Method Article
Spinal Sonography for Ultrasound-Guided Lumbar Neuraxial Anesthesia
In This Article
Summary
The protocol described here provides the basics of spine sonoanatomy and a quick, straightforward method for performing ultrasound-guided neuraxial anesthesia. Additionally, two handheld devices that enhance portability are presented, one of which uses pattern recognition software to aid in epidural space localization.
Abstract
Neuraxial anesthesia is one of the few remaining forms of regional anesthesia that relies on palpation and tactile feedback techniques to facilitate catheterization into the epidural space. Over two decades ago, spine ultrasonography was demonstrated to provide reliable guidance for locating the epidural space. Compared to the palpation technique, preprocedural ultrasonography has been shown to result in fewer needle punctures and fewer traumatic procedures, particularly in patients with abnormal or distorted spine anatomy (e.g., scoliosis, obesity). Despite its utility, the ultrasound-guided neuraxial technique is still marginally used, even for patients with abnormal anatomy. Some experts attribute this to cost, a relatively high success rate without ultrasound, and a lack of technical expertise, which is often tied to formal education and regular practice. Several proponents of the ultrasound technique emphasize that proficiency requires practice on patients with normal spine anatomy, though this training may not be as challenging as once thought. This protocol was designed to help all providers learn the basics of lumbar spine anatomy and how to apply this knowledge clinically. Through a series of videos, we will provide step-by-step instructions for performing neuraxial ultrasonography and offer practical tips for troubleshooting in cases of difficult anatomy.
Introduction
Lumbar epidural analgesia provides the dual benefit of providing effective labor analgesia and the best way of avoiding the use of general anesthesia1. The latter has been associated with anesthetic and surgical complications as well as an increased risk of postpartum depression2,3. Hence, it is not surprising that anesthesiologists have evaluated many techniques over the years to decrease the incidence of epidural catheter failures. Several techniques (e.g., combined spinal and dural puncture epidural) evaluated over the years have been shown to reduce the incidence of epidural catheter failures1,4,5. Yet, to the best of the authors understanding, the ultrasound-guided neuraxial technique is the only technique that has demonstrated a decrease in the rate of failed epidural catheters and the number of epidural attempts, particularly when performed by relatively inexperienced providers6.
There is mounting high-quality evidence to demonstrate that ultrasound-guided neuraxial anesthesia decreases the number of needle manipulations, provides an excellent correlation between the estimated and actual depth from skin to epidural space, and reduces traumatic procedures7,8,9,10,11,12. Besides, the traditional anatomical landmark approach has proven inferior to the ultrasound technique or imaging for identifying the desired interspace for instrumentation13,14. The abovementioned benefits are noticed in patients with normal and abnormal anatomy. Yet, the evidence suggests that patients with abnormal anatomy benefit the most from using ultrasound guidance9,11,15,16. Perhaps these advantages prompted the National Institute for Health and Excellence (NICE) to determine that there was enough evidence to recommend the routine use of ultrasound guidance for establishing neuraxial anesthesia6,17. Close to two decades after that recommendation, this technique is scarcely, rather than routinely utilized.
Some cited reasons for this slow embrace include a high success rate without ultrasound, lack of access to the technology, additional time to obtain imaging, and lack of formal training18,19,20,21. While it is conceivable that access to ultrasound and the image quality were less than optimal when this technique was first described by Cork et al. in 1980, imaging quality and accessibility to ultrasound have improved22,23. Besides availability, portability has also increased without compromising image quality24,25,26. Hence, we have overcome most of the obstacles that have slowed the acceptance of this technique. The hurdles to overcome are the relatively high success rate without ultrasound, additional time to obtain imaging, and lack of formal training.
While the overall success rate of epidurals is high, the number of needle attempts is not often reported. Given that ultrasound-guided neuraxial anesthesia has been shown to decrease the number of needle manipulations (attempts and redirections) and failed catheters, it is conceivable that this technique may also improve patient satisfaction16. Besides the high success rate, the last two hurdles are time and formal training15,16,27,28,29. Regarding formal training, this is perhaps the rate-limiting factor. The skepticism surrounding the use of this technique perpetuates the lack of formal training. With the protocol below and enough practice (in patients with normal anatomy), most providers will achieve proficiency and seize the benefits of this procedure, even in the most challenging cases9,17,21.
Protocol
All procedures involving human participants in the study were conducted in accordance with the ethical standards of the institutional guidelines for research and the 1964 Declaration of Helsinki, including its later amendments or comparable ethical standards. The protocol was developed based on input from previously published articles in the academic literature30,31,32. Imaging studies were conducted on the authors themselves for normal images and as part of routine educational spine sonoanatomy. The following sections discuss the use of preprocedural ultrasound-guided neuraxial anesthesia, but real-time ultrasound guidance is not addressed. Details of the equipment used in this study are listed in the Table of Materials.
1. Probe selection
- Select a low-frequency (2-5 MHz) curvilinear probe if utilizing a traditional ultrasound device. See Figure 1A.
- For a handheld device (see Table of Materials), select a curvilinear frequency probe. Click on presets and select abdomen. See Figure 1B and Figure 2A,B.
- For an automated device (see Table of Materials), choose the spine option from the scanning menu. See Figure 1C.
2. Machine preset
- Set the scanning depth at 8 cm.
NOTE: A study by Sutton et al.33 demonstrated that most patients would have a skin-to-epidural space distance of 4-6 cm (76%), whereas depths between 2-4 cm or >6 cm range from 16% to 2%, respectively. Given the current obesity epidemic, depths >6 cm are probably more common than the cited 2%. Another great reason to use ultrasound is to help discern when a needle longer than the typical 3.5-inch Tuohy needle is needed. If using the AU, the device will automatically adjust according to the estimated depth.
3. Scanning technique
- Ask the patient to sit in the traditional sitting position with shoulders relaxed, chin to chest, arms resting on their thighs, and a slouched posture (pushing the belly button towards the provider)34.
- Apply ultrasound gel to the transducer.
4. Longitudinal paramedian view
- Place the ultrasound probe angled towards the midline over the sacral area, about 3 cm from the midline (Figure 3).
- Move the probe cephalad until a solid hyperechoic line is visualized.
NOTE: A solid, continuous hyperechoic line will be noticed. This is the sacrum. - Continue to scan the cephalad until the "saw sign" is visible.
NOTE: The teeth of the "saw" are formed by the lamina. The space between represents the interspace. With a good ultrasound window, the interspace looks like an equal sign (Figure 2). The equal sign - the blue line in Figure 3 and Figure 4 represents the posterior complex (PC), composed of the ligamentum flavum and the posterior dura matter. The white line in Figure 3 and Figure 4 represents the anterior complex (AC) - formed by the anterior dura, posterior longitudinal ligament, and vertebral body. The sacrum is only visualized when utilizing this view. Hence, this is the ideal view for estimating at which level the block is being performed. After locating the sacrum, the levels are counted as they appear (e.g., L5-S1, L5-L4, L4-L3, L3-L2).
5. Transverse view
- Place the ultrasound probe at the predicted midline.
- Scan the cephalad or caudal until a long hypoechoic (dark) line is noted (Figure 5).
NOTE: This hypoechoic line represents the spinous process, and you can mark this as the midline. The spinous process may be noted to be at an angle for patients with scoliosis. Angle the probe to the left or to the right to correct for the curvature. This angle should be considered to guide your epidural needle's trajectory. - Continue to scan the cephalad or caudal until the pattern shown in Figure 4 is localized.
NOTE: The interspace can be recognized and marked when the articular processes, transverse processes, and posterior (PC) and anterior complex (AC) come into view. Once these structures have been identified, the interspace can be marked on the patient's back by drawing a line on the lateral side of the probe. - Place the device over the suspected midline (users utilizing the AU device).
NOTE: This device has pattern recognition software programmed to identify the previously described bony landmarks. A 2D and 3D spine representation image will appear on the upper and lower portion of the screen, respectively, of your screen, and a crosshair icon will show when the space is correctly identified.
6. Measurements
- Once the posterior complex (PC) has been located, select the caliper (traditional US) and adjust the measure from the skin to the posterior complex (Figure 6A,B).
- For BU users, click on freeze image (Figure 7A), then click on Actions, select line (Figure 7B), and measure as shown in the previous step.
- Draw a straight line from the skin to the PC.
NOTE: This measurement will be the estimated depth from skin to epidural space. It is important to ensure no pressure is applied between the probe and the skin; otherwise, the measurement will underestimate the actual depth. - For Automated device users (see Table of Materials), place the device on the patient's back, and the device will automatically provide the estimated depth (Figure 8).
NOTE: As described above, applying pressure on the skin should be avoided to improve measurement accuracy.
7. Epidural placement
- After obtaining the information from the steps above, clean and drape the patient following the institutional protocol.
- Ensure that the insertion point is the midline and interspace marking intercept.
NOTE: The midline refers to the vertical centerline along the patient's back. The needle must be placed here for optimal results. The vertebrae in the spine have small gaps (interspaces) between them. The needle should be inserted in one of these gaps. The interspace marking intercept refers to the specific point where these guidelines (midline and interspace) meet. - Proceed with the epidural placement utilizing the loss of resistance technique.
NOTE: The loss of resistance technique is a common method for identifying when the needle has reached the epidural space. The medical practitioner will gently push the needle and feel for a "loss of resistance" as the needle passes through the ligament and enters the epidural space, indicating the correct position for administering the anesthesia. The information from the steps above should be used as guidance, not as a substitute for your clinical judgment or practice. For instance, the estimated depth measure in step 18 could underestimate or overestimate the actual needle depth.
8. Follow-up procedures
- Ensure the catheter is securely taped to the patient's back to prevent it from moving or dislodging.
- Document the insertion site and note the catheter's depth for future reference.
- After completing epidural catheterization, evaluate patient's pain management. This is usually done 15-30 min after the procedure.
- Ensure the catheter is functioning properly and delivering medication consistently.
Results
The main results from this research have focused on image quality and proficiency in performing ultrasound-guided neuraxial anesthesia. When comparing the quality of images from the BU to those of a mid-range ultrasound machine, it was determined that the former is a good alternative for obtaining spine anatomy images26. In terms of proficiency, in a prospective cohort analysis, first attempt success rate (defined as the number of skin needle punctures), number of needle passes (defined as the number of needle redirection), needle attempts (skin punctures), block time (defined as the time needed to perform a block from the time of local anesthetic infiltration until loss of resistance), and the difference between actual needle depth (skin to epidural space distance) and ultrasound-guided estimated distance were evaluated. For the primary outcome (first-attempt success), utilizing the transverse view, the group demonstrated a first-attempt success rate of 60% and 84% (p < 0.001) for the landmark and ultrasound-guidance techniques, respectively. When assessing first-pass success (single skin puncture without the need for needle redirection), the ultrasound-guided group outperformed the landmark technique (75% vs. 46%, p < 0.001, respectively)29.
The block time, expressed as mean (standard deviation), was 342.20 s (414.62) vs. 184 s (174.28) for the landmark versus ultrasound technique, respectively. The mean (SD) image acquisition time was 82.10 s (65.25 s). After adding the image acquisition time, the ultrasound group resulted in overall faster epidurals (266 s (181.33 s) and 342.20 s (414.62 s), p = 0.04). When the impact of the level of training (years of experience) was considered, there were fewer needle attempts and passes with the ultrasound-guided technique compared to the landmark technique across all levels of training. Procedural time was noted to be faster with the ultrasound-guided technique versus the landmark approach for providers with 2-3 years of experience in anesthesia. In contrast, no statistical difference in block time was noted for those with ≥4 years of experience. Lastly, the mean difference between the actual needle depth and ultrasound-guided estimated depth was 0.39 cm (95% CI 0.32 to 0.46), p < 0.0129.
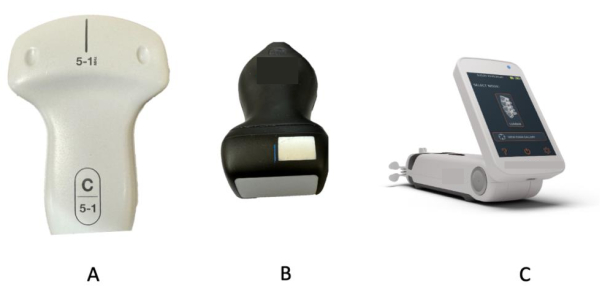
Figure 1: Equipment for spinal sonography. (A) Traditional curvilinear probes. (B) Handheld ultrasound probe. (C) Automated ultrasound device. These represent some of the available equipment alternatives for performing spinal sonography. Please click here to view a larger version of this figure.
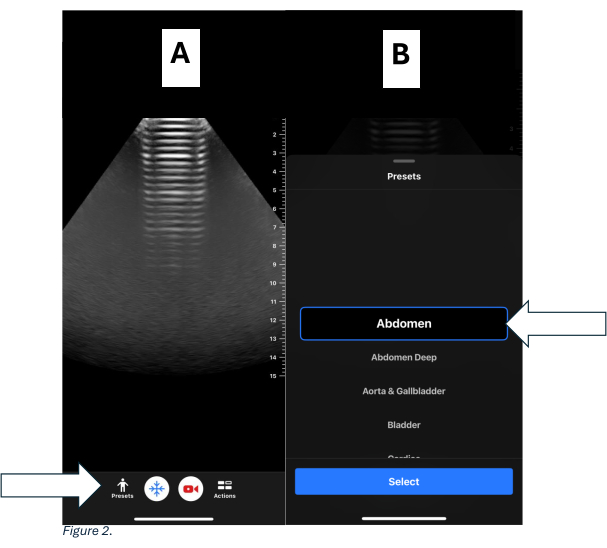
Figure 2: Presets for handheld device. (A) Home screen of the handheld device displaying presets on the lower left side. (B) Highlighted preset selection showing the recommended Abdomen Preset. Please click here to view a larger version of this figure.

Figure 3: Paramedian longitudinal view. (A) Paramedian placement of the ultrasound probe on a model. (B) Sonographic images captured from a paramedian longitudinal view. () Paramedian probe placement on a spine model. The blue arrow indicates the sacrum, blue triangles indicate the "sawtooth" pattern, the white box highlights the interspace, the blue line represents the posterior complex, and the white line represents the anterior complex. Please click here to view a larger version of this figure.
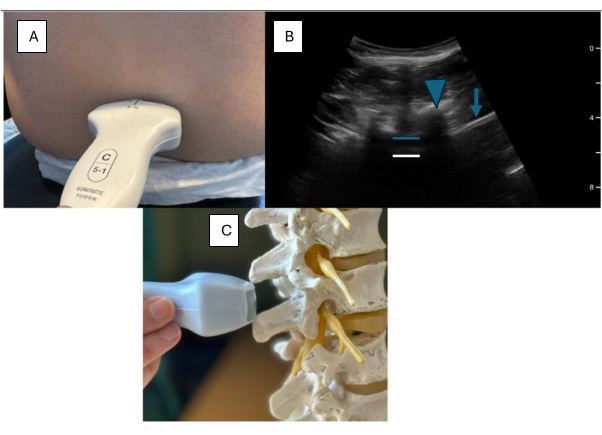
Figure 4: Transverse view. (A) Transverse probe placement on a model. (B) Sonographic transverse view of the lumbar spine. (C) Transverse view on a lumbar spine model. Blue arrows indicate the articular process, PC refers to the posterior complex, AC refers to the anterior complex, the blue arrowhead represents the articular process, and the blue arrow indicates the transverse process. The blue line highlights the posterior complex, and the white line highlights the anterior complex. Please click here to view a larger version of this figure.
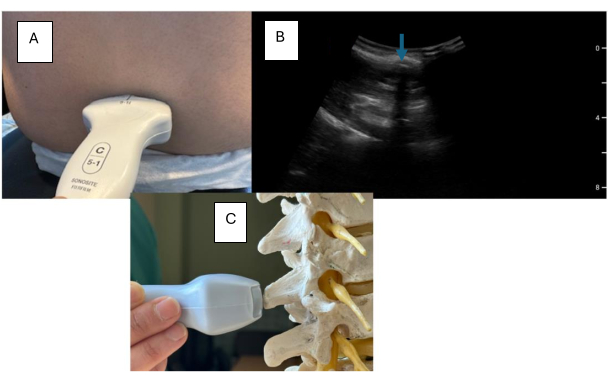
Figure 5: Transverse view with focus on spinous process. (A) Transverse probe placement on a model. (B) Sonographic lumbar spine transverse view highlighting the spinous process. (C) Transverse view on a lumbar spine model. The blue arrow indicates the spinous process. Please click here to view a larger version of this figure.
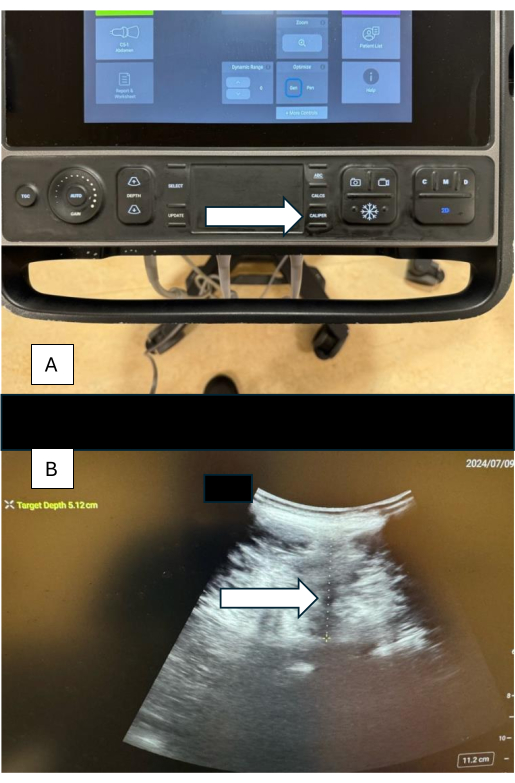
Figure 6: Measurements using calipers. (A) Caliper button on the sonographic device. (B) Sonographic image showing caliper measurement of the distance from skin to posterior complex, with the estimated depth shown in the left corner of the image. Please click here to view a larger version of this figure.
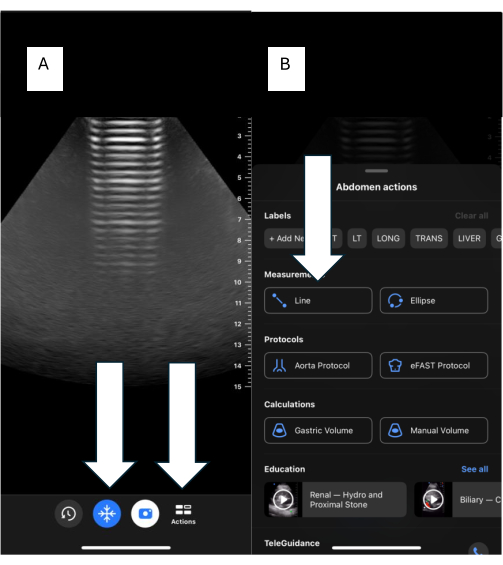
Figure 7: Measurements using handheld devices. (A) The main screen of the handheld device with "Freeze" and "Action" buttons. (B) Line Action feature for making measurements. Please click here to view a larger version of this figure.

Figure 8: Automated ultrasound device. The blue numbers on the right side of the screen represent the estimated distance from the skin to the spinous process, while the orange number represents the estimated distance from the skin to the epidural space. Please click here to view a larger version of this figure.
Discussion
The main findings of this research are that the use of US-guided neuraxial anesthesia results in an overall increased first-attempt success. That is, fewer needle attempts and passes are needed to identify the epidural space29. The abovementioned findings are in agreement with those of several meta-analysis studies when comparing preprocedural US-guided neuraxial anesthesia to the landmark technique7,8,9,10,11,12. Given the fewer needle attempts and redirections, it is not surprising that the use of ultrasound has also been associated with a lower incidence of back pain, traumatic injections, accidental dural puncture, and subsequent headaches7,8,9,10,11,12. Hence, as expected, patient satisfaction has been reported to be higher when comparing the ultrasound technique to the traditional landmark technique (Standardized Mean difference (SMD) - 0.25; 95% CrI 0.05 to 0.45)11.
As noted in the current research, experience level may play a role in the measured outcomes. When assessing the evidence, it is essential to analyze the methodology of these studies, particularly the sonographers' and proceduralist level of expertise. Generally speaking, for patients with normal anatomy and in the hands of experienced providers, ultrasound provides little to no benefits35,36. Similarly, ultrasound does not lead to marked benefits when relatively inexperienced providers obtain images and perform the procedure, particularly for patients with normal anatomy7,9,10,29. On the other hand, there is good evidence to suggest that patients with abnormal anatomy (e.g., obesity, scoliosis) benefit from the use of ultrasound15,28,37. Despite individual study differences, two recent meta-analysis studies demonstrated that US-guided benefits patients with normal and abnormal anatomy11,12.
Another significant benefit of US-guided neuraxial anesthesia is its ability to identify a particular interspace accurately. The clinical importance of this advantage relates to the avoidance of the L1 interspace, as the cord ends at this interspace in most patients. When aiming at a lumbar epidural, the avoidance of this space limits possible complications related to cord injury38. Compared to imaging techniques, the landmark technique has been shown to be correct 29% of the time for identifying a particular interspace. Furthermore, it was noted to be wrong by two interspaces 27% of the time. On the other hand, the US-guided technique was reported to be correct 68%-76% of the time and rarely wrong by more than one interspace9.
One perceived limitation of using ultrasound is that it will add time to the overall procedural (block) time18. As demonstrated by our group, the time to obtain images is <2 min when proficiency is obtained29. Of note, for this study, the transverse approach was utilized as this single view is as accurate as the combined transverse (for midline identification) and paramedian (for interspace identification) approach, allowing for the identification of midline and interspace30. These findings correlate with several studies that compared needle attempts and overall block time between the traditional landmark approach and the preprocedural ultrasound technique15,28,39. Furthermore, two recent meta-analyses have demonstrated that at the very least, ultrasound technology [including computer-aided devices (i.e., AU)] does not result in a difference in procedural time. If it does, it may decrease the overall procedural time by 30 s11,12.
This protocol and the results demonstrate that the ultrasound can provide a reliable estimated depth from skin to epidural space. This distance varies from 1-13 mm, with most studies reporting a mean difference of ≤3 mm9. Clinically, this information can be utilized to make decisions regarding equipment (i.e., needle size) and for guidance when performing or supervising a neuraxial technique. In most instances, the estimated depth will be less than the actual needle depth. This perceived disadvantage, which is expected secondary to tissue compression and differences in needle versus ultrasound beam trajectory, is considered by some experts to increase the safety margin of the procedure11,25.
Despite the skepticism and perceived limitations to the use of ultrasound, the only rate-limiting step to its routine use is a lack of formal training18. Learning the US-guided technique requires practice with accurate preprocedural marking, which is the precision needed to measure the distance from skin to epidural space, and to mark the optimal insertion point requires constant training, initially with a substantial number of patients with normal anatomy17,21,40. Paraphrasing Arzola et al.17, an anesthesiologist with over a decade of research in this topic and training clinicians practicing this technique in routine cases (with normal /palpable) anatomy is imperative to obtain technical proficiency. Only then will the technique prove to be helpful when utilized for the patient with abnormal anatomy.
The use of ultrasound has changed how we perform procedures. Over the last decade, most procedures solely based on anatomy or using nerve stimulators (e.g., central lines, regional blocks) have shifted to ultrasound-guided procedures. This transition has been slow for the adoption of US-guided neuraxial technique, and neuraxial anesthesia remains one of the few procedures based solely on anatomy and palpation. There is now ample objective (high-quality) evidence that demonstrates that the limitations of the use of ultrasound are mainly subjective8,9,10,11,12. In light of the evidence suggesting that US-guided neuraxial anesthesia may improve patient satisfaction, all anesthesiologists should consider utilizing the above protocol, if not routinely, at least frequently.
Disclosures
One of the authors (Antonio Gonzalez) is conducting a research project that is funded by the Butterfly Network. This author has provided his opinion and helped with the creation of educational material for Rivanna Medical (work not funded by the company).
Acknowledgements
We thank our fellows and residents who encourage us to keep up with our ever-changing practice.
Materials
| Name | Company | Catalog Number | Comments |
| ACCURO | Rivanna Medical | NA | Described throughout the manuscript as the automated device |
| Butterfly iQ+ | Butterfly Network | iQ+ | Described throughout the manuscript as the handheld device |
| Traditional ultrasound | SonoSite | SonositePX | Select a low-frequency (2-5 MHZ) curvilinear probe if utilizing a traditional ultrasound device. |
References
- Berger, A. A., Jordan, J., Li, Y., Kowalczyk, J. J., Hess, P. E. Epidural catheter replacement rates with dural puncture epidural labor analgesia compared with epidural analgesia without dural puncture: a retrospective cohort study. Int J Obstet Anesth. 52, 103590 (2022).
- Guglielminotti, J., Landau, R., Li, G. Adverse events and factors associated with potentially avoidable use of general anesthesia in cesarean deliveries. Anesthesiology. 130, 912-922 (2020).
- Guglielminotti, J., Li, G. Exposure to general anesthesia for cesarean delivery and odds of severe postpartum depression requiring hospitalization. Anesth Analg. 131 (5), 1421-1429 (2020).
- Booth, J. M., et al. Combined spinal-epidural technique for labor analgesia does not delay recognition of epidural catheter failures: A single-center retrospective cohort survival analysis. Anesthesiology. 125 (3), 516-524 (2016).
- Groden, J., Gonzalez-Fiol, A., Aaronson, J., Sachs, A., Smiley, R. Catheter failure rates and time course with epidural versus combined spinal-epidural analgesia in labor. Int J Obstet Anesth. 26 (C), 4-7 (2016).
- Vallejo, M. C., Phelps, A. L., Singh, S., Orebaugh, S. L., Sah, N. Ultrasound decreases the failed labor epidural rate in resident trainees. Int J Obstet Anesth. 19 (4), 373-378 (2010).
- Zhang, Y., Peng, M., Wei, J., Huang, J., Ma, W., Li, Y. Comparison of ultrasound-guided and traditional localization in intraspinal anesthesia: A systematic review and network meta-analysis. BMJ Open. 13 (11), e071253 (2023).
- Young, B., Onwochei, D., Desai, N. Conventional landmark palpation vs. preprocedural ultrasound for neuraxial analgesia and anesthesia in obstetrics - a systematic review and meta-analysis with trial sequential analyses. Anaesthesia. 76 (6), 818-831 (2021).
- Perlas, A., Chaparro, L. E., Chin, K. J. Lumbar neuraxial ultrasound for spinal and epidural anesthesia: A systematic review and meta-analysis. Reg Anesth Pain Med. 41 (2), 251-260 (2016).
- Shaikh, F., et al. Ultrasound imaging for lumbar punctures and epidural catheterizations: systematic review and meta-analysis. BMJ. 346, f1720 (2013).
- de Carvalho, C. C., et al. Efficacy and safety of ultrasound-guided versus landmark-guided neuraxial puncture: A systematic review, network meta-analysis and trial sequential analysis of randomized clinical trials. Reg Anesth Pain Med. , (2024).
- Kamimura, Y., et al. Comparative efficacy of ultrasound guidance or conventional anatomical landmarks for neuraxial puncture in adult patients: A systematic review and network meta-analysis. Br J Anaesth. 132 (5), 1097-1111 (2024).
- Watson, M. J. Could ultrasonography be used by an anesthetist to identify a specified lumbar interspace before spinal anesthesia. Br J Anaesth. 90 (4), 509-511 (2003).
- Furness, G., Reilly, M. P., Kuchi, S. An evaluation of ultrasound imaging for identification of lumbar intervertebral level. Anaesthesia. 57 (3), 277-280 (2002).
- Chin, K. J., et al. Ultrasound imaging facilitates spinal anesthesia in adults with difficult surface anatomic landmarks. Anesthesiology. 115 (1), 94-101 (2011).
- Li, M., et al. Ultrasound-assisted technology versus the conventional landmark location method in spinal anesthesia for cesarean delivery in obese parturients. Anesth Analg. 129 (1), 155-161 (2018).
- Arzola, C. Preprocedure ultrasonography before initiating a neuraxial anesthetic procedure. Anesth Analg. 124 (3), 712-713 (2017).
- Gambling, D. R. Lumbar ultrasound: Useful gadget or time-consuming gimmick. Int J Obstet Anesth. 20 (4), 318-320 (2011).
- Wong, J., et al. Barriers to learning and using point-of-care ultrasound: a survey of practicing internists in six North American institutions. Ultrasound J. 12 (1), 19 (2020).
- Chui, J., et al. Identifying barriers to the use of ultrasound in the perioperative period: A survey of southwestern Ontario anesthesiologists. BMC Health Serv Res. 19 (1), 214 (2019).
- Margarido, C. B., Arzola, C., Balki, M., Carvalho, J. C. A. Anesthesiologists' learning curves for ultrasound assessment of the lumbar spine. Can J Anesth. 57 (2), 120-126 (2010).
- Corr, R. C., Kryc, J. J., Vaughan, R. W. Ultrasonic localization of the lumbar epidural space. Anesthesiology. 52 (6), 513-515 (1980).
- Lee, A. Ultrasound in obstetric anesthesia. Semin Perinatol. 38 (6), 349-358 (2014).
- Weiniger, C. F., et al. A randomized trial to investigate needle redirections/re-insertions using a handheld ultrasound device versus traditional palpation for spinal anesthesia in obese women undergoing cesarean delivery. Int J Obstet Anesth. 49, 103229 (2021).
- Carvalho, B., Seligman, K. M., Weiniger, C. F. The comparative accuracy of a handheld and console ultrasound device for neuraxial depth and landmark assessment. Int J Obstet Anesth. 39, 68-73 (2019).
- Salimi, N., Aymen, A. Ultrasound image quality comparison between a handheld ultrasound transducer and mid-range ultrasound machine. POCUSJ. 7 (1), 154-159 (2022).
- Sahin, T., Balaban, O., Sahin, L., Solak, M., Toker, K. A randomized controlled trial of preinsertion ultrasound guidance for spinal anesthesia in pregnancy: Outcomes among obese and lean parturients. J Anesth. 28 (3), 413-419 (2014).
- Park, S. -. K., et al. Ultrasound-assisted versus landmark-guided spinal anesthesia in patients with abnormal spinal anatomy: A randomized controlled trial. Anesth Analg. 130 (3), 787-795 (2020).
- Fiol, A. G., et al. A prospective cohort study to evaluate needle passes using a portable ultrasound device versus traditional landmark approach for epidural anesthesia in a busy obstetric tertiary care center. POCUS J. 8 (2), 153-158 (2023).
- Arzola, C., Davies, S., Rofaeel, A., Carvalho, J. C. A. Ultrasound using the transverse approach to the lumbar spine provides reliable landmarks for labor epidurals. Anesth Analg. 104 (5), 1188-1192 (2007).
- Carvalho, J. C. A. Ultrasound-facilitated epidurals and spinals in obstetrics. Anesthesiol Clin. 26 (1), 145-158 (2008).
- Sivakumar, R. K., Karmakar, M. K. Spinal sonography and central neuraxial blocks. Best Pract Res Clin Anaesthesiol. 37 (2), 209-242 (2023).
- Sutton, D. N., Linter, S. P. K. Depth of extradural space and dural puncture. Anaesthesia. 46 (2), 97-98 (1991).
- Özhan, M. &. #. 2. 1. 4. ;., Çaparlar, C. &. #. 2. 1. 4. ;., Süzer, M. A., Eskin, M. B., Atik, B. Comparison of three sitting positions for combined spinal - epidural anesthesia: a multicenter randomized controlled trial. Braz J Anesthesiol. (English Edition). 71 (2), 129-136 (2021).
- Ansari, T., Yousef, A., Gamassy, A. E., Fayez, M. Ultrasound-guided spinal anesthesia in obstetrics: Is there an advantage over the landmark technique in patients with easily palpable spines. Int J Obstet Anesth. 23 (3), 213-216 (2014).
- Tawfik, M. M., Atallah, M. M., Elkharboutly, W. S., Allakkany, N. S., Abdelkhalek, M. Does preprocedural ultrasound increase the first-pass success rate of epidural catheterization before cesarean delivery? a randomized controlled trial. Anesth Analg. 124 (3), 851-856 (2017).
- Singla, P., et al. Feasibility of spinal anesthesia placement using automated interpretation of lumbar ultrasound images: a prospective randomized controlled trial. J Anesthe Clin Res. 10 (2), 878 (2019).
- Arnolds, D., Hofer, J., Scavone, B. Inadvertent neuraxial block placement at or above the L1-L2 interspace in the super-obese parturient: A retrospective study. Int J Obstet Anesth. 42, 20-25 (2019).
- Creaney, M., Mullane, D., Casby, C., Tan, T. Ultrasound to identify the lumbar space in women with impalpable bony landmarks presenting for elective cesarean delivery under spinal anaesthesia: A randomised trial. Int J Obstet Anesth. 28, 12-16 (2016).
- Arzola, C., Mikhael, R., Margarido, C., Carvalho, J. C. A. Spinal ultrasound versus palpation for epidural catheter insertion in labor. Eur J Anaesthesiol. 32 (7), 499-505 (2015).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved