Method Article
In Situ Exploration of Murine Megakaryopoiesis using Transmission Electron Microscopy
In This Article
Summary
Here, we present a protocol to analyze ultrastructure of the megakaryocytes in situ using transmission electron microscopy (TEM). Murine bone marrows are collected, fixed, embedded in epoxy resin and cut in ultrathin sections. After contrast staining, the bone marrow is observed under a TEM microscope at 120 kV.
Abstract
Differentiation and maturation of megakaryocytes occur in close association with the cellular and extracellular components of the bone marrow. These processes are characterized by the gradual appearance of essential structures in the megakaryocyte cytoplasm such as a polyploid and polylobulated nucleus, an internal membrane network called demarcation membrane system (DMS) and the dense and alpha granules that will be found in circulating platelets. In this article, we describe a standardized protocol for the in situ ultrastructural study of murine megakaryocytes using transmission electron microscopy (TEM), allowing for the identification of key characteristics defining their maturation stage and cellular density in the bone marrow. Bone marrows are flushed, fixed, dehydrated in ethanol, embedded in plastic resin, and mounted for generating cross-sections. Semi-thin and thin sections are prepared for histological and TEM observations, respectively. This method can be used for any bone marrow cell, in any EM facility and has the advantage of using small sample sizes allowing for the combination of several imaging approaches on the same mouse.
Introduction
Megakaryocytes are specialized large polyploid cells, localized in the bone marrow, responsible for platelet production1. They originate from hematopoietic stem cells through an intricate maturation process, during which megakaryocyte precursors progressively increase in size, while undergoing extensive concomitant morphologic changes in the cytoplasm and nucleus2. During maturation, megakaryocytes develop a number of distinguishable structural elements including: a polylobulated nucleus, invaginations of the surface membrane that form the demarcation membrane system (DMS), a peripheral zone devoid of organelles surrounded by the actin based cytoskeletal network, and numerous organelles including α-granules, dense granules, lysosomes, and multiple Golgi complexes. At the ultrastructural level, a major modification observed is the cytoplasmic compartmentalization into discrete regions delimited by the DMS3. This extensive supply of membranes will fuel the extension of long cytoplasmic processes in the initial phase of platelet production, which will then remodel into platelets inside the circulation. Any defect during megakaryocyte differentiation and maturation can affect platelet production in term of platelet count and/or platelet function.
Thin layer transmission electron microscopy (TEM) has been the imaging approach of choice for decades providing high-quality ultrastructure of megakaryocytes that have shaped our understanding of the physiology of thrombopoiesis4,5. This paper focuses on a standardized TEM method allowing to capture the process of platelet biogenesis occurring in situ within the native bone marrow microenvironment, which could also serve as a basis to analyze any bone marrow cell type. We provide ultrastructural examples of the development of megakaryocytes from immature to fully mature, which extend cytoplasmic processes into the microcirculation of sinusoids6. We also describe an easy procedure to quantify the different megakaryocyte maturation stages, instructing on the regeneration and platelet production capacity of the bone marrow.
Protocol
All animal experiments were performed in accordance with European standards 2010/63/EU and the CREMEAS Committee on the Ethics of Animal Experiments of the University of Strasbourg (Comité Régional d'Ethique en Matière d'Expérimentation Animale Strasbourg). The protocol is schematically shown in Figure 1.
1. Bone marrow collection and fixation ( Figure 1A)
CAUTION: This procedure includes carcinogenic, mutagenic, and/or toxic substances and is performed under a chemical extraction hood. Wear appropriate protective equipment such as gloves and protections glasses.
- Prepare the fixative solution consisting of 2.5% glutaraldehyde in cacodylate buffer (see Supplementary File).
- Bone marrow collection
- Use adult C57BL/6 mice of either sex 12-18 weeks of age. Euthanize the mice by CO2 asphyxiation and cervical dislocation.
- With a pair of thin scissors, cut the skin around the thigh and use tweezers to peel the skin off. Remove the extremity of the paw and then cut between the hip and thigh. Detach tibia from femur by cutting at the knee articulation and remove adherent tissue on tibias and femurs by using a scalpel.
- Remove the epiphyses with a sharp razor blade. While holding the femur with tweezers, use a 5 mL syringe filled with cacodylate buffer with a 21 G needle to flush the bone marrow into a 15 mL tube filled with 2 mL cacodylate buffer. To do so, insert the bevel of the needle into the bone marrow opening and slowly press the plunger until the marrow is expelled.
- Bone marrow fixation by rapid immersion into fixative.
- Immediately after flushing, use a plastic pipette to transfer the bone marrow cylinder into 1 mL of fresh glutaraldehyde fixative solution (previously prepared in 1.1) for 60 min at room temperature.
NOTE: To preserve the tissue, ensure that the entire process, from bone dissection to the fixation step, is completed in less than 10 min. For the fixation, ensure that the fixative solution is at room temperature to avoid heat shock.
- Immediately after flushing, use a plastic pipette to transfer the bone marrow cylinder into 1 mL of fresh glutaraldehyde fixative solution (previously prepared in 1.1) for 60 min at room temperature.
2. Embedding bone marrow in agarose
NOTE: Marrow tissue is not sufficiently cohesive to maintain its integrity during the different washing steps and material can be easily lost. To overcome this problem, the marrow is covered in a gel of agar before dehydration.
- Prepare the agarose solution as described in the Supplementary File.
- Wash the fixed marrow from section 1.3 in cacodylate buffer and transfer it carefully to a glass slide using a plastic pipette. Using a warm pipette, quickly apply a drop of 2% liquid agar to the bone marrow cylinder.
NOTE: The agar solidifies quickly while cooling. To ensure a homogenous covering of the bone marrow, the agar solution has to be kept warm until it is deposited onto the slide. - Quickly place the slide rapidly on ice until the agar solidifies (1-2 min).
- Under a microscope, use a sharp razor blade to cut and discard the extremities of the bone marrow cylinder because of possible tissue compression in these areas. Transfer the marrow blocks in 1.5 mL microcentrifuge tubes containing 1 mL of cacodylate buffer.
3. Embedding bone marrow in resin
CAUTION: Resin components are toxic; some are carcinogenic and must be handled with care under a chemical extraction hood. Use appropriate protective equipment such as gloves and protection glasses. Osmium tetroxide is highly volatile at room temperature and its vapors are very harmful to the eyes, nose, and throat. Before being discarded, 2% osmium tetroxide must be neutralized by adding twice its volume of vegetable oil.
- Prepare the epoxy resin as described in the Supplementary File.
- Resin embedding
NOTE: Keep the samples in the same microcentrifuge tubes during incubations in successive baths of osmium, uranyl acetate and ethanol. Aspirate the supernatants with a Pasteur pipette. The volume of solution used for each bath must equal at least 10x the volume of the sample.- Post-fix the blocks with 1% osmium in cacodylate buffer for 1 h at 4 °C, wash once in cacodylate buffer and then once in distilled water.
- Stain with 4% uranyl acetate in distilled water for 1 h, wash twice in distilled water.
- Dehydrate through a graded series of ethanol in distilled water: 4 times in 75% ethanol for 5 min, followed by 3 times in 95% ethanol for 20 min and then 3 times in 100% ethanol for 20 min. At this step, take one syringe of epoxy resin out from the freezer.
NOTE: The protocol can be paused in 100% ethanol for 1 h. - To obtain uniform infiltration and polymerization of epoxy resin inside the marrow, incubate first the blocks in 2 successive baths of propylene oxide for 15 min.
- Add a 1:1 mixture of 100% propylene oxide and epoxy resin and incubate for 1 h. Place the samples on a slow rotary shaker at room temperature.
- Add 100% epoxy resin leave the sample for overnight incubation under agitation.
- Add 100% epoxy resin for 2 h incubation, still under agitation.
- Under a microscope, place the marrow blocks into flat silicone molds. Orientate samples to permit subsequent transversal sectioning of the entire bone marrow. Fill the molds with epoxy resin and place them at 60 °C for 48 h.
NOTE: All solutions (except ethanol and propylene oxide) are filtered through 0.22 µm filter to avoid samples contamination. To ensure adequate polymerization of the resin, avoid bubbles while filling the molds.
4. Ultrathin sectioning (Figure 1B)
NOTE: Transmission EM requires thin tissue sections through which electrons can pass generating a projection image of the interior of cells, structure, and organization of inner organelles (granules, endoplasmic reticulum, Golgi) and the arrangement of intracellular cell membranes.
- Mount the sample block in an ultra-microtome support. Put it on the sample holder. Trim the samples at 45˚ in order to remove the excess of resin around the tissue with a rotating diamond or tungsten milling cutter.
- Mount the samples on the ultramicrotome with a diamond knife blade equipped with a water tank. Cut transverse sections of 500 nm and 100 nm thickness for histological and TEM analyses, respectively. Collect floating sections on the water-surface with a loop.
- Deposit the 500 nm thick section on a glass slide and 100 nm thick sections on 200 mesh thin-bar copper grids with a paper filter underneath. Prepare five grids for one condition: stain two grids first and keep the three remaining grids as a backup if necessary.
5. Toluidine blue staining for histology
NOTE: Staining sections for histology is important for three reasons: 1) to make sure that the tissue is actually cut and not the resin, 2) to check the quality of the inclusion, and 3) to rapidly evaluate the marrow sample. If this is not correct, cut deeper in the block.
- Dry the semi-thin sections slide on a heat plate (60 °C).
- Add filtered 1% toluidine blue/1 % sodium borate in distilled water on the slides and heat on a hot plate (60 °C) for 1-2 min. Wash the slides with distilled water and let it dry on the heat plate.
- Mount sections on coverslips with a drop of Poly(butyl methacrylate-co-methyl methacrylate) mounting medium and examine under a light microscope.
6. Heavy metal staining for TEM observation (Figure 1C)
NOTE: For the contrast, the upper side of the grids are inverted on 100 µL drops of each successive bath with a loop. Prior to use, each solution is 0.22 µm filtered. Remove the excess of liquid between each bath by gently contact the grid side on a filter paper.
- Stain with 4% uranyl acetate in distilled water for 5 min.
- Wash 3 times in distilled water for 5 min.
- Stain with lead citrate for 3 min.
- Wash 3 times in distilled water for 5 min.
- Deposit the grids by the lower side in contact with the filter paper to let them dry.
NOTE: Heavy metals react in the presence of carbon dioxide. To minimize the precipitates, avoid air displacement during the contrast, do not speak, keep the environment calm and turn off the air-conditioning.
7. TEM (Figure 1E)
NOTE: The sections are introduced in a TEM microscope and examined at 120 kV.
- First examine the sections at low magnification (< 500x) to appreciate the general aspect of the preparation (absence of hole in the resin, folds/compression in the sections, precipitates due to staining).
- Then examine the sections at higher magnification (~ 2000x allowing to distinguish the stage of maturation). Count manually the megakaryocytes from each stage of maturation over whole transversal sections (see Representative Results on how do visually identify each stage).
NOTE: Each square of the grids is defined as an area for examination (which equals 16000 µm2 for 200 mesh copper grids). - To assess the number of megakaryocytes, quantify only the squares that are fully covered with a section. To do so, use a model based on the screening of ranges. Observe a first range of squares from an extremity of the section to another, then another range in the same way, etc. Using this procedure, screen fully and systematically the whole marrow transversal section square by square.
- For each square, score the number of Stage I, II or III megakaryocytes.
NOTE: Higher magnifications are required to analyze the granules, the DMS organization, the size of cytoplasmic territories and the polylobulated nucleus.
Results
Bone marrow histology
Observation of the bone marrow toluidine blue histology under a light microscope is key to rapidly analyze the overall tissue architecture in terms of e.g., tissue compactness, microvessel continuity, and the size and shape of megakaryocytes (Figure 1D). It is performed before ultrathin sections to determine the need of cutting deeper in the bone marrow block. Due to their giant size and nuclear lobulation, the more mature megakaryocytes may be easily visualized with a 40x objective. This gives an excellent and rapid overview of the density of mature megakaryocytes in the tissue and their relative localization to the microvessels. Anomalies in megakaryocyte proliferation and maturation could already been detected in such semi-thin sections.
Bone marrow ultrastructure
On the basis of distinct ultrastructural characteristics, murine megakaryocytes are divided into 4 stages representing sequential stages in their maturation (Figure 2A). Stage I megakaryocytes are 10-15 µm in diameter with a large nucleus occupying most of the cell and containing abundant ribosomes and rough endoplasmic reticulum. The presence of the earliest detectable stage of the DMS, called pre-DMS, is also a key criterion for distinguishing stage I MKs in TEM analysis3. In the stage II of maturation, granule formation begins and the development of the DMS is initiated. Megakaryocytes increase in size, measuring 15-25 µm in diameter and develop nuclear lobulation. Mature stage III megakaryocytes are giant cells 25-50 µm in diameter. Their cytoplasm contains a well-developed DMS with clearly defined cytoplasmic territories and a peripheral zone devoid of organelles. At this stage, the nucleus is generally located eccentrically and appears very irregular with condensed chromatin located at the nuclear membrane. The last step is characterized by a naked nucleus, also called pyrenocyte, consisting of a large nucleus surrounded by a plasma membrane after the bulk of cytoplasm has been eliminated. In wild type C57BL/6 mice, the bone marrow comprises about 8% stage I, 20% stage II, 71% stage III megakaryocytes and < 1% pyrenocytes. The average number of megakaryocytes is between 1.7 and 2.2 cells per square. This arbitrary classification allows to conveniently monitor a continuous process of cell differentiation and detect its possible anomalies.
Beside these classical stages of maturation, observation of fixed megakaryocytes in the bone marrow allows to analyze the series of events occurring as megakaryocytes interact with the sinusoidal wall (Figure 2B). Megakaryocytes in contact with the endothelial cells are frequently observed in thin sections. On occasion one observes megakaryocytes forming short invasive protrusions penetrating the endothelium or extending large projection of its cytoplasm into the sinusoidal lumen7,8. Remarkably, these intravascular cytoplasmic processes display variable sizes, lengths, and diameters, illustrating the progressive platelet remodeling in the circulation. Platelets already present in the general circulation, having a discoid shape maintained by circumferential microtubule coils, are also visible in the lumen of the sinusoids. This typical morphology of the platelets is indicative of the correct fixation of the specimen.
Transmission electron microscopy has the level of resolution required to visualize ultrastructural details, such as nuclear lobulation, spatial organization of the DMS and granules in terms of size, shape, and distribution. Figure 2C is an example of the perinuclear region in a stage III megakaryocyte showing the presence of α granules, Golgi cisternae, mitochondria, and endoplasmic reticulum. Also noteworthy in Figure 2C is a multivesicular body, which represents an intermediate stage in the formation of alpha and dense granules, containing multiples exosomes measuring less than 200 Å in diameter9,10. Finally, transmission electron microscopy enables to visualize neutrophils and other hematopoietic cells present inside the megakaryocytes, (Figure 2D) following an uncommon process called emperipolesis whereby a cell penetrates another living cell11. This process, which concerns 4% of megakaryocytes in normal physiological condition, can be significantly increased in certain pathological conditions12.
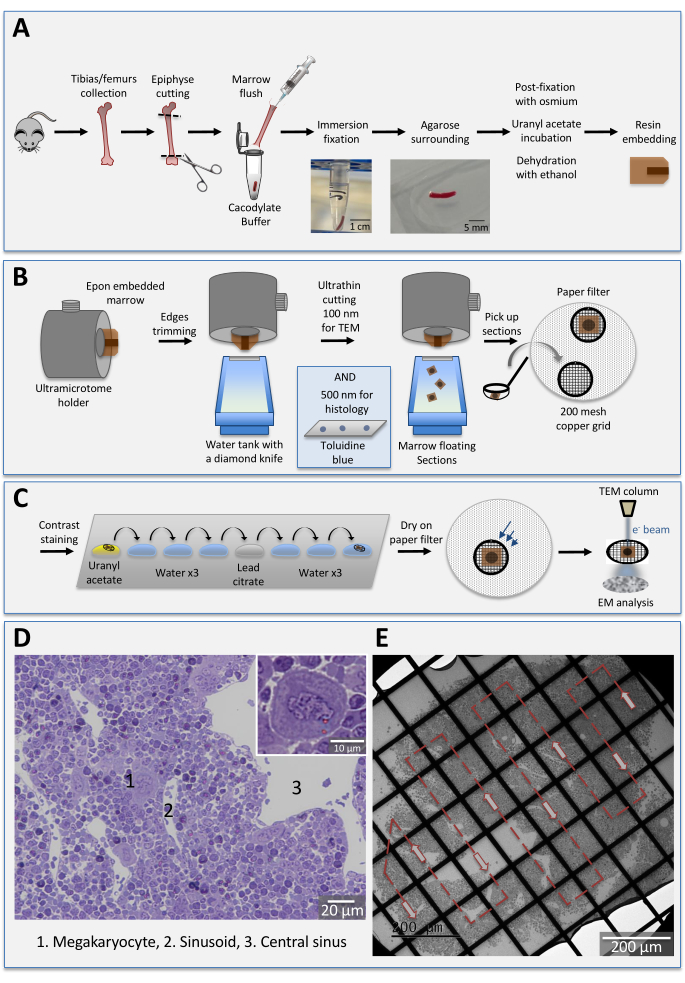
Figure 1: Schematic illustration of the experimental setup. (A) Bone marrow embedding procedure. The bone marrow is flushed and fixed by rapid immersion in glutaraldehyde solution. The photograph illustrates the typical appearance of the bone marrow cylinder following flushing. After 1 h fixation at room temperature, the marrow is surrounded in agarose, post-fixed in osmium tetroxide and incubated in uranyl acetate. The tissues are then rinsed in buffer, dehydrated in a series of graded ethanol, incubated in propylene oxide and infiltrated with epoxy resin. (B) Bone marrows blocks sectioning. The embedded bone marrow is mounted on an ultramicrotome holder, trimmed at 45° and cut either in semi-thin (500 nm) or thin (100 nm) sections. For ultrastructural studies, the floating sections are picked up with a loop and deposited on grids with a paper filter underneath. (C) Contrast staining for TEM observations. Grids are inverted on uranyl acetate drops, washed on distilled water drops and incubated on lead citrate before another run of washings. After drying (upper side with the sections), the samples are ready to be examined under the TEM. (D) Histology of a mouse femoral marrow section stained with toluidine blue. The giant cells correspond to mature megakaryocytes (1), some being in contact with sinusoids (2). The sinusoids converge on a large central sinus vein (3). Bar: 20 µm. Inset: Normal appearance of a mature megakaryocyte at 40x magnification. (E) Representative TEM image of a bone marrow section at low magnification.Cells are tightly packed with little extracellular space. Each grid square of the section is observed from an extremity to another by following the schematic arrowed path (red arrows). Bar: 200 µm. Please click here to view a larger version of this figure.
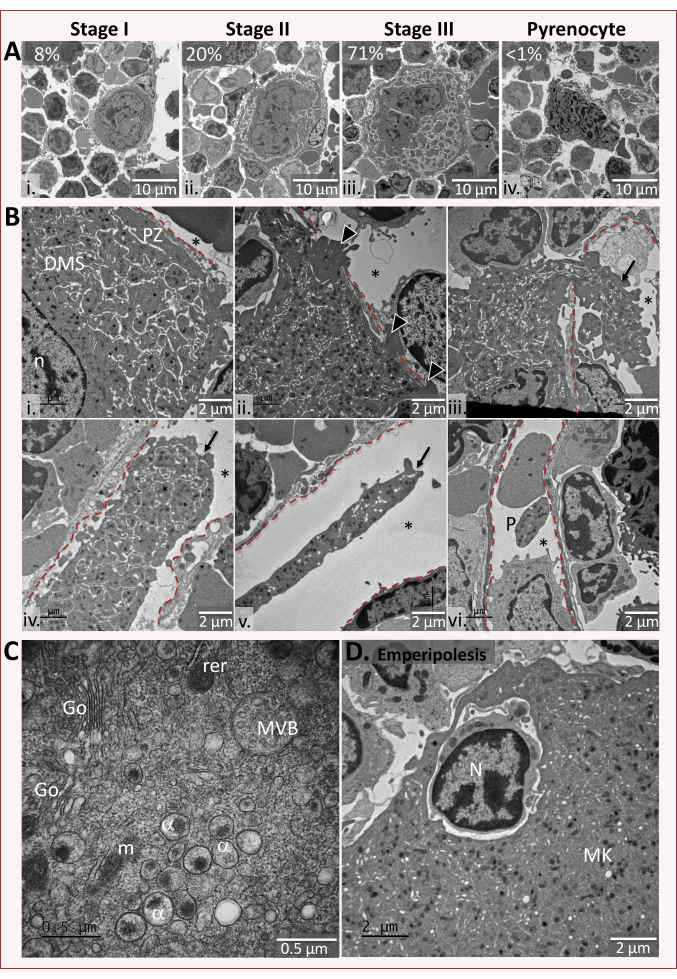
Figure 2: Representative in situ images of megakaryocytes ultrastructure. (A) Characteristic maturation stages of wild-type megakaryocytes. Megakaryocytes are classified in four maturation stages: Stage I, a cell 10-15 µm in diameter with a large nucleus; stage II, a cell 15-30 µm in diameter containing the DMS under development; stage III, a 30-50 µm cell containing a well-developed demarcation membrane system (DMS) defining cytoplasmic territories and having an organelle-free peripheral zone. A pyrenocyte corresponds to the naked polylobulated nucleus remaining in the bone marrow following full cytoplasmic extension. Bars: 10 µm (B) Megakaryocyte-endothelial cell interactions and intravascular cytoplasmic processes.(i) The peripheral zone (PZ) of a megakaryocyte is closely apposed to the abluminal surface of the sinusoidal endothelium. (ii) A megakaryocyte forming short invasive protrusions that penetrate deeply into the endothelial layer (arrowheads). (iii-v) The arrows indicate cytoplasmic processes of megakaryocytes with varying diameters, some of which are very large and have a peripheral zone that may represent fragments that have just entered the bloodstream. (vi) A typical discoid platelet (P) observed in the sinus lumen. In each micrograph, the red line indicates the luminal side of the endothelial barrier and the star indicates the sinusoid lumen. Bars: 2 µm. (C) Higher magnifications of the perinuclear region of a mature megakaryocyte. α, alpha granule; rer, rough endoplasmic reticulum; G, golgi; MVB, multivesicular body; m, mitochondria. Bar: 0.5 µm. (D) Example of a megakaryocyte showing emperipolesis. The engulfed neutrophil appears morphologically unaltered by the interaction with megakaryocytes. Bar: 2 µm. Please click here to view a larger version of this figure.
Supplementary File: Preparation of the reagents. Please click here to download this File.
Discussion
Direct examination of megakaryocytes in their native environment is essential to understand megakaryopoiesis and platelet formation. In this manuscript, we provide a transmission electron microscopy method combining bone marrow flushing and fixation by immersion, allowing to dissect in situ the morphology characteristics of the entire process of megakaryocyte morphogenesis taking place in the bone marrow.
The flushing of the bone marrow is a critical step of this method, as the success of a high-quality flushing depends on the practice and training of the operator. Although delicate, flushing the bone marrow is the best way to avoid removal of the mineralized bone, which usually requires a 2-week EDTA treatment for complete decalcification associated with significant artifacts on the megakaryocyte morphology. Additionally, a major advantage of collecting whole unfixed bone marrows from tibia and femurs is the ability to combine several imaging approaches to the same mouse. In practice, only a single bone marrow is required for the ultrastructural study, the other three specimens being available for complementary analyses. The second bone marrow can then be used for the preparation of fresh bone marrow explants, to study in real-time the dynamics of proplatelet formation of native megakaryocytes6. The third sample is usually designed for immunostaining studies on thick sections, providing 3D imaging and distribution of megakaryocytes within their natural environment. The last sample can be frozen and stored for further studies by immunogold electron microscopy, where the subcellular localization of proteins is investigated at high resolution4. These combined imaging methods, together with the availability of the targeted deletion/mutation of genes in a mouse, provide an important means of delineating in situ the biological role of a given protein in thrombopoiesis. However, it should be pointed out here that one limitation of this method is the withdrawal of the epiphyses needed to flush the marrow. Epiphyses are known to be important areas for hematopoiesis, and their removal therefore hinders any possibility of analyzing hematopoietic stem cells and the initial phases of engagement13. Another limitation is that progenitors of megakaryocytes before the immature stage I cannot be identified because these cells do not have specific ultrastructural features. To overcome this limitation, an EM immunogold approach could be used.
The second important step of the method is the bone marrow fixation by immersion. When performed under the conditions described here, i.e., fixation immediately after flushing out the compact bone marrow cylinder, it has the following advantages: (i) it is quick and easy to perform, (ii) it preserves an ultrastructure close to that observed following fixation by perfusion6, and (iii) it maintains free megakaryocyte processes and platelets in the sinusoid bloodstream which are otherwise flushed out/lost after perfusion. With this technique it is possible to investigate the entry of megakaryocytes into the sinusoidal circulation and to characterize all the intermediate forms of cytoplasmic processes from which platelets arise8. In line with this, it has recently been reported that the large protrusions intravasating from megakaryocytes in vivo are structurally distinct from the thin extensions formed by megakaryocytes in vitro, with notably a different arrangement of the microtubules7. Similarly, we have recently shown that the mechanism governing platelets formation in vivo differs from that identified in vitro14.
Important ultrastructural differences are increasingly recognized between in vitro cultured and in vivo generated native megakaryocytes, underscoring the need for the bone marrow microenvironment for a full megakaryocyte differentiation/maturation. Following combination of bone marrow flushing and fixation by immersion described in this article, conventional transmission electron microscopy still remains an invaluable tool to study megakaryocyte biology and platelet formation, under physiological and pathological conditions.
Disclosures
The authors have no conflicts of interests to declare.
Acknowledgements
The authors wish to thank Fabienne Proamer, Jean-Yves Rinckel, David Hoffmann, Monique Freund for technical assistance. This work was supported by ARMESA (Association de Recherche et Développement en Médecine et Santé Publique), the European Union through the European Regional Development Fund (ERDF) and by Grant ANR-17-CE14-0001-01 to H.d.S.
Materials
| Name | Company | Catalog Number | Comments |
| 2,4,6-Tri(dimethylaminomethyl)phenol (DMP-30) | Ladd Research Industries, USA | 21310 | |
| Agarose type LM-3 Low Melting Point Agar | Electron Microscopy Sciences, USA | 1670-B | |
| CaCl2 Calcium chloride hexahydrate | Merck, Germany | 2083 | |
| Copper grids 200 mesh thin-bar | Oxford Instrument, Agar Scientifics, England | T200-CU | |
| Dimethylarsinic acid sodium salt trihydrate | Merck, Germany | 8.20670.0250 | |
| Dodecenyl Succinic Anhydride (DDSA) | Ladd Research Industries, USA | 21340 | |
| Double Edge Stainless Razor blade | Electron Microscopy Sciences-EMS, USA | EM-72000 | |
| Ethanol absolut | VWR International, France | 20821296 | |
| Filter paper, 90 mm diameter | Whatman, England | 512-0326 | |
| Flat embedding silicone mould | Oxford Instrument, Agar Scientific, England | G3533 | |
| Glutaraldehyde 25% | Electron Microscopy Sciences-EMS, USA | 16210 | |
| Heat plate Leica EMMP | Leica Microsystems GmbH, Austria | 705402 | |
| Histo Diamond Knife 45° | Diatome, Switzerland | 1044797 | |
| JEOL 2100 Plus TEM microscope | JEOL, Japan | EM-21001BU | |
| Lead citrate - Ultrostain 2 | Leica Microsystems GmbH, Austria | 70 55 30 22 | |
| LX-112 resin | Ladd Research Industries, USA | 21310 | |
| MgCl2 Magnesium chloride hexahydrate | Sigma, France | M2393-100g | |
| Mounting medium - Poly(butyl methacrylate-co-methyl methacrylate) | Electron Microscopy Sciences-EMS, USA | 15320 | |
| Nadic Methyl Anhydride (NMA) | Ladd Research Industries, USA | 21350 | |
| Osmium tetroxide 2% | Merck, Germany | 19172 | |
| Propylene oxide (1.2-epoxypropane) | Sigma, France | 82320-250ML | |
| Saline injectable solution 0.9% NaCl | C.D.M Lavoisier, France | MA 575 420 6 | |
| Scalpel Surgical steel blade | Swann-Morton, England | .0508 | |
| Sodium tetraborate - Borax | Sigma, France | B-9876 | |
| Sucrose | Merck, Germany | 84100-1KG | |
| Syringe filter 0.2µm | Pall Corporation, USA | 514-4126 | |
| Toluidine blue | Ladd Research Industries, USA | N10-70975 | |
| Trimmer EM TRIM2 | Leica Microsystems GmbH, Austria | 702801 | |
| Ultramicrotome Ultracut UCT | Leica Microsystems GmbH, Austria | 656201 | |
| Uranyl acetate | Ladd Research Industries, USA | 23620 |
References
- Machlus, K. R., Italiano, J. E. The incredible journey: From megakaryocyte development to platelet formation. The Journal of Cell Biology. 201 (6), 785-796 (2013).
- Zucker-Franklin, D., Termin, C. S., Cooper, M. C. Structural changes in the megakaryocytes of patients infected with the human immune deficiency virus (HIV-1). American Journal of Pathology. 134 (6), 9 (1989).
- Eckly, A., et al. Biogenesis of the demarcation membrane system (DMS) in megakaryocytes. Blood. 123 (6), 921-930 (2014).
- Scandola, C., et al. Use of electron microscopy to study megakaryocytes. Platelets. , 1-10 (2020).
- Behnke, O., Forer, A. From megakaryocytes to platelets: platelet morphogenesis takes place in the bloodstream. European Journal of Haematology. 60, 3-23 (2009).
- Eckly, A., et al. Characterization of megakaryocyte development in the native bone marrow environment. Platelets and Megakaryocytes. 788, 175-192 (2012).
- Brown, E., Carlin, L. M., Nerlov, C., Lo Celso, C., Poole, A. W. Multiple membrane extrusion sites drive megakaryocyte migration into bone marrow blood vessels. Life Science Alliance. 1 (2), 201800061 (2018).
- Eckly, A., et al. Megakaryocytes use in vivo podosome-like structures working collectively to penetrate the endothelial barrier of bone marrow sinusoids. Journal of Thrombosis and Haemostasis. , 15024 (2020).
- Cramer, E. M., et al. Ultrastructure of platelet formation by human megakaryocytes cultured with the Mpl ligand. Blood. 89 (7), 2336-2346 (1997).
- Heijnen, H. F. G., Debili, N., Vainchencker, W., Breton-Gorius, J., Geuze, H. J. Multivesicular Bodies Are an Intermediate Stage in the Formation of Platelet α-Granules. Blood. 7 (7), 2313-2325 (1998).
- Gupta, N., Jadhav, K., Shah, V. Emperipolesis, entosis and cell cannibalism: Demystifying the cloud. Journal of Oral and Maxillofacial Pathology. 21 (1), 92 (2017).
- Centurione, L., et al. Increased and pathologic emperipolesis of neutrophils within megakaryocytes associated with marrow fibrosis in GATA-1low mice. Blood. 104 (12), 3573-3580 (2004).
- Ellis, S. L., et al. The relationship between bone, hemopoietic stem cells, and vasculature. Blood. 118 (6), 1516-1524 (2011).
- Bornert, A., et al. Cytoskeletal-based mechanisms differently regulate in vivo and in vitro proplatelet formation. Haematologica. , (2020).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved