Method Article
Synthesis of Graphene-Hydroxyapatite Nanocomposites for Potential Use in Bone Tissue Engineering
In This Article
Summary
Novel nanocomposites of graphene nanoribbons and hydroxyapatite nanoparticles were prepared using solution-phase synthesis. These hybrids when employed in bioactive scaffolds can exhibit potential applications in tissue engineering and bone regeneration.
Abstract
Developing novel materials for bone tissue engineering is one of the most important thrust areas of nanomedicine. Several nanocomposites have been fabricated with hydroxyapatite to facilitate cell adherence, proliferation, and osteogenesis. In this study, hybrid nanocomposites were successfully developed using graphene nanoribbons (GNRs) and nanoparticles of hydroxyapatite (nHAPs), that when employed in bioactive scaffolds may potentially improve bone tissue regeneration. These nanostructures can be biocompatible. Here, two approaches were used for preparing the novel materials. In one approach, a co-functionalization strategy was used where nHAP was synthesized and conjugated to GNRs simultaneously, resulting in nanohybrids of nHAP on GNR surfaces (denoted as nHAP/GNR). High-resolution transmission electron microscopy (HRTEM) confirmed that the nHAP/GNR composite is comprised of slender, thin structures of GNRs (maximum length of 1.8 µm) with discrete patches (150-250 nm) of needle-like nHAP (40-50 nm in length). In the other approach, commercially available nHAP was conjugated with GNRs forming GNR-coated nHAP (denoted as GNR/nHAP) (i.e., with an opposite orientation relative to the nHAP/GNR nanohybrid). The nanohybrid formed using the latter method exhibited nHAP nanospheres with a diameter ranging from 50 nm to 70 nm covered with a network of GNRs on the surface. Energy dispersive spectra, elemental mapping, and Fourier transform infrared (FTIR) spectra confirmed the successful integration of nHAP and GNRs in both nanohybrids. Thermogravimetric analysis (TGA) indicated that the loss at elevated heating temperatures due to the presence of GNRs was 0.5% and 0.98% for GNR/nHAP and nHAP/GNR, respectively. The nHAP-GNR nanohybrids with opposite orientations represent significant materials for use in bioactive scaffolds to potentially promote cellular functions for improving bone tissue engineering applications.
Introduction
Graphene has sheet-like two-dimensional structures composed of sp-hybridized carbon. Several other allotropes can be attributed to the extended honeycomb network of graphene (e.g., the stacking of graphene sheets forms 3D graphite while rolling off the same material results in the formation of 1D nanotubes1). Likewise, 0D fullerenes are formed due to wrapping2. Graphene has attractive physicochemical and optoelectronic properties that include an ambipolar field-effect and a quantum Hall effect at room temperature3,4. Detection of single-molecule adsorption events and extremely high carrier mobility add to the attractive attributes of graphene5,6. Further, graphene nanoribbons (GNRs) with narrow widths and a large mean free path, low resistivity with a high current density, and high electron mobility are considered promising interconnecting materials7. Hence, GNRs are being explored for applications in a myriad of devices, and more recently in nanomedicine, particularly tissue engineering and drug delivery8.
Among various traumatic ailments, bone injuries are considered one of the most challenging due to difficulties in stabilizing the fracture, regeneration and replacement with new bone, resisting infection, and re-aligning bone non-unions9,10. Surgical procedures remain the only alternative for femoral shaft fractures. It should be noted that almost $52 million is spent every year on treating bone injuries in Central America and Europe11.
Bioactive scaffolds for bone tissue engineering applications can be more effective by incorporating nano-hydroxyapatite (nHAP), as they resemble the micro and nano architectural properties of the bone itself12. HAP, chemically represented as Ca10(PO4)6(OH)2 with a Ca/P molar ratio of 1.67, is the most preferred for biomedical applications, particularly for treating periodontal defects, the substitution of hard tissues, and fabricating implants for orthopedic surgeries13,14. Thus, the fabrication of nHAP-based biomaterials reinforced with GNRs can possess superior biocompatibility and may be advantageous due to their ability to promote osseointegration and be osteoconductive15,16. Such hybrid composite scaffolds can preserve biological properties such as cell adherence, spreading, proliferation, and differentiation17. Herein, we report the fabrication of two new nanocomposites for bone tissue engineering by rationally altering the spatial arrangement of nHAP and GNRs as illustrated in Figure 1. The chemical and structural properties of the two different nHAP-GNRs arrangements were evaluated here.
Protocol
1. Synthesis of nHAP by precipitation
- Synthesize the pristine nHAP using 50 mL of the reaction mixture containing 1 M Ca(NO3)2∙4H2O and 0.67 M (NH4)H2PO4 followed by the dropwise addition of NH4OH (25%) to maintain a pH around 1018.
- Thereafter, agitate the reaction mixture by ultrasound irradiation (UI) for 30 min (500 W power and 20 kHz ultrasound frequency).
- Allow the resulting solution to mature for 120 h at room temperature until the white precipitate of nHAP settles out. Recover the nHAP by centrifugation at 1398 x g for 5 min at room temperature.
- Wash the precipitate with deionized (DI) water 3x and lyophilize for 48 h. Store the dry powder at 4 °C.
2. Preparation of nHAP/GNR nanocomposites
NOTE: The following describes two approaches for fabricating nHAP/GNR (i.e., nHAP on GNR surfaces) and GNR/nHAP (GNR-coated nHAP) nanocomposites that represent two different spatial arrangements of nHAP and GNRs (Figure 1).
- Synthesis of nHAP/GNR
- To prepare the nHAP/GNRs nanocomposite, use a co-functionalization strategy where nHAP can be synthesized and conjugated to GNRs simultaneously, as follows.
- Dissolve 5 mg of GNRs (Table of Materials) in a mixture of 1 M calcium nitrate tetrahydrate [Ca(NO3)2·4H2O] and 0.67 M diammonium hydrogen phosphate [(NH4)2HPO4] to a 50 mL final volume19.
- During this reaction, add 25% of NH4OH dropwise to maintain the pH at ~10. Agitate the resulting mixture by UI for 30 min.
- After completion of the reaction, leave the solution undisturbed for 120 h at room temperature until maturation.
- Observe the formation of a gelatinous precipitate of nHAP which coats the GNRs, following which a white precipitate of nHAP/GNRs settles.
- Wash the precipitate 3x by centrifugation at 1398 x g for 5 min at room temperature followed by re-dispersion in DI water.
- Lyophilize the recovered washed precipitate for 48 h. Store the dry powder at 4 °C.
- Use pristine nHAP and GNRs as control samples.
- Synthesis of GNR/nHAP nanocomposite
- Suspend commercially available nHAP at a concentration of 5 mg/mL in 50 mL of DI water supplemented with 5 mg of GNRs.
- Agitate the resulting mixture by UI for 30 min and thereafter leave the mixture undisturbed for 120 h at room temperature.
- After maturation, recover the white precipitate of the resulting GNR/nHAP by centrifugation at 1398 x g for 5 min at room temperature.
- Wash the sample 3x using DI water, lyophilize it for 48 h, and store the dry powder at 4 °C for further use.
3. Characterization of nHAP, nHAP/GNR, and GNR/nHAP
- Use a high-resolution transmission electron microscope (HRTEM) (see Table of Materials) to characterize the morphology and size of the nanocomposites11.
- Analyze the elemental composition of the nanocomposites employing energy dispersive spectroscopy (EDS) and perform elemental mapping using the scanning transmission electron microscope (STEM)11.
- Perform Fourier transform infrared (FTIR) spectroscopy for the neat samples at wavenumbers of 500-4000 cm−1 to analyze the chemical groups in the nanocomposite16.
- Perform powder X-ray diffraction (XRD) analysis of the as-synthesized nHAP using an X-ray wavelength of 1.5406 Å, current and voltage settings of 40 mA and 40 kV, respectively, and 2θ ranging from 20° to 90°.
- Evaluate the percent loading of GNR in the nanocomposite using thermogravimetric analysis (TGA) by heating the samples from room temperature to 1000 °C at a rate of 10 °C/min under nitrogen flow.
Results
HRTEM analysis
Individually, GNRs were slender bamboo-like structures with some bends at some distance as observed in Figure 2. The longest GNR was 1.841 µm while the smallest bent GNR was 497 nm. The nanoribbons often showed a visible variation in width that might be attributed to twisting to form helical configurations in many places. Such unidirectional alignment of GNRs may help to obtain attractive features such as magnetic properties, conductivity, or heat transport7.
The nHAPs synthesized using calcium nitrate tetrahydrate and diammonium hydrogen phosphate at room temperature (step 1) were rod-shaped or needle-like with a size ranging from 40 nm to 50 nm (Figure 3). The as-synthesized nanomaterials were found in clumps due to aggregation and crystalline growth. On the other hand, the commercially available nHAPs used were spherical (Figure 4); these nanospheres were 50-70 nm in diameter and present in discrete clusters of 15-20 spheres.
nHAP was deposited in situ on the GNRs (represented as nHAP/GNR) in the co-functionalization strategy (step 2.1). The resulting nanocomposites of GNRs and nHAP consisted of highly porous interconnected nanostructures. The predominance of needle-like nHAPs covering the GNR surface in patches (Figure 5) is attributed to the GNRs that serve as a nano-featured scaffold for nHAP nucleation. The nHAP patches were found to be between 150 nm and 250 nm in length and width (Figure 5A,B). Elemental mapping confirmed that the intermediated nodal patches on the GNRs were indeed nHAP due to the presence of elemental calcium and phosphorous (Figure 5C).
In the other method (step 2.2), pre-formed nHAP was conjugated to the GNRs which led to the formation of GNR-coated nHAP (represented as GNR/nHAP, i.e., with a reverse orientation compared with nHAP/GNR composite). In this case, the GNRs formed thin films on the surface of the spherical nHAP nanoparticles (Figure 6).
Interestingly, the bends and convolutions noticed at the periphery of the GNRs as evident in Figure 2A are mostly attributed to low stability properties that might have significantly enhanced the mechanical interaction and attachment with the nHAP as seen in Figure 5 and Figure 6. Moreover, the large surface area of the pristine GNRs also helps in more nHAP loading. Also, the aging of the composite solutions for 120 h resulted in the complete conversion of the apatite into highly crystalline hydroxyapatite (Figure 3 and Figure 5). The oxygen-based functional groups of the GNR surface electrostatically interact with Ca2+, serving as the receptor site. Apatite nanostructures can be obtained further due to the in situ reaction between these anchored cations and the phosphate ions (step 2.1). The orientation of the microstructured nHAP on the GNR surface is controlled by several factors that include the amount and type of oxygenous groups on the graphene-based templates, the relative concentration of the precursors (Ca2+ and HPO42-), pH of the reaction mixture, and the maturation time. The cumulative effect of the reaction conditions resulted in the wrapping of the transparent GNRs onto the surface of the nHAP nanospheres possibly due to non-covalent physical adsorption.
Energy dispersive spectra (EDS) analysis
To confirm the major components and the elemental composition of the nanocomposites, an energy dispersive spectral analysis was performed. In Figure 7A, the EDS spectra of pristine GNRs showed a carbon peak that corresponds to the GNRs while no other peaks were observed except for the copper that was attributed to the grid used for mounting samples during HRTEM analysis. Figure 7B shows the EDS spectrum of commercially available pre-formed nHAP nanospheres where the carbon and copper peaks are attributed to the carbon-coated copper grids used for mounting the samples during HRTEM analysis. In Figure 7C, a clear increase in the carbon content was attributed to the GNRs, while the other peaks specific to calcium and phosphorous were due to nHAP in the GNR/nHAP nanocomposites. Figure 8 shows the EDS spectra of the as-synthesized nHAP (step 1) (Figure 8A) and nHAP/GNR composite (Figure 8B). The marked increase in the carbon content in the nHAP/GNR spectrum is due to the majority of the GNRs onto which only small patches of freshly synthesized nHAP were observed.
FTIR analysis
Conjugation of nHAP with the GNRs was confirmed through FTIR spectra. Figure 9 shows the FTIR spectra of nHAP, GNR, nHAP/GNR, and GNR/nHAP. The OH out of plane bending peak at 600 cm-1 is seen in the FTIR of GNR12. The peak at 1030 cm-1, attributed to P-O stretching was observed in nHAP, confirming its chemical composition15. Notably, the characteristic P-O stretching peak of nHAP was also found in nHAP/GNR and GNR/nHAP, indicating the presence of nHAP in both the composites. The other two peaks, 1413 and1447 cm-1 found only in the composites are attributed to δCH2 vibrations and carbonate group (CO32−), respectively, which confirm the conjugation of GNR and nHAP16.
X-ray diffraction (XRD) analysis
The XRD pattern of the HAP (step 2.1) is shown in Figure 10. The strong peaks indicated good crystallinity of the material. The peak positions were in good agreement with those in the ICDD standard data (PDF2 card: 00-009-0432). This further confirmed the hexagonal crystal structure (P63/m space group) of the nHAP, having lattice parameters values of a = b = 0.940 nm and c = 0.615 nm. Some of the salient, strong peaks at 2θ values of 25.8°, 28.2°, 31.8°, 32.9°, 34.1°, 39.7°, 43.9°, 46.6°, and 49.4° corresponding to (002), (102), (211), (300), (202), (310), (113), (222), and (213) planes, respectively, confirmed the purity of the as-synthesized nHAP 16,20,21.
Thermogravimetric analysis (TGA)
Thermogravimetric analysis (TGA) was employed to estimate the loading percentage in the conjugates (Figure 11). Three prominent losses in mass were evident during TGA analysis. The initial loss in mass at temperatures up to 100 °C is due to the entrapped physical water. The second loss between 100 °C to 200 °C is due to the decomposition of the GNR into carbon soot. The steady decrease in mass thereafter up to 500 °C was due to the crystallization of nHAP. Further heating led to the decomposition of the complexes. Loss due to the presence of GNRs was found to be between 0.5% and 0.98% in GNR/nHAP and nHAP/GNR, respectively. Hence, it is in good agreement with our previous analysis where HAP was found as the major component and the GNRs were surface-oriented within the GNR/nHAP. On the other hand, the GNRs were abundant in nHAP/GNR wherein the nHAP formed discrete patches on the long stretches of GNRs.
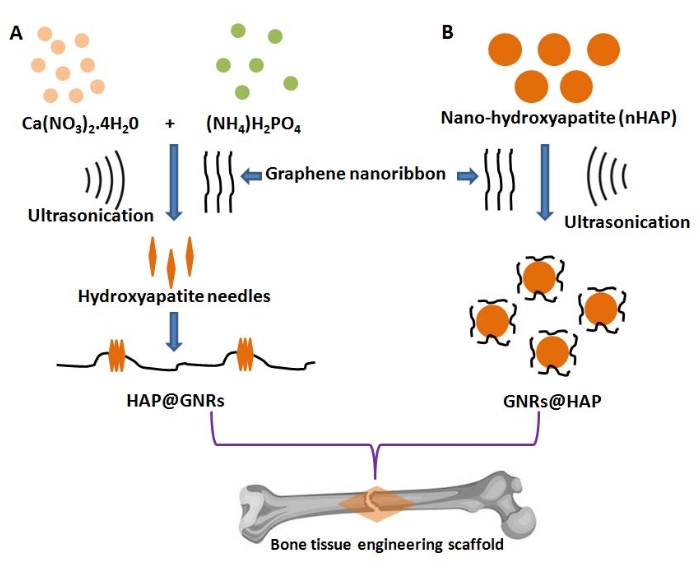
Figure 1: Schematic representation for synthesizing reverse-oriented graphene nanoribbon-hydroxyapatite hybrid composites: (A) nHAP/GNR and (B) GNR/nHAP. Please click here to view a larger version of this figure.

Figure 2: Structural analysis of GNRs: (A) HRTEM analysis of bare GNRs; (B) Scanning transmission electron mode (STEM) images of GNRs; and (C) Elemental mapping of the GNRs, where red, green, yellow, and blue colors denote carbon, oxygen, phosphorous, and calcium, respectively. Please click here to view a larger version of this figure.

Figure 3: Structural analysis of as-synthesized nHAP: (A) HRTEM analysis of nHAP; (B) Scanning transmission electron mode (STEM) images of nHAP with the inset scale bar representing 100 nm; and (C) Elemental mapping of the nHAP where red, green, yellow, and blue colors denote carbon, oxygen, phosphorous, and calcium, respectively. Please click here to view a larger version of this figure.
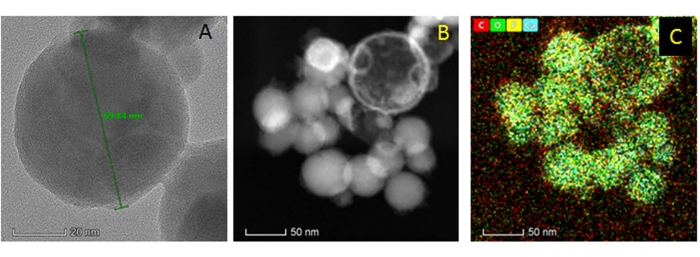
Figure 4: Structural analysis of the commercially available pre-formed nHAP: (A) HRTEM analysis of nHAP; (B) Scanning transmission electron mode (STEM) images of nHAP; and (C) Elemental mapping of the nHAP where red, green, yellow, and blue colors denote carbon, oxygen, phosphorous, and calcium, respectively. Please click here to view a larger version of this figure.

Figure 5: Structural analysis of nHAP/GNR synthesized by the co-functionalization strategy: (A) HRTEM analysis of nHAP/GNR; (B) Scanning transmission electron mode (STEM) images of nHAP/GNR; and (C) Elemental mapping of the nHAP/GNR where red, green, yellow, and blue colors denote carbon, oxygen, phosphorous, and calcium, respectively. Please click here to view a larger version of this figure.

Figure 6: Structural analysis of GNR/nHAP: (A) HRTEM analysis of GNR/nHAP; (B) Scanning transmission electron mode (STEM) images of GNR/nHAP with inset scale bar representing 50 nm; and (C) Elemental mapping of the GNR/nHAP where red, green, yellow, and blue colors denote carbon, oxygen, phosphorous, and calcium, respectively. Please click here to view a larger version of this figure.

Figure 7: EDS analysis of the GNR/nHAP nanocomposite: (A) GNRs, (B) commercially available pre-formed nHAP, and (C) GNR/nHAP nanocomposite. Please click here to view a larger version of this figure.
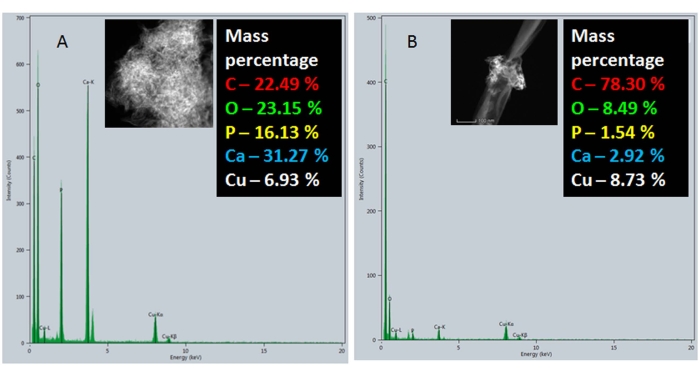
Figure 8: EDS analysis of the nHAP/GNR nanocomposite: (A) As-synthesized nHAP and (B) nHAP/GNR. Please click here to view a larger version of this figure.

Figure 9: FTIR analysis of the nanocomposites. Please click here to view a larger version of this figure.

Figure 10: X-ray diffraction (XRD) analysis of nHAP. Please click here to view a larger version of this figure.

Figure 11: Thermogravimetric analysis of the nanocomposites. Please click here to view a larger version of this figure.
Discussion
Although various metals, polymers, ceramics, and their combinations have been researched as orthopedic implants and fixation accessories, HAP is considered to be one of the most preferable materials due to its chemical similarity to the bone itself and consequent high cytocompatibility20,21,22. In this study, the orientation of HAP was varied, which can have a significant impact on its unique properties, such as promotion of osteogenesis, osseointegration, and osteoconductivity. Moreover, changing the orientation of HAP can impact the nanocomposites' mechanical properties to further mimic that of natural bone, since long bones in the body possess an anisotropic alignment of HA with collagen, while cuboidal bones possess a random arrangement of HA with collagen. It should be noted that although natural HAP is the main constituent of human teeth and bones, its physical properties are largely dependent on reaction conditions such as the reaction time, pH, phosphate concentration, and chemical nature of the CaP phase23. Hence, in this study, a wet chemical method was used to synthesize nHAP at a pH of 10 under ultrasound irradiation (UI). Barbosa et al. (2013) also reported that UI in conjunction with aqueous precipitation without calcination is a simple, rapid, and efficient method that generates nHAP with high crystallinity and specificity18.
It is significant to note that artificially fabricated HAP-associated biomaterials often exhibit poor mechanical properties which include intrinsic brittleness, low fracture toughness, and wear22. Hence, nHAP is reinforced here with GNRs to facilitate: (i) surface functionalization associated with the nano-particles, (ii) electrostatic interactions at the interface in the complex, and (iii) transfer of stress to the nano-fillers from the matrix of the scaffolds24,25,26. The wet chemical synthesis followed here resulted in pristine nHAP predominantly in small acicular particles agglomerated into larger particles (~40 nm). This result is well in agreement with the report by Barbosa et al. (2013), where it was speculated that UI played a critical role in inducing nucleation by forming bubble walls in close proximity, referred to as "hot-spots"18,27.
It is interesting to note that a decrease in the particle size of both the host and guest particles can enhance the flowability only up to a certain limit. Thereafter, further reduction in the dimension of the host particle may lead to agglomeration that negatively impacts the flowability28. Apart from inducing primary nucleation in a virtually particle-free solution, UI prevents high supersaturation levels. Further, UI-mediated reduction in the elapsed time between establishing supersaturation and the onset of nucleation and crystallization may be key in the shape evolution of nHAP and the pattern of functionalization on the GNRs. The nHAP/GNR structure might be attributed to the cumulative effect of the reaction temperature, pressure associated due to collapse of the bubble, and shock waves in addition to highly energetic agitation created in spatially concentrated regions. Similarly, the GNR/nHAP structure synthesized by the simultaneous addition of pristine GNRs and nHAP in the presence of UI might be attributed to the subsequent rapid local cooling rates enhancing supersaturation. A localized increment in pressure can also decrease the temperature of crystallization, while the barrier created by activation energy can be significantly surmounted by the transfer of energy due to cavitation during surface functionalization of the GNRs18,27.
One report shows that the excessive application of UI (~30-120 min) during synthesis reduces the crystallinity and/or the size of the nHAP crystals29. This may further determine the orientation of the functionalization as observed in our study. Even in this study, results showed that UI for a relatively long exposure time (30 min) during the synthesis of nHAP led to nHAP deposition on GNRs. On the other hand, UI for 30 min with pre-formed nHAP and GNRs led to the deposition of GNRs on the spherical nHAPs. Hence, this method is ideal for the large-scale production of nHAP to obtain GNR-incorporated composites for scaffold fabrication30,31. Such novel reverse-oriented composites with superior mechanical properties can be significant for bone tissue engineering. In particular, Fan et al. (2014) reported that introducing graphene can significantly improve the hardness and Young's modulus of nHAP that in turn exhibit higher osseointegration with surrounding bone (i.e., superior biocompatibility), compared to pristine graphene and nHAP, individually32. Thus, nanocomposites comprised of GNRs and nHAP with superior mechanical properties and biocompatibility can be promising biomaterials for numerous orthopedic applications33,34,35.
However, the major challenge in the fabrication of nanohybrids with opposite orientations is that the reaction parameters should be strictly followed to obtain the desired orientation of nanocomposites36,37. Moreover, in the co-functionalization strategy, fewer amounts of the needle-shaped nHAP were deposited on the GNRs, which can reduce their potential for bone tissue regeneration and mechanical strength. The shapes of the nHAP in both methods are different, which can significantly influence the amount of osteogenesis and hence cause various properties pertinent for biomedical applications.
In conclusion, here, we synthesized nanocomposites comprised of GNRs and nHAP with contrasting spatial arrangements that may have potential applications in orthopedics. Results showed that nHAP morphology and functionalization time (i.e., whether functionalization takes place after synthesis or at the same time as nHAP synthesis) determined the orientation of nHAP and GNRs in the nanocomposites. Co-functionalization during synthesis resulted in nHAP/GNRs, while functionalization with pre-formed nHAP resulted in GNRs/nHAP. These nanocomposites might be applicable to develop scaffolds to promote osteogenesis and, thus, have significant promise in regenerative nanomedicine warranting their further investigation.
Disclosures
The authors have no conflicts of interest.
Acknowledgements
Dr. Sougata Ghosh acknowledges the Department of Science and Technology (DST), Ministry of Science and Technology, Government of India, and Jawaharlal Nehru Centre for Advanced Scientific Research, India for funding under the Post-Doctoral Overseas Fellowship in Nano Science and Technology (Ref. JNC/AO/A.0610.1(4) 2019-2260 dated August 19, 2019). Dr. Sougata Ghosh acknowledges Kasetsart University, Bangkok, Thailand for a Post-Doctoral Fellowship, and funding under the Reinventing University Program (Ref. No. 6501.0207/10870 dated November 9, 2021). The authors would like to thank the Kostas Advanced Nano-Characterization Facility (KANCF) for assistance with the characterization experiments. KANCF is a shared multidisciplinary research and educational facility within the Kostas Research Institute (KRI) at Northeastern University.
Materials
| Name | Company | Catalog Number | Comments |
| Ammonium phosphate monobasic | Sigma-Aldrich | 216003-100G | Synthesis |
| Calcium nitrate tetrahydrate | Sigma-Aldrich | 237124 | Synthesis |
| Centrifuge | Hettich | EBA 200S | Recovery |
| Fourier transform infrared spectrometer | Brucker | Vertex 70 | Characterization |
| Graphene nanoribbon | Sigma-Aldrich | 922714 | Synthesis |
| High resolution transmission electron microscope | Thermo Fisher Scientific | Themis Titan 300 | Characterization |
| Magnetic stirrer | IKA | C-MAG HS7 S68 | Functionalization |
| Micropipettes | TreffLab | 06H35687 | Reagent preparation |
| pH meter | Eutech pH5+ | ECPH503PLUSK | Reagent preparation |
| Thermogravimetric analyzer | TA Instruments | SDT Q600 | Characterization |
| Ultrasonic bath | Bandelin | DT100 | Functionalization |
| Universal Oven | Memmert | UF55 | Functionalization |
| Weighing balance | Precisa | XB220A | Reagent preparation |
| X-ray diffractometer | Brucker | D8-Advanced | Characterization |
References
- Novoselov, K. S., et al. Electric field effect in atomically thin carbon films. Science. 306 (5696), 666-669 (2004).
- Novoselov, K. S., et al. Unconventional quantum Hall effect and Berry's phase of 2π in bilayer graphene. Nature Physics. 2 (3), 177-180 (2006).
- Zhang, Y. B., Tan, Y. W., Stormer, H. L., Kim, P. Experimental observation of the quantum Hall effect and Berry's phase in graphene. Nature. 438 (7065), 201-204 (2005).
- Ozyilmaz, B., et al. Electronic transport and quantum hall effect in bipolar Graphene p−n−p junctions. Physical Review Letters. 99 (16-19), 166804 (2007).
- Morozov, S. V., et al. Giant intrinsic carrier mobilities in graphene and its bilayer. Physical Review Letters. 100 (1-11), 016602 (2008).
- Han, M., Ozyilmaz, B., Zhang, Y., Jarillo-Herero, P., Kim, P. Electronic transport measurements in graphene nanoribbons. Physica Status Solidi B: Basic Solid State Physics. 244 (11), 4134-4137 (2007).
- Talyzin, A. V., et al. Synthesis of graphene nanoribbons encapsulated in single-walled carbon nanotubes. Nano Letters. 11 (10), 4352-4356 (2011).
- Allen, M. J., Tung, V. C., Kaner, R. B. Honeycomb carbon: A review of graphene. Chemical Reviews. 110 (1), 132-145 (2010).
- Ghosh, S., Webster, T. J. Metallic nanoscaffolds as osteogenic promoters: Advances, challenges and scope. Metals. 11 (9), 1356 (2021).
- Ghosh, S., Webster, T. J. Mesoporous silica based nanostructures for bone tissue regeneration. Frontiers in Materials. 8, 213 (2021).
- Medeiros, J. S., et al. Nanohydroxyapatite/graphene nanoribbons nanocomposites induce in vitro osteogenesis and promote in vivo bone neoformation. ACS Biomaterials Science and Engineering. 4 (5), 1580-1590 (2018).
- Faniyi, I. O., et al. The comparative analyses of reduced graphene oxide (RGO) prepared via green, mild and chemical approaches. SN Applied Sciences. 1 (10), 1-7 (2019).
- Neelgund, G. M., Oki, A., Luo, Z. In situ deposition of hydroxyapatite on graphene nanosheets. Materials Research Bulletin. 48 (2), 175-179 (2013).
- Rajkumar, M., Sundaram, N. M., Rajendran, V. Preparation of size controlled, stoichiometric and bioresorbable hydroxyapatite nanorod by varying initial pH, Ca/P ratio and sintering temperature. Digest Journal of Nanomaterials and Biostructures. 6 (1), 169-179 (2011).
- Mondal, S., et al. Hydroxyapatite coated iron oxide nanoparticles: A promising nanomaterial for magnetic hyperthermia cancer treatment. Nanomaterials. 7 (12), 426 (2017).
- Oliveira, F. C., et al. High loads of nano-hydroxyapatite/graphene nanoribbon composites guided bone regeneration using an osteoporotic animal model. International Journal of Nanomedicine. 14, 865-874 (2019).
- Murugan, N., Sundaramurthy, A., Chen, S. -. M., Sundramoorthy, A. K. Graphene oxide/oxidized carbon nanofiber/mineralized hydroxyapatite based hybrid composite for biomedical applications. Materials Research Express. 4 (12), 124005 (2017).
- Barbosa, M. C., Messmer, N. R., Brazil, T. R., Marciano, F. R., Lobo, A. O. The effect of ultrasonic irradiation on the crystallinity of nano-hydroxyapatite produced via the wet chemical method. Materials Science and Engineering C. 33 (5), 2620-2625 (2013).
- Rodrigues, B. V. M., et al. Graphene oxide/multi-walled carbon nanotubes as nanofeatured scaffolds for the assisted deposition of nanohydroxyapatite: characterization and biological evaluation. International Journal of Nanomedicine. 11, 2569-2585 (2016).
- Sharma, M., Nagar, R., Meena, V. K., Singh, S. Electro-deposition of bactericidal and corrosion-resistant hydroxyapatite nanoslabs. RSC Advances. 9 (20), 11170-11178 (2019).
- Kamrujjaman, M., Khandaker, J. I., Haque, M. M., Rahman, M. O., Rahman, M. M. Study of the dependency of pH values on HAp synthesis. Journal of Nanomaterials & Molecular Nanotechnology. 7, 4 (2019).
- Baradaran, S., et al. Mechanical properties and biomedical applications of a nanotube hydroxyapatite-reduced graphene oxide composite. Carbon. 69, 32-45 (2014).
- Sassoni, E. Hydroxyapatite and other calcium phosphates for the conservation of cultural heritage: A review. Materials. 11 (4), 557 (2018).
- Tang, H., Ehlert, G. J., Lin, Y., Sodano, H. A. Highly efficient synthesis of graphene nanocomposites. Nano Letters. 12 (1), 84-90 (2012).
- Walker, L. S., Marotto, V. R., Rafiee, M. A., Koratkar, N., Corral, E. L. Toughening in graphene ceramic composites. ACS Nano. 5 (4), 3182-3190 (2011).
- Rafiee, M. A., et al. Enhanced mechanical properties of nanocomposites at low graphene content. ACS Nano. 3 (12), 3884-3890 (2009).
- Luque de Castro, M. D., Priego-Capote, F. Ultrasound-assisted crystallization (sonocrystallization). Ultrasonics Sonochemistry. 14 (6), 717-724 (2007).
- Azhari, A., Toyserkani, E. Additive manufacturing of graphene-hydroxyapatite nanocomposite structures. International Journal of Applied Ceramic Technology. 12 (1), 8-17 (2015).
- Li, H., Wang, J., Bao, Y., Guo, Z., Zhang, M. Rapid sonocrystallization in the salting-out process. Journal of Crystal Growth. 247 (1-2), 192-198 (2003).
- Zou, Z., Lin, K., Chen, L., Chang, J. Ultrafast synthesis and characterization of carbonated hydroxyapatite nanopowders via sonochemistry-assisted microwave process. Ultrasonics Sonochemistry. 19 (6), 1174-1179 (2012).
- Rouhani, P., Taghavinia, N., Rouhani, S. Rapid growth of hydroxyapatite nanoparticles using ultrasonic irradiation. Ultrasonics Sonochemistry. 17 (5), 853-856 (2010).
- Fan, Z., et al. One-pot synthesis of graphene/hydroxyapatite nanorod composite for tissue engineering. Carbon. 66, 407-416 (2014).
- Ghosh, S., Mostafavi, E., Thorat, N., Webster, T. J., Liu, H., Shokuhfar, T., Ghosh, S. Nanobiomaterials for three- dimensional bioprinting. Nanotechnology in Medicine and Biology. , 1-24 (2021).
- Ghosh, S., Sanghavi, S., Sancheti, P., Balakrishnan, P., Sreekala, P., Thomas, S. Metallic biomaterial for bone support and replacement. Fundamental Biomaterials: Metals. Vol 2. Woodhead Publishing Series in Biomaterials. , 139-165 (2018).
- Hazra, A., Basu, S. Graphene nanoribbon as potential on-chip interconnect material-A Review. C Journal of Carbon Research. 4 (3), 49 (2018).
- Zanin, H., et al. Fast preparation of nano-hydroxyapatite/superhydrophilic reduced graphene oxide composites for bioactive applications. Journal of Materials Chemistry B. 1 (38), 4947-4955 (2013).
- Lobo, A. O., et al. Fast preparation of hydroxyapatite/superhydrophilic vertically aligned multiwalled carbon nanotube composites for bioactive application. Langmuir. 26 (23), 18308-18314 (2010).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved