Rodent Handling and Restraint Techniques
Genel Bakış
Source: Kay Stewart, RVT, RLATG, CMAR; Valerie A. Schroeder, RVT, RLATG. University of Notre Dame, IN
It has been demonstrated that even minimal handling of mice and rats is stressful to the animals. Handling for cage changing and other noninvasive procedures causes an increase in heart rate, blood pressure, and other physiological parameters, such as serum corticosterone levels. Fluctuations can continue for up to several hours. The methods of restraint required for injections and blood withdrawals also cause physiological changes that can potentially affect scientific data. Training in the proper handling of mice and rats is required to minimize the effects to the animals.1 Mice and rats can be restrained manually with restraint devices, or with chemical agents. Manual methods and the use of restraint devices are covered in this manuscript. All restraint methods include the process of lifting the animals from their home cage.
İlkeler
Common methods for removing either a mouse or a rat from its cage involve lifting the animal by the tail, using forceps to grab the scruff of the neck or base of the tail, using a tube or other enrichment device,2 grasping around the body, or scooping the animal into the palm.3,4
When lifting a mouse or rat by the tail, it is imperative that the tail be grasped at the base near the rump of the animal. Should the animal be lifted from the end of the tail, the skin of the tail can deglove and be pulled off by the stress of the body weight; a degloving injury will result in the tail being amputated. 3 Moving an animal from one cage to another, or to a working surface, should be done quickly and steadily. Suspending the animal in the air for any length of time will not only cause distress, but can also cause the animal to twist or struggle, possibly resulting in harm to the tail. The handler should not place their hand under the animal because-as it is lifted by its tail-it is instinctive for the animal to try to grasp the hand for security, resulting in a bite.
In many mouse production facilities, forceps are used to remove animals from their cages. This is done for biosecurity, as it is believed that there is less chance for cross-contamination from animal to animal. The forceps tips should be stored in alcohol between handling of individual animals, or groups of animals. There are a variety of forceps used to grasp mice. The most commonly used include long dressing forceps with atraumatic tips-or with rubber, plastic, or silicone tubing placed over the ends of the forceps to provide a cushion-or tongue forceps with rubber grips.4
Forceps can grasp a mouse either at the scruff of the neck or at the base of the tail. Most mice become accustomed to this method quickly and do not struggle. However, patience and practice is necessary to become adept at catching the animals. Beginners will need to be trained on how tightly to grasp the mice, especially at the scruff, as this could interfere with breathing. When using forceps on the tail, care must be taken to place the forceps near the base of the tail to avoid injury.
For animals that are anxious or exhibit stereotypies, placing tubes into the cage may decrease handling difficulty, and reduce the flight/fright response to daily animal husbandry. A tube made of a nonporous material can be added for enrichment, and serve as a place for the animal to shelter or seek safe refuge. Such tubes can be open-ended or capped on one end. Most mice or rats will willingly go into the tubes when their cage is opened, or they can be easily guided to the tube. Once the animal is inside the tube, the open end is covered to transport the animal to the new location. When placed in a fresh cage, the tube can be gently tipped upward to encourage the animals to leave it. Animals are easily conditioned to this method of cage changing and will immediately enter the tubes, making this method as quick and easy as others for moving animals from one place to another. Although tubes do not have to be present in the home cage to be effective, having a tube in the primary enclosure increases recognition of a familiar safe site, as well as provides cage enrichment.2
Some institutions prefer that animals be lifted by the body for routine handling, especially for handling rats. Animals must be habituated to this method from an early age. Young rats between 2-4 weeks of age tend to jump straight up. Placing a hand over the rat's back will cause it to jump into the palm of the handler, allowing it to be grasped. A second hand may be needed to prevent the rat from squirming out of grasp before placing it into another cage. Adult rats are gently grasped around the thorax, lifted, and quickly placed into another cage, or onto a surface.
When changing cages with neonate mice or rats, it is often necessary to remove them while keeping the nest intact. With the use of two hands to scoop the nest and neonates from the bottom of the cage, the intact nest is moved into a new cage. However, to avoid dropping pups, the fingers of each hand must be held closely together. Once moved to a new cage, it is important to verify that the pups are all present. It is advisable to count the pups before and after moving them. A plastic scoop may be used instead of the hands. If this method is used, a hand should be placed over the top of the scoop to prevent neonates from wiggling or jumping out of the scoop. This method of transferring pups, compared to the individual handling of the pups, is less distressing to both the pups and the dams.
Restraint for technical procedures requires a confident and firm, but gentle, touch. Tentative approaches can result in handler bites. If the handler recoils as an animal squeals, that animal quickly learns how to avoid restraint. However, aggressive handling can result in the injury or death of rodents. A balance of an assertive yet gentle approach is the goal for rodent handling.
Mice and small rats can be restrained by grasping the skin at the nape of the neck, referred to as scruffing. Precautions for this method include both grasping the skin too firmly or too loosely. If the skin is grasped too tightly, the airway can become constricted, which can lead to death. If grasped too loosely, the animal will be able to turn its head and potentially bite the handler.
Animals that are agitated or extremely fearful are much more likely to bite. It is best to calm the animal prior to handling. There are various calming techniques that have been tried with varying success. The most reliable method is to use some sort of chemical restraint, most often an inhalation anesthetic. Isoflurane or sevoflurane are short-acting inhalation anesthetics that can be delivered with only the handling required to transfer the animal from its cage to an induction chamber. Once the animal is anesthetized, they can be manipulated or manually restrained for the procedure.
A method for calming a rat is to wrap it in a thick terry towel, allowing it to hide under the towel. Placing it on a lab coat sleeve so that they can bury their head in the folds at the elbow may also provide a sense of safe security to the animal. This same result can be achieved by placing the animal in a dark area for a few minutes.
Prosedür
1. Scruffing
Mice are most often restrained using the scruffing technique, but young rats can also be restrained with this method. Adult rats are more difficult to restrain with this technique due to a more muscular neck, a reduced amount of loose skin, and an aversion to this method.
- The one-handed restraint method is utilized most often in mouse strains that are very calm, such as the athymic nude, SCID, and some GEM strains. However, there is a greater risk for being bitten with this method. Novice animal handlers should become comfortable with the two-handed restraint technique before attempting the one-handed method.
- Place the animal on a surface that they can grip, such as a wire bar cage top or a mat.
- Hold the tail between the third and fourth fingers of the nondominant hand, and apply gentle backward traction. This causes the animal to hold onto the surface, and results in the body being stretched and elongated.
- Turn the hand so that the palm is facing the body of the animal.
- With the index finger and thumb, pin the rodent over the shoulders.
- Gently slide the thumb and index finger forward, and position them at the base of the skull. Grasp the loose skin at the neck and lift the animal.
- Use the middle finger to stabilize the animal along the back by pinning the dorsal skin against the base of the thumb.
- Two-handed restraint
- Place the animal on a surface that they can grip.
- Hold the tail at the base (within 1-2 cm of the body) with the dominant hand, and apply gentle backward traction. This causes the animal to hold onto the surface, and results in the body being stretched and elongated.
- With the index finger and thumb of the other hand, pin the rodent over the shoulders.
- Gently slide the thumb and index finger forward, and position them at the base of the skull. Grasp the loose skin at the neck and lift the animal.
- Use the remaining fingers to stabilize the animal along the back by pinning the dorsal skin against the base of the thumb.
- Secure the hindquarters by pinning the tail against the palm with the fourth finger, or by allowing the hind limbs to rest on a solid surface.
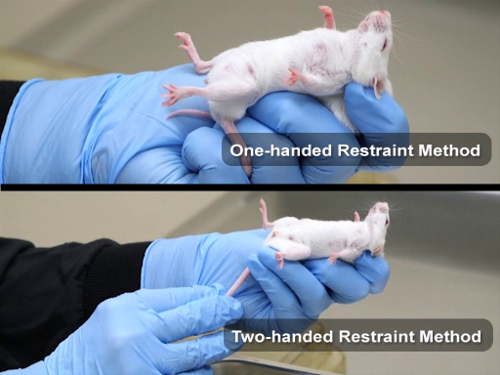
Figure 1: One-handed and two-handed restraint method for mice
2. Gloves
There are a variety of gloves available for handlers to wear for protection from rat bites. They are generally unsuitable for work with mice due to the loss of dexterity. Often, the disadvantages of many glove types outweigh the advantages.
- Cotton canvas work gloves are not bite resistant, but provide a small amount of protection from teeth and nails. They do not provide good grip as they slide easily over the fur.
- Good quality leather gloves are bite resistant. However, their stiffness causes a reduction in dexterity. The thickness of the leather, though protective, reduces tactile acuity and hand mobility.
- Chain mail gloves provide a psychological sense of security for the animal handler. However, they are heavy and most rats' teeth can penetrate between the links. In addition, these gloves decrease manual dexterity, and if used improperly are more dangerous for both the animal and the handler.
- Rubber-insulated gloves that are commonly used in homes for washing dishes provide a textured palmar surface, and allow for a secure grip on the hair coat of rodents. The stretchy latex surface is thick enough to resist puncture by rodent incisors, and thin enough to be able to feel and control the animal. The major disadvantage is that they are made of latex, which is a concern for persons with latex allergies. Furthermore, these gloves deteriorate in light, and need to be replaced regularly.
3. Body Restraint
- The "T. rex grip" is a two-handed restraint method for rats.
- Place a rat on a surface that can be gripped.
- Holding the base of the tail with one hand, bring the other hand up and over the back with the index and middle fingers split.
- Place the hand over the shoulder with the index finger on one side of the head, and the middle finger on the other side. The fingers on each side of the head restrict side-to-side movement of the head.
- Encircle the body behind the forelegs with the third finger, the fourth finger, and the thumb. Care must be taken to avoid compressing the chest and compromising respirations. This grip prevents the rat from moving forward or backward out of the hand.
- Stabilize the tail and hindquarters by grasping the base of the tail with or without holding onto the hind feet.
- This restraint works well with larger rats, and when access to the head is needed.
- The forelimb crisscross method is a two-handed restraint method.
- Place the animal on a surface that they can grip.
- Hold the tail at the base (within 1-2 cm of the body) with the dominant hand and apply gentle backward traction. This causes the animal to grip onto the surface, and results in the body being stretched and elongated.
- Bring the other hand from behind over the back.
- Grasp the rat directly behind the shoulders, with the fingers on one side and the thumb on the other side of the chest, and slide the hand forward, thus forcing the forelegs forward.
- The forelegs will cross under the rat's chin, creating a physical barrier and preventing the rat from moving its head down toward the fingers.
- Take care to avoid compressing the chest and compromising respirations.

Figure 2: T-rex grip and forelimb crisscross method for restraining rats
4. Restraint devices

Figure 3: Broome-style restraining device

Figure 4: Flat-bottomed rodent restrainer
- Rigid devices come in a variety of types, and are generally made of nonporous material that is easily disinfected.
- The Broome-style restraint is designed to provide access to the tail for intravenous injections. It is constructed with a slot that runs along the full length of the device, which allows the animal to be pulled into the restrainer hindquarters first. A plastic nosepiece is inserted to hold the animal in place.
- Remove the nosepiece from the body of the restraint tube by loosening the screw.
- Grasp the animal at the base of the tail, and orient the restraint tube so that the slit that runs the length of the tube is facing up. Gently pull the animal into the restraint device hindquarters first.
- It is often advantageous to place the animal on a smooth surface to facilitate its placement into the tube.
- Once the animal is fully in the tube-and has been drawn to the closed end-slide the plastic nosepiece into the tube to occlude the opening.
- Place the nosepiece assembly so that the animal's nose is in the center opening. Do not position the nosepiece so tightly that the animal cannot breathe.
- The flat-bottomed rodent restrainer is a half cylinder with openings that allow access from the top and the bottom of the restraint device. A rigid plastic gate is inserted into one of several graduated slots to hold the animal within the device.
- Hold the animal by the base of the tail with the dominant hand on a smooth surface, or on a cage top.
- Hold the flat-bottomed restraint tube in the other hand, and angle it so that the open end is at the animal's head at a 45° angle to the table/cage top surface.
- Some animals will immediately enter the tube, while others are reluctant and require the handler to steer them into the tube.
- Place the tube over the animal's head. Immediately tap the animal's rump and maintain pressure to prevent the animal from backing up.
- Once the animal is in the restraint device, slide the plastic gate into the appropriate slot to hold the animal in the restraint device.
- Tube restraints are Plexiglass cylinders with one closed end containing holes or slits for air circulation. Some are modified with a wide slothalf the length of the cylinderto accommodate exposure of a hind limb for intramuscular injections, saphenous and femoral blood collection, or access to the dorsal surface for subcutaneous injections.
- Grasp the animal by the base of the tail with the dominant hand on a smooth surface, or on a cage top.
- Hold the restraint tube in the other hand and angle it so that the open end is at the animal's head at a 45° angle to the table/cage top surface.
- Some animals will immediately enter the tube. Other animals are reluctant and require the handler to steer them into the tube.
- Place the tube over the animal's head. Immediately tap the animal's rump and maintain pressure to prevent the animal from backing up.
- Scruff and manually guide unmanageable animals into the tube, allowing the skin being held to slide through the slit in the tube.
- Once the animal is in the restraint device, it is necessary to occlude the opening to prevent the animal from backing up.
- Depending on the procedure, several fingers are placed across the opening.
- Stuff a large latex glove into the opening. The gloves are usually textured and will grip the Plexiglass surface.
- Some restraint tubes will have closures specific to that style or size.
- The Broome-style restraint is designed to provide access to the tail for intravenous injections. It is constructed with a slot that runs along the full length of the device, which allows the animal to be pulled into the restrainer hindquarters first. A plastic nosepiece is inserted to hold the animal in place.

Figure 5: Tube restraining device
- Flexible restrainers are disposable flexible plastic cones. They are available in either transparent or opaque plastic. The plastic is thin enough to allow penetration by a needle to accomplish injections without removing the animal from the restraint device. They come in a variety of sizes to fit mice and rats, both adult and weanling.
- Cut the plastic with scissors to allow access to the limbs, the tail, and other injection sites.
- Select a cone size appropriate for the animal. The cone should be long enough to extend 2-3 inches beyond the animal's rump.
- Insert the animal into the cone
- Open the cone and grasp it around the upper half.
- Grasp the animal by the base of the tail with the dominant hand, placing it on a smooth surface or a cage top.
- Hold the restraint cone in the other hand, and angle it so that the open end is at the animal's head at a 45° angle to the table/cage top surface.
- Some animals will immediately enter the cone. Other animals are reluctant and require the handler to steer them into the restraint cone.
- Place the cone over the animal's head. Immediately tap the animal's rump and maintain pressure to prevent the animal from backing up. Due to the larger diameter of the cone opening, animals are much more likely to turn around to exit the cone.
- As soon as the animal enters the cone, grasp the open end and seal it to force the animal to the end.
- Secure the opening by folding the plastic to one side of the tail and applying the binder clip to the folded plastic, being careful to avoid clamping the tail or skin. Alternatively, gather the plastic around the tail evenly, and place the twist tie as close to the body as possible to secure the opening.

Figure 6: Flexible restrainer
5. Restraint methods for specific technical procedures
- Ventral exposure is required for intraperitoneal injections.
- Scruffing
- Scruff the animal and turn the hand so that the abdomen is exposed.
- Tilt the animal so that the head is pointing downward at a 30° angle.
- Secure the hindquarters by pinning the tail against the palm using the fourth finger.
- Alternatively, the hindquarters can be immobilized by pinning the excess skin along the back between the fingers and the base of the thumb.
- T. rex grip and forelimb crisscross restraint methods
- Grasp the animal (rats only) using either of the above techniques.
- With the free hand, grasp both hind legs above the hock, and extend them caudally.
- Support the weight of the animal with the palm of the hand on the back.
- Tilt the animal so that the head is pointing downward at a 30° angle.
- A second person performs the injection.
- Rigid restrainer
- Most rigid restraint devices cannot prevent the animal from instinctively rolling to remain upright.
- If a rigid restrainer is employed, turn the animal so that its head is pointed down and is perpendicular to the tabletop.
- Flexible restrainer
- When placed properly in a flexible plastic cone, the animal is unable to turn over or around.
- Care must be taken to position the animal such that the hind limbs are sufficiently separated to allow access to the abdomen.
- As the animal is compressed, it is imperative that the injection be low enough on the ventral surface to avoid puncture of the spleen, liver, kidney, or stomach.
- Scruffing
- Dorsal exposure is required for injections into the subcutaneous space.
- Scruffing
- Scruff the animal and allow its hind legs to rest on a solid, flat surface.
- Lift the scruff to create a tent of skin over the neck and shoulders.
- Place the weight of the hand on the table when injecting mice. Placing weight on the mouse can cause suffocation.
- Hold rats in place with weight on the hindquarters, but never the chest.
- Inject the substance into the space below the fingers on the body of the animal.
- Rigid restrainers
- Place the animal in a restraint device with an opening large enough to pull the skin up.
- It may be necessary to use forceps to grasp the skin.
- Most animals remain in the proper posture unless the restraint is rotated.
- Do not inject a volume that prevents the skin from receding from the opening once the injection is complete.
- Scruffing
- The restraint of the hind limb is necessary for intramuscular injection; blood withdrawal from the saphenous vein, femoral vein, or femoral artery; and injection into the footpad.
- Scruffing of mice and small rats (under 200 g)
- Scruff the animal and turn the hand so that the abdomen is exposed.
- Access the hind foot by placing the foot between the fingers.
- This method is best used only for footpad injections.
- T. rex grip or forelimb crisscross
- This method requires two people: one for restraint of the body, and one for restraint of the limb and the performance of the injection.
- Extend the rat's hind leg by grasping the foot for access to the saphenous vein, femoral vein or artery, or the muscle for injection.
- Hold the leg just above the hock for injection of the footpad.
- Rigid restrainers
- Place the animal in a restraint tube head first.
- Expose the tail and hindquarters by gently pulling them backward from the tube.
- Immobilize the leg by grasping the skin of the flank and locking the knee straight. This provides access to the quadriceps muscle for injection in the mouse or rat, and prevents the animal from exiting the restraint device.
- In devices with large slots, position the leg outside the tube for access to the blood vessels for sampling or footpad injection.
- Those restraint tubes with flat end pieces allow the tube to rest on the table and stabilize the device during the procedure.
- Because the animal is secured in this manner, it is possible to perform injections or blood sampling of rats by a single technician.
- Flexible restraint
- Flexible plastic restraints can be modified by cutting openings to accommodate the exposure of limbs.
- The openings made can be customized for each animal, or for specific procedures.
- As the animal is secured in this manner, it is possible for a single technician to perform injections or blood sampling of rats.
- Scruffing of mice and small rats (under 200 g)
Başvuru ve Özet
Routine handling for cage changing and technical procedures is a cause of stress for experimental animals. Although this type of stress is not a threat to the overall wellbeing of the animal, it can cause fluctuations in physiologic parameters that can have an adverse effect on the research data. The use of skilled personnel, proper techniques, and equipment can mitigate some of the stress.
Atla...
Bu koleksiyondaki videolar:

Now Playing
Rodent Handling and Restraint Techniques
Lab Animal Research
176.0K Görüntüleme Sayısı

Basic Care Procedures
Lab Animal Research
28.1K Görüntüleme Sayısı

Fundamentals of Breeding and Weaning
Lab Animal Research
35.9K Görüntüleme Sayısı

Rodent Identification I
Lab Animal Research
55.1K Görüntüleme Sayısı

Rodent Identification II
Lab Animal Research
25.7K Görüntüleme Sayısı

Compound Administration I
Lab Animal Research
101.4K Görüntüleme Sayısı

Compound Administration II
Lab Animal Research
35.2K Görüntüleme Sayısı

Compound Administration III
Lab Animal Research
31.7K Görüntüleme Sayısı

Compound Administration IV
Lab Animal Research
52.1K Görüntüleme Sayısı

Blood Withdrawal I
Lab Animal Research
172.8K Görüntüleme Sayısı

Blood Withdrawal II
Lab Animal Research
74.0K Görüntüleme Sayısı

Anesthesia Induction and Maintenance
Lab Animal Research
51.1K Görüntüleme Sayısı

Considerations for Rodent Surgery
Lab Animal Research
22.6K Görüntüleme Sayısı

Diagnostic Necropsy and Tissue Harvest
Lab Animal Research
58.5K Görüntüleme Sayısı

Sterile Tissue Harvest
Lab Animal Research
35.0K Görüntüleme Sayısı
JoVE Hakkında
Telif Hakkı © 2020 MyJove Corporation. Tüm hakları saklıdır