that assume constant temperatures and that no heat escapes from the system during measurement. Both of these are false assumptions for real measurements. The Netzsch LFA 457 is often run using the Cowan model. This model corrects the ideal model; it takes energy and heat loss into consideration and gives more accurate fitting for many different material scans. This model is used here for an iron standard material.
Procedure
- Turn on the machine and wait for the warm-up process to end (approximately 2 hours).
- Fill up the detector compartment with liquid nitrogen using a small funnel until the nitrogen vapor can be seen coming from the detector. Let the liquid settle until there is no more vapor coming out and close the detector.
- Measure the thickness of your sample with a micrometer over several spots and calculate the average thickness and the standard deviation. The edges of the sample should be between 6mm and 25.4mm, with a flat geometry either round or rectangular. Additionally, the thickness of the sample should be uniform and between 1mm and 4mm. High thermal diffusivity samples work best with thicker samples. Here, we are using a standard iron disc sample.
- In order to maximize the absorbance of the sample and ensure uniform emissivity, spray a thin coating of graphite on the sample using colloidal graphite. Repeat three times allowing sample to dry between passes. Once done with the first side, carefully flip the sample and spray the other side.
- Once dry, place the sample in the bottom half of the small sample support and cover it with the top half of the sample support.
- Open the furnace by simultaneously pressing the safety button on the right side of the machine and the button on the front side of the machine labeled furnace with a down arrow. Rotate the detector around clockwise looking down in order to have more mobility around the furnace.
- The sample stage in the furnace has three locations designed to hold the samples. Put the sample support containing the sample in one of the three locations (take note of which one) then realign the detector and the furnace before closing the furnace. To do so, press the safety button and the labeled furnace with an up arrow.
- Before turning on the vacuum pump, make sure that the vent valve located to the right behind the detector is closed. Once closed, turn on the vacuum pump. Slowly open the vacuum valve and pump a vacuum until the pressure indicator light on the front side of the machine is stabilized to its lowest level. A vacuum is pulled to remove all air from the chamber before purging with inert gas.
- Open the regulator on the Argon cylinder and make sure the pressure is set between 5 psi and 10 psi. Close the vacuum valve, open the backfill valve then press the purge button to purge the sample space so there is no trapped gas from the sample.
- Repeat steps 8 and 9 three times to make sure that there is no air left in the chamber. This is to eliminate the chance of oxygen, nitrogen or other air constituents reacting with compounds present at the surface of the sample, particularly at elevated temperatures.
- The furnace should be left with a very slight positive pressure from the purge gas in order to ensure that air does not flow back into the furnace.
- Launch the machine's software from the desktop icon labeled "LFA 457". Select Service → Hardware Info → Switches then click the box to turn on the purge. This should turn on the Purge light on the front of the LFA-457.
- Open the vent valve while the purge light is on.
- Open a database or create a new one and enter all the necessary information, including all the necessary fields in the tabs General, Autosampler Position, Initial Conditions, Temperature Steps & Final Conditions.
- If the experiment takes longer than 8 hours, the detector will need to be filled up again. This might happen, especially if multiple samples are being run.
- Samples are then removed in a similar fashion to how they were inserted. The software automatically displays the results, here shown from an iron standard material.
Results
Figures 1, 2, and 3 show the data from an LFA run of an iron standard sample. Figures 1 and 2 show laser pulse vs time plots for two temperatures (48.2°C and 600°C); the blue trace shows the collected laser pulse from the iron sample and the thin red line shows the calculated pulse from the Cowan model. Both temperature pulses fit well to the model because this is a well-defined standard material. Generally, experimentally calculated values match the Cowan model best at high temperatures, as shown by the greater deviation from the model trace for the laser pulses at low temperatures (Figure 1) vs high temperatures (Figure 2). Low temperatures fit relatively well to the model for this standard material but deviate more than high temperature results because the lower set temperatures may not be reached in the time allowed for equilibration between each pulse. Each data point (red circle) in Figure 2 represents one laser pulse; the closer the data points fit the Cowan model, the better and more accurate the resulting thermal diffusivity values.

Figure 1: Laser signal vs time plot at 48.2 °C for an iron standard run in the LFA 457. The blue trace represents the signal from the laser hitting the sample. The thin red line represents the calculated pulse for the Cowan model.

Figure 2: Laser signal vs time plot at 600.6 °C for an iron standard run in the LFA 457. The blue trace represents the signal from the laser hitting the sample. The thin red line represents the calculated pulse for the Cowan model.
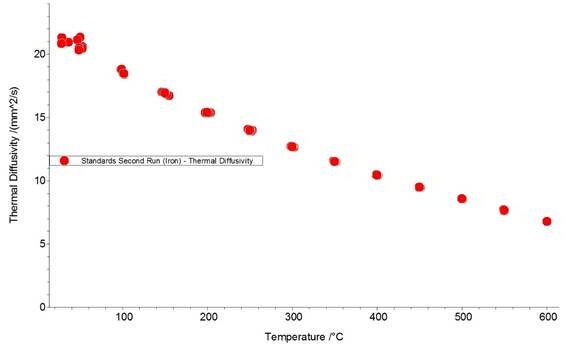
Figure 3: Thermal diffusivity (α) vs temperature plot for an iron standard disk, run in the LFA 457. Each red circle represents one laser pulse.
Application and Summary
The laser flash method is a widely used technique for determination of thermal diffusivity which consists of radiating one side of a sample with thermal energy (from a laser source) and placing an IR detector on the other side to pick up the pulse. The wide range in temperature of different models enables measurement on various types of samples. The LFA requires relatively small samples. Other tools that measure thermal conductivity directly, rather than thermal diffusivity, include the Guarded Hot Plate, Heat Flow Meter and others. The Guarded Hot Plate system can hold relatively large square samples (300mm x 300mm) and requires careful calibration in order to calculate thermal flux necessary for thermal conductivity calculation. Neither of these tools can measure thermal diffusivity to high temperatures and typically operate below 250oC.
Thermal diffusivity is an important property that needs to be known when choosing the appropriate material for any applications involving heat flow or that are sensitive to heat fluctuations. For example, thermal conductivity, aong with diffusivity, also play an important role in insulation. When selecting a material to use for insulation, it is important to be able to measure and compare the thermal properties of different materials. These thermal properties are even more critical in aerospace. Thermal protection tiles play an important role in a spacecraft's successful atmospheric re-entry. When entering the atmosphere, a spacecraft is exposed to extremely high temperatures and would melt, oxidize, or burn without a protective layer. Thermal protection tiles are typically made of pure silica glass fibers with tiny air-filled pores. These two components have low thermal conductivity and therefore minimize heat flux across the tiles. The thermal conductivity of materials with a high porosity (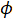 ) can be calculated with the following Maxwell's relation :
) can be calculated with the following Maxwell's relation :
 (Equation 2)
(Equation 2)
Skip to...
Videos from this collection:

Now Playing
Thermal Diffusivity and the Laser Flash Method
Materials Engineering
13.2K Views

Optical Materialography Part 1: Sample Preparation
Materials Engineering
15.3K Views

Optical Materialography Part 2: Image Analysis
Materials Engineering
10.9K Views

X-ray Photoelectron Spectroscopy
Materials Engineering
21.5K Views

X-ray Diffraction
Materials Engineering
88.2K Views

Focused Ion Beams
Materials Engineering
8.8K Views

Directional Solidification and Phase Stabilization
Materials Engineering
6.5K Views

Differential Scanning Calorimetry
Materials Engineering
37.1K Views

Electroplating of Thin Films
Materials Engineering
19.6K Views

Analysis of Thermal Expansion via Dilatometry
Materials Engineering
15.6K Views

Electrochemical Impedance Spectroscopy
Materials Engineering
23.0K Views

Ceramic-matrix Composite Materials and Their Bending Properties
Materials Engineering
8.0K Views

Nanocrystalline Alloys and Nano-grain Size Stability
Materials Engineering
5.1K Views

Hydrogel Synthesis
Materials Engineering
23.5K Views
Copyright © 2025 MyJoVE Corporation. All rights reserved