Method Article
Analyzing DNA-Protein Interactions with Streptavidin-Based Biolayer Interferometry
In This Article
Summary
This article describes a protocol for studying DNA-protein interactions using a streptavidin-based biolayer interferometry (BLI) system. It outlines the essential steps and considerations for utilizing either basic or advanced binding kinetics to determine the equilibrium binding affinity (KD) of the interaction.
Abstract
Protein-DNA interactions underpin essential cellular processes. Understanding these interactions is critical for elucidating the molecular mechanisms of various pathways. Key factors such as the structure, sequence, and length of a DNA molecule can significantly influence protein binding. Bio-layer interferometry (BLI) is a label-free technique that measures binding kinetics between molecules, offering a straightforward and precise approach to quantitatively study protein-DNA interactions. A major advantage of BLI over traditional gel-based methods is its ability to provide real-time data on binding kinetics, enabling accurate measurement of the equilibrium dissociation constant (KD) for dynamic protein-DNA interactions. This article presents a basic protocol for determining the KD value of the interaction between a DNA replication protein, replication protein A (RPA), and a single-stranded DNA (ssDNA) substrate. RPA binds ssDNA with high affinity but must also be easily displaced to facilitate subsequent protein interactions within biological pathways. In the described BLI assay, biotinylated ssDNA is immobilized on a streptavidin-coated biosensor. The binding kinetics (association and dissociation) of RPA to the biosensor-bound DNA are then measured. The resulting data are analyzed to derive precise values for the association rate constant (ka), dissociation rate constant (kd), and equilibrium binding constant (KD) using system software.
Introduction
Cellular proteins play a pivotal role in orchestrating the complex biological processes that occur within living organisms. The optimal functioning of these pathways is dependent on the interplay between proteins and other biomolecules inside the cell, including interactions with partner proteins and nucleic acids1. Thus, comprehending the intricacies of cellular processes necessitates a deep understanding of the dynamics of protein-nucleic acid interactions.
Traditionally, protein-nucleic acid interactions have been studied using electrophoretic mobility gel shift assays (EMSAs)2. In this assay, proteins are incubated with synthetic oligonucleotides (either DNA/RNA, containing specific lengths or sequences) for a short period, and the reaction is then electrophoresed on a native polyacrylamide (PAGE) gel3,4,5. To enable visualization of the protein-oligonucleotide interaction, the oligonucleotides are typically radioactively labeled with 32P or tagged with fluorescent molecules. If the protein interacts and binds the oligonucleotide, then the binding of the protein slows down the mobility of the nucleic acid within the gel6,7. Thus, this method is also called the gel shift or gel retardation assay. Though this method has been extensively used, there are a few limitations to consider while using this method to obtain KD values, including low resolution from either weak or dynamic binding, the requirement for a significantly high concentration of proteins, and more effort. Additionally, EMSAs are not real-time assays and, therefore, cannot accurately measure binding kinetics7,8.
Innovative techniques such as surface plasmon resonance (SPR) and biolayer interferometry (BLI) have emerged to overcome these limitations9,10,11,12. Both methods measure association/dissociation rate constants and affinity constants between molecules in a label-free manner. Since the protein is not required to be tagged, these techniques eliminate the risk of altering the properties of the protein or blocking the binding site. In SPR, polarized light interacts with a sensor (a metal conducting film, typically gold), generating an electron charge density wave called plasmon. This interaction diminishes the reflected beam's intensity and a detector measures the change in the specific angle, known as the resonance angle13. To study ligand (nucleic acid) - analyte (protein) interactions, the ligand is immobilized in one flow cell on the sensor chip, and the analyte is injected into the flow cell containing the immobilized ligand. On binding the analyte to the ligand, there is a detectable change in the refractive index near the sensor surface, thus allowing for the measurement of molecular interactions14,15,16.
Bio-Layer Interferometry (BLI) measures the pattern of light interference as light passes through an optical fiber with a biolayer, composed of a ligand (bait) and its binding partner (analyte), at the bottom surface of biosensor's tip. Light is transmitted through the tip and reflected at both ends of the biolayer due to the biolayer's properties. The biolayer thickness is proportional to the number of bound molecules that influence the pattern of the reflected light. By comparing the change of the curve of relative intensity vs. wavelength caused by interference between the reflected light from the reference interface and from the biolayer/buffer interface, the thickness change of the biolayer can be determined. When more molecule binds, a greater shift occurs, making BLI a powerful tool for studying biomolecular interactions in real time. The change in the interference pattern is measured and represented on a sensorgram as a spectral shift17,18 (Figure 1A). The nature of the binding interaction can be accurately determined by using appropriate controls, such as a setup lacking the ligand-varying concentrations of the binding partner19,20,21. Compared to SPR, BLI is cost-effective and user-friendly, rendering it accessible to a broad range of researchers. Additionally, samples used in BLI remain intact if there is no degradation or aggregation. This allows them to be possibly recovered and reused, thereby minimizing waste. The BLI instrument operates without the use of microfluidics, thereby eliminating the disadvantages of the fluidics system, such as the need for maintenance/care, clogging, or use of degassed buffers. It also minimizes the risk of contaminating the instrument due to unfiltered or crude protein samples.
In a BLI experiment, the biolayer is established by immobilizing the bait molecule on the biosensor. The biolayer of biosensors can interact with various tags, making it possible to study the interactions between molecules (including nucleic acids, proteins, antibodies, viruses, small molecules, etc.)22. Various capturing strategies, including biotin/streptavidin, 6X-His-tag/Ni-NTA, FLAG/anti-FLAG, GST/anti-GST, and antibody/anti-Fc, can be employed to establish this immobilization. Maintaining the structure and activity of the immobilized ligand is crucial when choosing the biosensor. The changes in the interference pattern are influenced by the amount of bound molecule as well as the matrix23. Protein-nucleic acid interactions are studied using biotinylated oligonucleotide probes that can be immobilized on streptavidin-coated biosensors. Protein samples expected to interact with the immobilized bait (oligonucleotide) are in contact with the oligonucleotide for a specific period in a binding buffer to measure association and subsequently switched to a blank binding buffer to measure dissociation. A schematic representation of analyte binding and corresponding changes in the binding curve is shown in Figure 1B. Additionally, protein-nucleic interactions can also be studied by a reversed immobilization route, in which the protein (bait) is captured on the biosensor and interacts with the nucleic acid (analyte).
Currently, multiple instruments are available commercially that operate on the principle of biolayer interferometry. A straightforward and cost-effective instrument is known as the Octet N1 system, which features a single channel for data throughput. It requires manual operation, consumes minimal sample volume (4 µL), and performs sample analysis at ambient temperatures9,23. This single-channel instrument can detect binding efficiencies of proteins larger than 10 kDa and measures affinities in the micromolar (µM) to nanomolar (nM) range11,12. Some instruments with 2, 8, 16, and 96 channels for automated data reading are also available and compatible with both 96 or 384 well formats19,20. Some of these instruments can also operate between 4 °C to 40 °C, detect biomolecules with molecular weight as low as 150 Da, and measure affinities within the millimolar to picomolar range19,21. While the described system is cost-effective, the pricier instruments with multiple channels offer high-throughput automatic processing. These advanced systems are commonly employed in characterizing and developing biological drug molecules9,23.
The current protocol describes the steps involved in measuring the binding parameters of replication protein A (RPA) to single-stranded DNA (ss-DNA) using the single-channel, manual biolayer interferometry system. RPA is a heterotrimeric, ssDNA-binding protein complex that plays a critical role in almost all aspects of DNA metabolism, including DNA replication, repair, and recombination24. Due to its high affinity to ssDNA (measured to be in the sub-nanomolar range), it can rapidly bind to ssDNA generated during various DNA transactions, protect it from nucleolytic degradation, and prevent impromptu binding of other downstream proteins. RPA also plays a critical role in preventing the formation of non-canonical secondary and tertiary structures, such as G-quadruplexes25. Human RPA is made up of three subunits: RPA70 (70 kDa), RPA32 (32 kDa), and RPA14 (14 kDa), also called RPA 1, RPA 2, and RPA 3, respectively24,26,27. These subunits house six oligonucleotide/oligosaccharide binding folds (OB-folds) labeled A to F. Among these, the DNA binding domains (DBDs) are on the RPA1 (DBD-A, DBD-B, DBD-C) and RPA2 (DBD-D) subunits28. It was first thought that depending on the DNA's length, RPA exhibits different binding modes where different DBDs were engaged to specific DNA lengths29. Data from recent structural and single molecule studies have helped refine these models to suggest that the binding of different DBDs of RPA is more dynamic than initially suggested30,31,32,33,34,35,36. This feature of the dynamic binding property is essential to the function of RPA because RPA should be able to bind tightly to the DNA substrate during certain DNA transactions; however, it should also be able to displace from the substrate to hand over the substrate to the next protein during the biological process. Using different biochemical techniques, the KD for RPA has been determined to be about 0.4 nM for a ss-(polydT)30 substrate, and 80 nM and 200 nM for ss-(polydA)257 and dG-(polydG)602 substrates respectively, as measured by fluorescence polarization anisotropy (FPA)37,38. RPA also shows about a 50-fold higher preference for binding to pyrimidine rich sequences compared to purines. Though different biochemical techniques have determined differing KD measurements for RPA, all measurements have been in the nanomolar range, suggesting RPA's high binding affinity for ssDNA. Employing total internal reflection fluorescence microscopy (TIRFM), it was discovered that based on the length of the DNA, RPA associated with at least two binding modes: one that was defined at fast dissociating (Kd, 680 pM) and a slow dissociating (Kd, 60 pM) complex33,39. Given its pivotal involvement in nearly all DNA metabolic pathways, there has been a keen interest in creating inhibitors that can hinder the interaction between RPA and single-stranded DNA (ssDNA). These chemical inhibitors are vital in disrupting the DNA damage response, rendering cancer cells more susceptible to DNA-damaging agents employed in clinical therapies. Utilizing the BLI assay allows for a precise quantitative evaluation of inhibitor efficacy in modulating RPA's binding function40,41.
BLI setup allows for distinct settings: basic and advanced kinetics. In basic kinetics mode, the assay comprises three primary stages: baseline establishment, association, and dissociation. However, before performing this assay, the biosensors need to be coated separately, using either the instrument or manually on the laboratory bench. Conversely, the advanced kinetics mode extends beyond the fundamental three steps, allowing for the incorporation of additional steps. These supplementary steps can serve various purposes, such as conditioning the biosensor to the buffer alterations or facilitating the detection of subsequent protein interactions. Advanced kinetics also allows stripping the biosensor of the analyte by a good regeneration method for potential reuse, provided the bait and the biosensor remain intact. Generally, advanced kinetics is used over basic kinetics when multiple steps are involved in the assay, or the assay needs to be performed in a more complex format.
This protocol outlines both methods using the same bait [3'Bio-ss-poly (dT)32] and analyte (RPA). A streptavidin-coated biosensor will be used to immobilize the bait, and KD measurements for the analyte will be obtained. This protocol describes a complete approach to perform a BLI assay using a single-channel instrument biolayer interferometry, covering biosensor-bait preparation, buffer considerations, and a step-by-step outline of the procedure.
Protocol
Details of the reagents and the equipment used in the study are listed in the Table of Materials.
1. Preparation of bait, analyte, buffers and drop-holder cleaning
- Setting up the instrument: Turn the instrument on at least 1 h before starting the experiment. This will allow the lamp to warm up.
- Preparation of stripping and cleaning buffers
- Stripping buffer: Prepare 50 mL of 0.15 M Phosphoric acid [pH 2.0]).
- Cleaning buffer: Prepare 10 mL of 0.5 N HCl.
- Preparation of BLI buffer
- Prepare 50 mL of 1x BLI buffer (50 mM Tris pH 7.5, 1 mM EDTA, 100 mM NaCl, 0.1 mg/mL BSA, 1 mM DTT, 0.05% Tween 20). Store this buffer at 4 °C. Before setting up this assay, bring the buffer to room temperature.
NOTE: It is highly recommended that fresh buffers be prepared for the assay, as aged buffers may undergo pH alterations or formation of aggregates and may have microbial contaminations, potentially compromising the reproducibility and accuracy of results. It is not recommended to use buffers that have been stored for more than two weeks.
- Prepare 50 mL of 1x BLI buffer (50 mM Tris pH 7.5, 1 mM EDTA, 100 mM NaCl, 0.1 mg/mL BSA, 1 mM DTT, 0.05% Tween 20). Store this buffer at 4 °C. Before setting up this assay, bring the buffer to room temperature.
- Preparation of bait/substrate
- Prepare 12.5 nM of 3'Bio-ss-poly (dT)32 substrate (5' TTT TTT TTT TTT TTT TTT TTT TTT TTT TTT TT 3'Bio) using BLI buffer.
- Preparation of protein concentration stocks
- Prepare four concentrations of RPA (5 nM, 10 nM, 20 nM, 40 nM) using BLI buffer. The highest concentration of the protein (40 nM) is obtained by diluting the stock protein in BLI buffer. Subsequent dilutions are prepared by serial dilution of the 40 nM working solution. Ensure all protein stocks are kept on ice throughout the experiment to maintain stability.
- Cleaning the drop holder
- Add 4 µL of distilled water and clean the drop holder with a lint-free wipe (repeat 3x). Next, repeat this step with 4µL of 70% ethanol and clean with a lint-free wipe. This step ensures the removal of surface contaminants and dust particles.
- Add 4µL of 0.5N HCl and leave it in the drop holder for 1 min. Clean it with a lint-free wipe and repeat this step once more. This ensures deep cleaning of the drop holder. Deep cleaning is especially useful to clean the drop holder of dried-up drops from previous use.
- 1Repeat step 1 to wash the drop holder with distilled water and 70% ethanol and wipe it dry with a lint-free wipe. The drop holder is now ready to be used.
2. Basic kinetics
- Setting up the instrument and the software
- Open the software and select Basic Kinetics on the left panel (Supplementary Figure 1).
- Remove the rack of biosensors from the tray (obtained from commercial sources) and place a 96-well plate in the tray. Add 200 µL of BLI buffer to the first well. Next, position the tray of biosensors back on top of the 96-well plate, ensuring each biosensor is inserted into its corresponding well. The first biosensor should now be submerged in the BLI buffer in the first well.
- On the software, click on Hydrate and turn the timer on for 10 min, allowing the single biosensor to hydrate for at least 10 min. The step hydrates the biosensor to make it ready for BLI assay.
- While the biosensor is being hydrated, pipette 250 µL of BLI buffer into two 0.5 mL tubes. Label one as "A" (association) and the other one as "D" (dissociation).
- Fill in the details on the software (Name: Substrate Coating and description of the assay: RPA- 3'Bio-ss-poly (dT)32: Basic Kinetics).
- Adjust the run setting for each step: Baseline (30 s), Association (120 s), Dissociation (120 s) (Table 1). Keep the shaker on for the tube and drop holder. The shaker speed is set at 2200 rpm (Range, 1000–2600 rpm), which prevents the mass transport effects caused by the limited diffusion near the biosensor surface.
NOTE: Optimizing the duration for each step (baseline, loading, associating, dissociating) when setting up a binding kinetics assay is recommended to improve data acquisition.
- Establishing a baseline curve
- Place tube A in the tube holder and attach the hydrated biosensor to the BLI system. Slide the tube holder under the biosensor (position A, Figure 2).
NOTE: Biosensors must not be completely dried at any time during the experiment. If left to dry, readings of the experiment can be significantly skewed. - Hit Run and follow the instructions in the prompt message.
- After completing the initial baseline, open the lid and add 4 µL of BLI buffer to the drop holder. Slide it to the right under the same biosensor (position B, Figure 2). Close the lid and monitor the BLI curve on the software (Figure 3, blue curve).
- After completion, open the lid to replace tube 'A' with 'D' and slide it under the biosensor (position A, Figure 2). Close the lid and proceed with the dissociation step. After completion, open the lid, detach the biosensor, place it back in the tray, and ensure the biosensor is dipped in BLI buffer.
- Remove the sample/buffer, clean the drop holder with a buffer before drying it with lint-free wipes, and remove tube 'D' from the tube holder.
- Place tube A in the tube holder and attach the hydrated biosensor to the BLI system. Slide the tube holder under the biosensor (position A, Figure 2).
- Substrate coating
- Place tube A in the tube holder and attach the hydrated biosensor to the BLI system. Slide the tube holder under the biosensor and hit Run.
- After the initial baseline step, add 4 µL of 12.5 nM substrate [3'Bio-ss-poly (dT)32] to the drop holder and slide it under the biosensor. Close the lid and monitor the BLI curve on the software. Ensure that this response is higher than the baseline (Figure 3).
NOTE: Substrate concentration can be optimized by coating biosensors with different concentrations (only one concentration per biosensor) and observing the binding curves. A binding curve with a visibly higher signal compared to the baseline is enough to confirm substrate coating. Overloading the biosensor may lead to steric hindrance or crowding on the surface. Figure 3shows the curves for five concentrations of biotinylated 3'Bio-ss-poly (dT)32 substrate. Additionally, after the bait loading step is completed, the biosensors can be dipped in streptavidin-blocking buffer (biocytin) for 30 s, followed by washing in BLI buffer. This will block the unbound streptavidin surface and prevent non-specific binding. - After completing the association step, open the lid and replace tube 'A' with tube 'D.' Slide it under the biosensor and close the lid.
- After the dissociation step is completed, open the lid, detach the biosensor, and place it in the biosensor tray containing the BLI buffer. Ensure that the biosensor is completely dipped in the buffer and is adequately hydrated for the next steps. Clean the drop holder with assay buffer, dry it with lint-free wipes, and remove tube 'D' from the tube holder. The biosensor is now coated with the 3'Bio-ss-poly (dT)32 substrate.
- Protein binding
- Pipette 250 µL of BLI buffer into ten 0.5 mL black microfuge tubes. Label them A1 to A5 and D1 to D5.
- In another 96-well plate, add 200 µL of stripping buffer into the well, corresponding with the hydrated biosensor's position.
- Before starting the assay, clean the drop holder 3x with BLI buffer.
- Open a new file on the software and select Basic Kinetics on the left panel. Name the assay and add description as needed (Supplementary Figure 2). Adjust the run settings as required (Table 1).
- Place the tube labeled A1 in the tube holder and attach the 3'Bio-ss-poly (dT)32-coated biosensor to the BLI system. Slide the tube under the biosensor and hit Run.
- After completing the initial baseline step, open the lid, add 4 µL of the BLI buffer to the drop holder, and slide it under the biosensor. This will be the 0 nM protein concentration and serve as the reference curve.
- After completing the association step, replace tube A1 with D1 and slide it under the biosensor.
- Once the dissociation step is completed, remove the biosensor and place it back in its tray. Ensure that the biosensor is completely dipped in the buffer and adequately hydrated for the next steps. Remove tube D1, clean the drop holder with assay buffer, and dry it with a lint-free wipe.
- Next, the binding curve for 5 nM RPA stock will be obtained. Fill out sample details (Concentration: 5 nM, Molecular weight: 116 KDa], and press Calc. This will calculate the protein concentration in µg/µL.
- Attach the 3'Bio-ss-poly (dT)32-coated biosensor and put tube A2 in the tube holder. Slide it under the biosensor and hit Run.
- After baseline, add 4 µL of 5 nM RPA onto the drop holder and slide it under the biosensor. Close the lid and monitor the association curve. Protein binding to bait can be visualized by a curve running higher than the baseline curve. After the association step, replace A2 with D2, slide it under the biosensor, and complete the dissociation step.
- Remove the biosensor, dip it in the stripping buffer for 30 s, and then dip it in the BLI buffer for 3 min.
NOTE: The regeneration efficiency depends upon the immobilized bait and the disrupted analyte. Some immobilized biosensors can withstand ten or more regeneration cycles. We could strip biotinylated 3'Bio-ss-poly (dT)32 substrate more than three times (Supplementary Figure 3) without observing a noticeable change in response. - Repeat steps 2.4.9–2.4.12 for the other three concentrations of RPA 10 nM, 20 nM, and 40 nM. Make sure to strip the biosensor for each concentration.
NOTE: For BLI assays, experts recommend using four (excluding the reference curve) concentrations, spanning roughly from 0.1x to 10x the expected KD for accurate kinetic measurements. Here, the four concentrations were obtained by serial dilutions.
- Analyzing data and calculating KD values
- Before calculating the KD value, it is necessary to visually inspect the sensorgram for each concentration of RPA binding on the 3'Bio-ss-poly (dT)32 substrate. Ensure that the BLI signal increases with increasing concentration of RPA until the immobilized DNA is saturated (Figure 4).
NOTE: For reliable results, ensure that at least four protein concentrations are bound successfully to the substrate, excluding the 0 nM analyte concentration. Refer to the discussion section to troubleshoot potential errors. - In the table 'Run List,' check Ref for 0 nM analyte concentration to serve as a reference curve. Check Analyze for all the concentrations of the analyte.
- Next, select both Start of association and Start of dissociation for step correction. This will align the beginning of association with the end of the baseline and the beginning of dissociation with the end of association, which is usually caused by different reflection patterns between the drop holder and tube if the buffer matches well.
- Select Global Fitting (1:1) for this assay. Global analysis calculates the KD values for an entire set of protein concentrations, while local analysis is for a single protein concentration.
NOTE: The software accompanying the instrument only allows for 1:1 fitting. This could be a potential caveat when the bait and analyte are expected to fit into other fitting models. Data from this software may be exported to other plotting software and fit into required fitting models. - Next, select Analyze. This will calculate the KD, ka/kd values (Table 2). The ka and kd error within 10% of the value is generally considered acceptable.
NOTE: Ensure these values are within reasonable limits. If the error is less than 10%, it suggests that the fitting of data is reliable for studying interactions between biological molecules. Additionally, the calculated KD (equilibrium dissociation constant) using these ka and kd values should be sufficiently accurate for most practical applications18. - To export the fitted data, right-click on the graph generated using the analyzed data. Choose Export Data, then select Text/Data. Click on File and Browse to save data in '.dat' format. This file can be opened in Microsoft Excel and used in other software for graph plotting.
NOTE: Global fitting considers all the binding curve data within the group, while local fitting focuses solely on the kinetic parameters for each individual analyte concentration.
- Before calculating the KD value, it is necessary to visually inspect the sensorgram for each concentration of RPA binding on the 3'Bio-ss-poly (dT)32 substrate. Ensure that the BLI signal increases with increasing concentration of RPA until the immobilized DNA is saturated (Figure 4).
3. Setting up the instrument to run advanced kinetics
- Open the software and select Advanced Kinetics on the left panel (Supplementary Figure 1).
- Place a 96-well plate in the tray holding the biosensors. Add 200 µL of BLI buffer in five wells. Place the tray of biosensors on the 96-well plate so that five biosensors dip into the well containing the buffer.
- On the software, click on Hydrate and turn the timer on for 10 min, allowing the single biosensor to hydrate for at least 10 min.
- While the biosensors are hydrating, pipette 250 µL of BLI buffer into ten 0.5 mL black centrifuge tubes. Label them as A1 to A5 and D1 to D5.
- Fill in the details on the software (Name: Substrate Coating and description of the assay: RPA-3'Bio-ss-poly (dT)32: Advanced Kinetics).
- Input the duration for each step (Supplementary Figure 4). Initial Baseline (30 s), Loading (120 s), Baseline (30 s), Association (120 s), Dissociation (120 s). Adjust the run settings as required (Table 1).
- Fill in the details on the software (Concentration: 0 nM, Molecular Weight: 116 kDa) and press Calc.
- Place tube A1 in the tube holder and attach the biosensor. Slide the tube holder under the biosensor and press Run to obtain the initial baseline.
- Add 4 µL of 12.5 nM substrate into the drop holder and slide it under the biosensor. Close the lid. This will load the substrate onto the biosensor. Replace A1 with D1 in the tube holder and slide it under the biosensor. This will give the baseline curve.
- Clean the drop holder with a lint-free wipe and load 4 µL of the BLI buffer. Slide it under the biosensor and close the lid. This will be the 0 nM protein concentration and will serve as the reference curve.
- After completing the association step, slide the D2 tube under the biosensor. Proceed with the dissociation step. Allow dissociation to complete.
- Detach the biosensor, remove D2 from the tube holder, and clean the drop holder with a lint-free wipe.
- Enter the details (Concentration: 5 nM, Molecular weight: 116 kDa) for the next run and press Calc.
- Attach the second biosensor to the BLItz system, place tube A2 in the tube holder, and slide it under the biosensor.
- After establishing the initial baseline, add 4 µL of 12.5 nM substrate into the drop holder. Slide it under the biosensor and close the lid. Replace tube A2 with D3 and slide it under the biosensor. Next, add 4 µL of 10 nM stock of RPA into the tube holder and slide it under the biosensor.
- After association, slide D4 under biosensor for dissociation.
- Repeat steps 3.1.12–3.1.15 for the subsequent three concentrations of RPA (10 nM, 20 nM, 40 nM). Binding curves for these concentrations are shown in Figure 5.
- Data analysis and KD value can be fitted in the same procedure as the BLI basic kinetics (step 2.5, Table 2).
Results
In BLI, white light is reflected from the interface of biolayer/buffer and the internal reference interface to the spectrometer. The resulting interference pattern is recorded, and the spectral shift is measured over a period of time and depicted as binding curves response in nm. A representative figure showing the biosensor tip with and without analyte binding and the corresponding spectral wavelength shift (nm) is shown in Figure 1. The binding curves correspond to the baselines, bait loading, and analyte association followed by analyte dissociation, as shown in Figure 2.
For this assay, BLI kinetics buffer was prepared as mentioned in the protocol and used for making 3'Bio-ss-poly (dT)32 stocks and the RPA protein stocks. It is recommended that the same buffer is used for all dilutions throughout the assay. The streptavidin biosensor was hydrated in BLI kinetics buffer at room temperature and then coated with 3'Bio-ss-poly (dT)32 substrate for basic kinetics. Four concentrations were evaluated to optimize the concentration of the substrate, along with a negative control (0 nM) (Figure 4). The sensorgram for 0 nM substrate will serve as a reference curve. For kinetics assay, it is recommended to use lower ligand/bait density that provides sufficient signal for accurate results17,18. In Figure 3, the steep pattern of the sensorgrams for 25 nM, 50 nM, and 100 nM substrate concentration may be due to measuring the responses in drop holder and tube, which have slightly different conditions (reflection patterns, distance from sensor surface to samples surface, etc.) for the biosensor. However, the loading step is not part of the binding kinetics data for fitting, and it does not impact the result. The biosensor was loaded with 12.5 nM biotinylated 3'Bio-ss-poly (dT)32 for 120 s and was utilized to obtain binding curves for five concentrations of RPA.
The sensorgram for RPA binding using basic kinetics is shown in Figure 4. The binding curve for 0nM protein will serve as the reference for subtraction. The BLI signal strength will increase with increasing concentrations of RPA until the immobilized DNA is saturated. In this assay, a single-loaded biosensor was utilized to study the binding kinetics of one concentration of RPA, after which the biosensor underwent a regeneration process and was reused for the next concentration. Regeneration was conducted by dipping the biosensor in 0.15 M phosphoric acid for 30 s, followed by neutralization in BLI buffer for 2 min.
The sensorgram for RPA binding using advanced kinetics is shown in Figure 5. Five biosensors were hydrated with BLI buffer for at least 10 min. Subsequently, each biosensor was loaded with a 12.5nM 3'Bio-ss-poly (dT)32 substrate for 120 s and was utilized to assess the binding kinetics of a singular concentration of RPA. Specifically, one biosensor is allocated for each concentration of the analyte.
After selecting the reference curves, the analyzed data will only depict sensorgrams for the protein concentrations (5 nM, 10 nM, 20 nM, and 40 nM). The reference curves will be used to negate the background signal before aligning the association and dissociation curves.
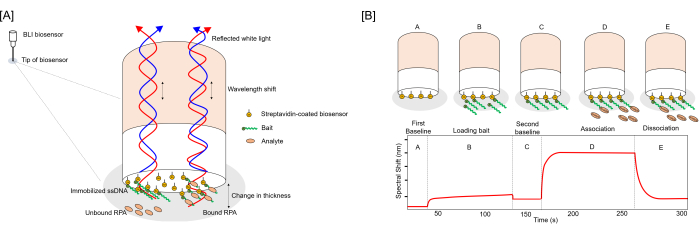
Figure 1: Schematic representations of biosensor binding and ligand interaction assay. (A) Diagram illustrating bait-coated biosensors in unbound and analyte-bound states. Binding of the analyte alters the biomolecular layer thickness on the biosensor surface, shifting the interference pattern of incident white light and producing a wavelength shift in the relative intensity/wavelength curve. Binding curves are generated by measuring spectral shifts (in nm) over time. SA: streptavidin, B: bait. (B) Diagram detailing ligand binding to biotinylated ssDNA bait loaded on streptavidin biosensors and corresponding changes in the binding curve measured through advanced kinetics. The assay involves several steps: [A] Establishing an initial baseline for the biosensors, [B] Immobilizing the 3′Bio-ss-poly(dT)32 bait on the biosensor, [C] Dipping biosensors into BLI buffer to assess bait loading levels, [D] Measuring analyte-bait association by immersing the biosensors in BLI buffer with the analyte, and [E] Measuring analyte dissociation by dipping the biosensor back into the BLI buffer. Please click here to view a larger version of this figure.

Figure 2: Slider positions for tube holder and drop holder with respect to the black arrowhead. Position A will have the tube holder under the biosensor. Position B will have the drop holder under the biosensor. Please click here to view a larger version of this figure.
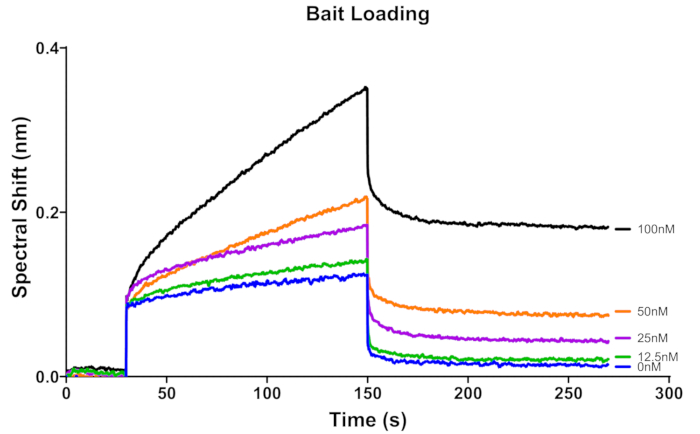
Figure 3: Binding sensorgram for five concentrations (0 nM, 12.5 nM, 25 nM, 50 nM, 100 nM) of the 3'Bio-ss-poly (dT)32 bait on the streptavidin-coated biosensor. These sensorgrams represent raw data. Time 0-30 s represents baseline of the biosensor before loading, 30-150 s represents loading of bait, and 150-270 s represents the second baseline to ensure that some molecules are stably bound to the sensor. Please click here to view a larger version of this figure.

Figure 4: BLI sensorgram obtained with basic kinetics.Binding curves were obtained for four concentrations of RPA (5 nM, 10 nM, 20 nM, 40nM) bound to immobilized 3' Bio-ss-poly (dT)32 substrate. The black lines indicate fitting curves fitted with the 1:1 model. The purple line represents the sensorgram corresponding to each concentration. Time 0-150 s represents association, and 150-270 s represent dissociation. Please click here to view a larger version of this figure.
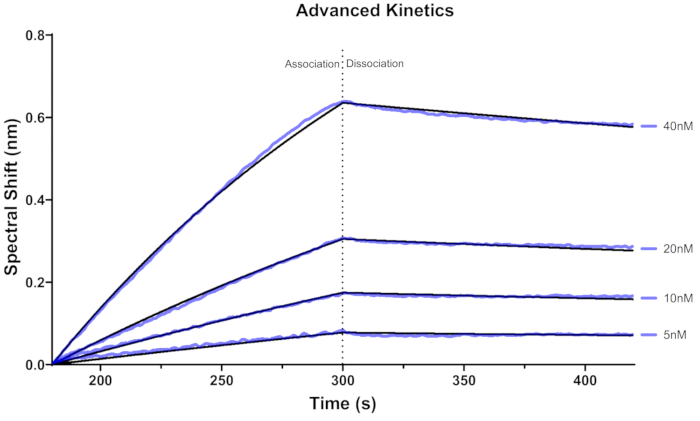
Figure 5: BLI sensorgram obtained with advanced kinetics. Binding curves for four concentrations of RPA (5 nM, 10 nM, 20 nM, 40 nM) bound to immobilized 3' Bio-ss-poly (dT)32 substrate. The black lines indicate fitting curves fitted with the 1:1 model. The purple line represents the sensorgram corresponding to each concentration. Time 0-300 s represents the association, and 300-450 s represents dissociation. Please click here to view a larger version of this figure.
| (A) | |||
| Step Type | Duration (s) | Position | |
| 1 | Baseline | 30 | Tube |
| 2 | Association | 120 | Drop |
| 3 | Dissociation | 120 | Tube |
| (B) | |||
| Step Type | Duration (s) | Position | |
| 1 | Initial Baseline | 30 | Tube |
| 2 | Loading | 120 | Drop |
| 3 | Baseline | 30 | Tube |
| 4 | Association | 120 | Drop |
| 5 | Dissociation | 120 | Tube |
Table 1: Run settings. The run settings for (A) basic kinetics and (B) advanced kinetics.Step durations and biosensor position for each step performed during the assay.
| KD (nM) | KD Error (nM) | ka (1/Ms) | ka Error | kd (1/s) | kd Error | |
| Basic Kinetics | 5.5 | 0.2 | 2.24E+05 | 1.58E+04 | 1.23E-03 | 7.19E-05 |
| Advanced Kinetics | 6.8 | 0.2 | 1.18E+05 | 6.59E+03 | 8.11E-04 | 2.26E-05 |
Table 2: Analyzed data for basic and advanced kinetics.The analyzed data table shows the calculated values for KD, ka, and kd, as well as their respective errors.
| Issue | Possible causes | Suggested solutions |
| Inconsistent results | Improper instrument setup | Avoid placing the BLI instrument near a centrifuge or any other sort of vibrations that might interfere with readings. |
| Abnormal Curves | Old/worn out internal light source. | Replace the internal light source/lamp. |
| Avoid keeping the instrument in the direct sun. | ||
| Old/expired biosensors | Ensure that the biosensors are kept sealed with silica gel packets and stored in dry cool and dark place. | |
| Spike/Drops in data, unstable baseline, curves running lower than the baseline or baseline drift | Buffer Mismatch | Use freshly prepared buffers. |
| Make all dilutions in the same buffer. | ||
| Ensure that the same buffers are used throughout the assay. | ||
| Bubbles in the sample | Centrifuge and pipette the sample carefully. | |
| Filter the sample using a 0.22uM filter. | ||
| Low signal | Uneven bait loading | Increase the duration of bait loading. |
| Low/high concentration of bait | Optimize the bait concentrations by testing a range. | |
| Skewed association curves | Non-specific analyte-biosensor interaction | Quench biosensor using an appropriate quencher, for example, biocytin. |
| No change in the signal during dissociation | Tight analyte binding | Normal for analyte with slow off rate. Increase the duration of dissociation. |
| Initial updrift during dissociation | Unstable bait-analyte interaction | Optimize the concentration of the bait and analyte. Adjust the duration of association. |
| Maintain consistent buffer composition and prevent protein aggregation. | ||
| Signal reduction following initial bait loading | Unstable bait/Bait dissociating from the biosensor. | Increase bait concentration and the duration of the dissociation step. |
Table 3: Guide to troubleshooting BLI assays. Possible issues and recommended solutions.
Supplementary Figure 1: Equipment software home page. The software screenshot shows the 'BASIC KINETICS' and 'ADVANCED KINETICS' options on the left panel. Please click here to download this figure.
Supplementary Figure 2: Software page for basic kinetics. The software screenshot shows the experiment details and duration setting for basic kinetics. Please click here to download this figure.
Supplementary Figure 3: Representative figure showing analyte binding on the bait immobilized biosensor after being regenerated multiple times. Each colored box represents one bait loading, analyte binding, followed by a regeneration event. Please click here to download this figure.
Supplementary Figure 4: Software page advanced kinetics. The software screenshot shows the experiment details and duration setting for advanced kinetics. Please click here to download this figure.
Discussion
The ability to analyze the binding kinetics of any protein to its substrate using BLI provides the means to isolate and characterize the specific factors (such as sequence, structure, or length of a DNA) governing protein-DNA interactions within the cell19. The Octet N1 system, which relies on the principles of biolayer interferometry, allows for quantitative measurement of protein-protein and protein-nucleic acids interactions. Further, interactions between lipids, antibodies, and small molecules can be determined using this methodology42,43,44. Though fairly simple and straightforward to use, there are some nuances to consider when using the system to measure binding kinetics.
The current protocol measured the binding kinetics of the protein complex RPA to ssDNA - an interaction that has been assessed over the past many decades. Since RPA has multiple DNA binding domains, studies have also focused on the engagement of the different domains when exposed to different length ssDNA substrates. Modular binding studies showed that RPA engages an 8 nt ssDNA with low affinity and switches to high affinity binding when it interacts with a ssDNA ~28-30 nt in length33,39. Using the BLI assay, for a 32 nt poly dT substrate, the KD was calculated to be 5.5 nM using basic kinetics and 6.8 nM using advanced kinetics. Studies using other techniques such as EMSA, fluorescence polarization anisotropy (FPA), and single-molecule total internal reflection fluorescence microscopy (smTIRF) have all consistently detected the KD value within the low nanomolar range. However, there's an approximately 10-fold variation between different assays. This discrepancy highlights that while these studies provide valuable insights into binding affinities, they are not absolute values.
Assessing the binding curve obtained in Figure 4 and Figure 5, it is evident that the analyte (RPA) does not completely dissociate from the bait [3' Bio-ss-poly(dT)32] within a limited time. Given that RPA is known to dissociate from the substrate very slowly in the absence of competing proteins, it is not surprising that we don't observe significant dissociation in the assay. This is also consistent with our recent report wherein we had to use a 1000-fold excess of competing ssDNA to show RPA dissociation once bound to a 28 nt ssDNA substrate45. Weeramange et al. employed an alternative approach for analyzing binding kinetics characterized by slow dissociation, specifically utilizing steady-state fitting. This method allows for the determination of the dissociation constant (KD) based solely on the binding response at the equilibrium, corresponding to the plateau of the binding curve46. To achieve more accurate KD values, assays should be conducted using varying concentrations of the analyte, and the resulting data should be fit globally.
Given the sensitive nature of BLI kinetics, keeping the drop holder of the instrument contaminant-free is paramount. Before beginning the experiment, the drop holder is cleaned with 70% ethanol and water to remove surface contaminants. HCl (0.5 N) is then used for deep cleaning the drop holder. This is to ensure the removal of any dried-up droplets from previous experimental runs. Another wash with ethanol and water removes the HCl. Finally, the drop holder is washed with buffer and is ready for the experiment. The drop holder is washed with the buffer between runs to remove residual bait/protein. The four-step cleaning protocol involving ethanol, water, HCl, and buffer is not necessary between runs of the same experiment but is recommended before the start of a new experiment. This initial preparation is crucial as it ensures that all the equipment is free from contaminants, setting a solid foundation for reliable results.
Similarly, prior to setting up a BLI assay, it requires careful consideration of various parameters such as the choice of buffer, choice of biosensor, optimum bait loading concentration, and range of analyte concentration. While BLI is compatible with a wide range of buffers, it is crucial to ensure that each component is compatible and does not interfere with the measurements. An unstable baseline may be due to inappropriate buffer contents or precipitates in the buffer. Certain detergents may tend to cause abnormal protein binding, and thus, concentrations of these agents should be kept below critical micelle concentration in order to enhance their solubilization properties47,48. It is also important to maintain the same buffer throughout the experiment to establish the baseline curve and make bait and analyte dilutions. Buffer mismatch can lead to spikes or drops in the data curve and result in baseline drift due to changes in the biolayer's environment. It is recommended to start with a buffer that has previously worked for the protein of interest and use the same buffer throughout the assay for the hydration of biosensors and analyte and bait preparation. BLI kinetics can accommodate different buffers by establishing a baseline before each buffer usage. To prevent non-specific binding, BSA (range: 0.1-10 mg/mL) or Tween20 (range: 0.005%-0.05%) can be added to the buffer. However, it is essential to optimize their concentrations in the buffer19,21. Additionally, commercially available blocking buffers (e.g., Biocytin) can also be utilized.
The initiation of analysis of biomolecular interactions begins with the immobilization of the bait on the biosensor, highlighting the importance of selecting the appropriate biosensor. It is imperative to consider the sensitivity of these biosensors, their interactions with bait and analyte, potential non-specific binding, and their ability to maintain biological activity post-immobilization. Biotin/streptavidin interactions are most commonly utilized, but other biosensors, such as anti-FLAG/FLAG, anti-GST/GST, and anti-Fc/IgG probes, can also be used depending on the bait.
Appropriate controls pertaining to the assay are crucial for validating experimental results and accurately accessing the data. For most runs, these include a no bait and a no analyte control. Impurities in the analyte or sample aggregation can also alter the sensorgram and result in an unstable baseline. The concentration and the shape of the binding curve depend on the affinity of the analyte. Depending on the individual nature of the bait and the analyte, these timings can be adjusted and optimized. Additionally, non-specific binding can also be monitored by measuring the interaction between the analyte and the uncoated biosensor. In basic kinetics, a single-coated biosensor can be used to determine the binding curve for at least four different analyte concentrations with regeneration between different samples in our assays. With regeneration, the biosensor is stripped of the bound analyte and reused for the next analyte concentration. Advanced kinetics could also use one biosensor for several analyte concentrations with regeneration but allow the regeneration step to be included within the assay49,50.
Streptavidin biosensors employed in the experiment immobilize biotinylated bait through a non-covalent, nearly irreversible, and stable interaction. To obtain accurate data, optimizing the density of the immobilized ligand on the biosensor is necessary. A steep loading curve could suggest uneven loading. Over-saturation of the biosensor may lead to weak non-specific interactions at higher analyte concentrations or re-binding at lower analyte concentrations. Conversely, loading a low concentration of the bait may yield a lower response signal that is too low to detect. Typically, gradual loading is preferred over fast loading to ensure proper coating of the biosensor and prevention of assay artifacts17. Optimal results can be achieved by selecting the lowest concentration of the bait that produces a response signal distinguishable from the established baseline during the analyte association step. Generally, in label-free assays such as BLI or SPR, using the lowest concentration that still provides a sufficient signal yields more accurate results, as lower loading minimizes mass transport effects and other artifacts49,50,51.
Analyte concentrations range can be chosen based on their known KD value. The choice of analyte concentration to be used in the binding assay should range from 0.1-10 times the expected or estimated KD value. If the KD value is unknown, the concentration range can be assessed by experimentally acquiring BLI data at different analyte concentrations. Increasing the concentration of the ligands should result in a corresponding change in the binding curve. If there is no binding for a known sample, it could be due to several factors, such as degradation/immobilization of the bait, stripping of the bait, inability to interact due to aggregation of the ligand, or poor quality of biosensor tips. A guide to troubleshooting potential problems is listed in Table 3.
While BLI requires low equipment maintenance and has a user-friendly operation, it falls short in sensitivity compared to SPR. Additionally, it lacks strict control over evaporation17,49. When testing very tight binding (KD = below pM range), the assay usually requires long dissociation (for several hours), and evaporation could be an issue that introduces variation. Despite these challenges, real-time, label-free BLI analysis provides quick and accurate kinetics parameters, making it a robust quantitative assay. BLI is a diverse tool for research and analytical applications, making it a valuable resource for studying complex molecular interactions.
Disclosures
Authors have no conflict of interest to declare.
Acknowledgements
This work was funded by grants from the National Science Foundation (1929346) and the American Cancer Society (RSG-21-028-01). We would also like to thank members of the Balakrishnan laboratory for helpful discussions.
Materials
| Name | Company | Catalog Number | Comments |
| 0.5 mL Micro Centrifuge Tubes | Globe Scientific | 111554A | |
| 96 Well Standard Black Microplate | Dot Scientific | 4ti-0223 | |
| Biotinylated poly dT Oligonucleotide | IDT | ||
| Bovine Serum Albumin (BSA) | Sigma-Aldrich | A2153-10G | |
| Dithiothreitol (DTT) | Dot Scientific | DSD11000-10 | |
| Ethylenediaminetetraacetic acid (EDTA) | Dot Scientific | DSE57020-500 | |
| Hydrochloric Acid | Fisher Scientific | A144-500 | |
| Kimtec Science Kimwipes | Kimtech | 34120 | |
| Octet N1 Software | Sartorius | 1.4.0.13 | |
| Octet SA Biosensor | Sartorius | 18-5019 | |
| PBS pH 7.2 (10x) | Gibco | 1666711 | |
| Personal Assay Octet N1 System | Sartorius | ||
| Phosphoric Acid | Ward's Science | 470302-024 | |
| Sodium Chloride (NaCl) | Dot Scientific | DSS23020-5000 | |
| Tris Base | Dot Scientific | DST60040-5000 | |
| Tween20 | Bio-Rad | 170-6531 |
References
- Kalodimos, C. G., et al. Structure and flexibility adaptation in non-specific and specific protein-DNA complexes. Science. 305 (5682), 386-389 (2004).
- Fried, M., Crothers, D. M. Equilibria and kinetics of lac repressor-operator interactions by polyacrylamide gel electrophoresis. Nucleic Acids Res. 9 (23), 6505-6525 (1981).
- Lane, D., Prentki, P., Chandler, M. Use of gel retardation to analyze protein-nucleic acid interactions. Microbiol Rev. 56 (4), 509-528 (1992).
- Dyer, R. B., Herzog, N. K. Immunodepletion EMSA: A novel method to identify proteins in a protein-DNA complex. Nucleic Acids Res. 23 (16), 3345-3346 (1995).
- Dudley, R. K. A laboratory guide to in vitro studies of protein-DNA interactions. FEBS Lett. 306, 2-3 (1991).
- Tsai, C., Smider, V., Hwang, B. J., Chu, G. Electrophoretic mobility shift assays for protein-DNA complexes involved in DNA repair. Methods Mol Biol. 920, 53-78 (2012).
- Hellman, L. M., Fried, M. G. Electrophoretic mobility shift assay (EMSA) for detecting protein-nucleic acid interactions. Nat Protoc. 2 (8), 1849-1861 (2007).
- Heffler, M. A., Walters, R. D., Kugel, J. F. Using electrophoretic mobility shift assays to measure equilibrium dissociation constants: GAL4-p53 binding DNA as a model system. Biochem Mol Biol Educ. 40 (6), 383-387 (2012).
- Shah, N. B., Duncan, T. M. Bio-layer interferometry for measuring kinetics of protein-protein interactions and allosteric ligand effects. J Vis Exp. (84), e51383 (2014).
- Wallner, J., Lhota, G., Jeschek, D., Mader, A., Vorauer-Uhl, K. Application of bio-layer interferometry for the analysis of protein/liposome interactions. J Pharm Biomed Anal. 72, 150-154 (2013).
- Abdiche, Y., Malashock, D., Pinkerton, A., Pons, J. Determining kinetics and affinities of protein interactions using a parallel real-time label-free biosensor, the Octet. Anal Biochem. 377 (2), 209-217 (2008).
- Yang, D., Singh, A., Wu, H., Kroe-Barrett, R. Comparison of biosensor platforms in the evaluation of high affinity antibody-antigen binding kinetics. Anal Biochem. 508, 78-96 (2016).
- Schasfoort, R. B. M., Tudos, A. J. . Handbook of surface plasmon resonance. , (2008).
- Douzi, B. Surface plasmon resonance: A sensitive tool to study protein-protein interactions. Methods Mol Biol. 2715, 363-382 (2024).
- Douzi, B. Protein-protein interactions: Surface plasmon resonance. Methods Mol Biol. 1615, 257-275 (2017).
- Drescher, D. G., Selvakumar, D., Drescher, M. J. Analysis of protein interactions by surface plasmon resonance. Adv Protein Chem Struct Biol. 110, 1-30 (2018).
- Martin, S. R., Ramos, A., Masino, L. Biolayer interferometry: Protein-RNA interactions. Methods Mol Biol. 2263, 351-368 (2021).
- Apiyo, D. . Biomolecular binding kinetics assays on the Octet BLI platform. , (2022).
- Barrows, J. K., Van Dyke, M. W. Biolayer interferometry for DNA-protein interactions. PLoS One. 17 (2), e0263322 (2022).
- Desai, M., Di, R., Fan, H. Application of biolayer interferometry (BLI) for studying protein-protein interactions in transcription. J Vis Exp. (149), e59687 (2019).
- Sultana, A., Lee, J. E. Measuring protein-protein and protein-nucleic acid interactions by biolayer interferometry. Curr Protoc Protein Sci. 79, 19.25.1-19.25.26 (2015).
- Petersen, R. L. Strategies using bio-layer interferometry biosensor technology for vaccine research and development. Biosensors (Basel). 7 (4), 49 (2017).
- Concepcion, J., et al. Label-free detection of biomolecular interactions using biolayer interferometry for kinetic characterization. Comb Chem High Throughput Screen. 12 (8), 791-800 (2009).
- Wold, M. S. Replication protein A: A heterotrimeric, single-stranded DNA-binding protein required for eukaryotic DNA metabolism. Annu Rev Biochem. 66, 61-92 (1997).
- Qureshi, M. H., Ray, S., Sewell, A. L., Basu, S., Balci, H. Replication protein A unfolds G-quadruplex structures with varying degrees of efficiency. J Phys Chem B. 116 (19), 5588-5594 (2012).
- Wold, M. S., Kelly, T. Purification and characterization of replication protein A, a cellular protein required for in vitro replication of simian virus 40 DNA. Proc Natl Acad Sci U S A. 85 (8), 2523-2527 (1988).
- Bochkareva, E., Korolev, S., Lees-Miller, S. P., Bochkarev, A. Structure of the RPA trimerization core and its role in the multistep DNA-binding mechanism of RPA. EMBO J. 21 (7), 1855-1863 (2002).
- Brosey, C. A., et al. NMR analysis of the architecture and functional remodeling of a modular multidomain protein, RPA. J Am Chem Soc. 131 (18), 6346-6347 (2009).
- Fanning, E., Klimovich, V., Nager, A. R. A dynamic model for replication protein A (RPA) function in DNA processing pathways. Nucleic Acids Res. 34 (15), 4126-4137 (2006).
- Gibb, B., et al. Protein dynamics during presynaptic-complex assembly on individual single-stranded DNA molecules. Nat Struct Mol Biol. 21 (10), 893-900 (2014).
- Nguyen, B., et al. Diffusion of human replication protein A along single-stranded DNA. J Mol Biol. 426 (19), 3246-3261 (2014).
- Brosey, C. A., et al. Functional dynamics in replication protein A DNA binding and protein recruitment domains. Structure. 23 (6), 1028-1038 (2015).
- Chen, R., Subramanyam, S., Elcock, A. H., Spies, M., Wold, M. S. Dynamic binding of replication protein a is required for DNA repair. Nucleic Acids Res. 44 (12), 5758-5772 (2016).
- Kemmerich, F. E., et al. Force regulated dynamics of RPA on a DNA fork. Nucleic Acids Res. 44 (12), 5837-5848 (2016).
- Pokhrel, N., et al. Dynamics and selective remodeling of the DNA-binding domains of RPA. Nat Struct Mol Biol. 26 (2), 129-136 (2019).
- Wang, Q. M., et al. Human replication protein A induces dynamic changes in single-stranded DNA and RNA structures. J Biol Chem. 294 (38), 13915-13927 (2019).
- Wyka, I. M., Dhar, K., Binz, S. K., Wold, M. S. Replication protein A interactions with DNA: Differential binding of the core domains and analysis of the DNA interaction surface. Biochemistry. 42 (44), 12909-12918 (2003).
- Kim, C., Snyder, R. O., Wold, M. S. Binding properties of replication protein A from human and yeast cells. Mol Cell Biol. 12 (7), 3050-3059 (1992).
- Caldwell, C. C., Spies, M. Dynamic elements of replication protein A at the crossroads of DNA replication, recombination, and repair. Crit Rev Biochem Mol Biol. 55 (5), 482-507 (2020).
- Mishra, A. K., Dormi, S. S., Turchi, A. M., Woods, D. S., Turchi, J. J. Chemical inhibitor targeting the replication protein A-DNA interaction increases the efficacy of Pt-based chemotherapy in lung and ovarian cancer. Biochem Pharmacol. 93 (1), 25-33 (2015).
- Gavande, N. S., et al. Structure-guided optimization of replication Protein A (RPA)-DNA interaction inhibitors. ACS Med Chem Lett. 11 (6), 1118-1124 (2020).
- Ana Jug, T. B., Janez Ilaš, . Biolayer interferometry and its applications in drug discovery and development. TrAC Trends Anal Chem. 176, 117741 (2024).
- . Using BLI for viral and lipid-based vector analytics Available from: https://www.news-medical.net/whitepaper/20230322/Using-BLI-for-viral-and-lipid-based-vector-analytics.aspx (2023)
- Noy-Porat, T., et al. Characterization of antibody-antigen interactions using biolayer interferometry. STAR Protoc. 2 (4), 100836 (2021).
- Onyekachi Ononye, S. S., et al. Biochemical impact of p300-mediated acetylation of replication protein A: Implications for DNA metabolic pathway choice. bioRxiv. , (2024).
- Weeramange, C. J., Fairlamb, M. S., Singh, D., Fenton, A. W., Swint-Kruse, L. The strengths and limitations of using biolayer interferometry to monitor equilibrium titrations of biomolecules. Protein Sci. 29 (4), 1018-1034 (2020).
- Artem Stetsenko, A. G. An Overview of the top ten detergents used for membrane protein crystallization. Crystals. 7 (7), 197 (2017).
- Hubbe, M. A., Szlek, D. B., Vera, R. E. Detergency mechanisms and cellulosic surfaces: A review. BioResources. 17 (4), 7167-7249 (2022).
- . Research the Industry Standard for Label-Free of Biomolecular Interactions Analysis (BIA) Available from: https://www.sartorius.com/en/products/biolayer-interferometry/bli-resources (2021)
- . Octet NTA Biosensor Kinetic Assays Available from: https://www.sartorius.com/resource/blob/552398/b27ccc2fdfde7a9320909572ced3f6a1/ni-nta-biosensor-kinetic-assays-technical-note-en-sartorius-data.pdf (2024)
- . Application Guides: Kinetics and affinity measurements with Biacore systems Available from: https://cdn.cytivalifesciences.com/api/public/content/digi-33042-pdf (2021)
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved