Method Article
Segmenting Growth of Endothelial Cells in 6-Well Plates on an Orbital Shaker for Mechanobiological Studies
* These authors contributed equally
In This Article
Summary
This protocol describes a coating method to restrict endothelial cell growth to a specific region of a 6-well plate for shear stress application using the orbital shaker model.
Abstract
Shear stress imposed on the arterial wall by the flow of blood affects endothelial cell morphology and function. Low magnitude, oscillatory and multidirectional shear stresses have all been postulated to stimulate a pro-atherosclerotic phenotype in endothelial cells, whereas high magnitude and unidirectional or uniaxial shear are thought to promote endothelial homeostasis. These hypotheses require further investigation, but traditional in vitro techniques have limitations, and are particularly poor at imposing multidirectional shear stresses on cells.
One method that is gaining increasing use is to culture endothelial cells in standard multi-well plates on the platform of an orbital shaker; in this simple, low-cost, high-throughput and chronic method, the swirling medium produces different patterns and magnitudes of shear, including multidirectional shear, in different parts of the well. However, it has a significant limitation: cells in one region, exposed to one type of flow, may release mediators into the medium that affect cells in other parts of the well, exposed to different flows, hence distorting the apparent relation between flow and phenotype.
Here we present an easy and affordable modification of the method that allows cells to be exposed only to specific shear stress characteristics. Cell seeding is restricted to a defined region of the well by coating the region of interest with fibronectin, followed by passivation using passivating solution. Subsequently, the plates can be swirled on the shaker, resulting in exposure of cells to well-defined shear profiles such as low magnitude multidirectional shear or high magnitude uniaxial shear, depending on their location. As before, the use of standard cell-culture plasticware allows straightforward further analysis of the cells. The modification has already allowed the demonstration of soluble mediators, released from endothelium under defined shear stress characteristics, that affect cells located elsewhere in the well.
Introduction
Responses of vascular cells to their mechanical environment are important in the normal function of blood vessels and in the development of disease1. The mechanobiology of the endothelial cells (ECs) that line the interior surface of all blood vessels has been a particular focus of mechanobiological research because ECs directly experience the shear stress generated by blood flow over them. Various phenotypic changes such as inflammatory responses, altered stiffness and morphology, the release of vasoactive substances, and the localization and expression of junctional proteins depend on EC exposure to shear stress2,3,4. Shear-dependent endothelial properties may also account for the patchy development of diseases such as atherosclerosis5,6,7.
It is useful to study the effect of shear on ECs in culture, where stresses can be controlled, and ECs can be isolated from other cell types. Commonly used in vitro devices for applying shear stress to ECs include the parallel-plate flow chamber and the cone-and-plate viscometer, but only uniaxial steady, oscillatory, and pulsatile flow can be applied8,9. Although modified flow chambers with tapered or branching geometries and microfluidic chips that mimic a stenotic geometry have been developed, their low-throughput and the relatively short culture duration that is possible pose a challenge10, 11.
The orbital shaker (or swirling well) method for the study of endothelial mechanotransduction, in which cells are grown in standard cell culture plasticware placed on the platform of an orbital shaker, is gaining increasing attention because it is capable of chronically imposing complex, spatially varying shear stress patterns on ECs with high throughput (see review by Warboys et al.12). Computational Fluid Dynamics (CFD) simulations have been employed to characterize the spatial and temporal variation of shear stress in a swirling well. The swirling motion of culture medium caused by the orbital motion of the shaker platform on which the plate is placed leads to Low Magnitude Multidirectional Flow (LMMF, or putatively pro-atherogenic flow) at the center and High Magnitude Uniaxial Flow (HMUF, or putatively atheroprotective flow) at the edge of the wells of a 6-well plate. For example, time-averaged wall shear stress (TAWSS) is approximately 0.3 Pa at the center and 0.7 Pa at the edge of a 6-well plate swirled at 150 rpm with a 5 mm orbital radius13. The method requires only commercially available plasticware and the orbital shaker itself.
There is, however, a drawback to the method (and to other methods of imposing flows in vitro): ECs release soluble mediators and microparticles in a shear-dependent manner14,15,16 and this secretome may affect ECs in regions of the well other than the one in which they were released, due to the mixing in the swirling medium. This may mask the actual effects of shear stress on EC phenotype. For example, Ghim et al. have speculated that this accounts for the apparently identical influence of different shear profiles on transcellular transport of large particles17.
Here we describe a method for promoting human umbilical vein endothelial cell (HUVEC) adhesion in specific regions of a 6-well plate using fibronectin coating while using Pluronic F-127 to passivate the surface and prevent growth elsewhere. The method resolves the limitation described above because, by segmenting cell growth, ECs experience only one kind of shear profile, and are not influenced by secretomes from ECs exposed to other profiles elsewhere in the well.
Protocol
1. Fabrication of devices and preparation of reagents
- Fabrication of stainless-steel module
- Fabricate the stainless-steel module from a grade 316 stainless-steel using a CNC milling machine according to the engineering drawing provided (Figure 1).
- 3D printing of a polydimethylsiloxane (PDMS) mold
- Prepare a 3D computer aided design (CAD) model of the PDMS mold using SolidWorks according to the engineering drawing provided (Figure 2).
- Export the CAD model to an STL file and import the STL file to Cura 2.6.2.
- Slice the model into layers with a print speed of 50 mm/s and infill density of 60%.
- Export the file as a G-code and upload it to an Ultimaker2 3D printer for printing. Use polylactic acid (PLA) as the printing material.
- Casting of PDMS ring
- Mix the PDMS base and curing agent (both from a silicone elastomer kit) with the ratio of 90.9% base and 9.1% curing agent.
- Pour approximately 2.6 mL of the well-mixed solution into the 3D printed mold.
- Remove bubbles in a vacuum degassing chamber.
- Cure it for 1 h in an 80 °C furnace.
- Allow the PDMS ring to cool to room temperature, then remove the cured PDMS ring carefully from the mold. The engineering drawing of PDMS ring is shown in Figure 3.
- Preparation of 1% Pluronic F-127
- Weigh out 5 g of Pluronic F-127, pour it into a glass bottle, then add 100 mL of sterile water into the glass bottle. This gives a 5% Pluronic F-127 solution.
- Ensure that all Pluronic F-127 powder is submerged in the water, close the cap and autoclave it using a liquid sterilisation cycle program.
- After autoclave, let the solution cool to room temperature before use.
- Add 10 mL of 5% Pluronic F-127 solution to 40 mL of autoclaved sterile water to make 1% Pluronic F-127 solution. Perform the dilution in a biosafety cabinet (BSC) hood.
- Store both 1% and 5% Pluronic F-127 at room temperature.
- Preparation of 4% paraformaldehyde (PFA)
- Add 800 mL of phosphate-buffered saline (PBS) to a glass beaker and heat it to 60 °C whilst stirring (keep stirring from step 1.5.1 to 1.5.5).
- Weigh 40 g of PFA powder and add it to the warm PBS solution.
CAUTION: PFA is hazardous, perform steps 1.5.2 to 1.5.6 in a fume hood. - Add 1 M sodium hydroxide (NaOH) slowly dropwise into the PFA solution until the solutions turns clear.
- Adjust the pH of the PFA solution to approximately 7.4 with 1 M of hydrochloric acid (HCl).
- Top up the solution to 1 L with 1X PBS. This gives 1 L of 4% PFA.
- Filter the PFA solution with 0.2 µm filters to remove any particulates, aliquot and freeze in a -20 °C freezer.
- Preparation of 0.1% Triton-X
- Add 50 µL of pure Triton-X into 50 mL of PBS to make 0.1% Triton-X solution.
- Preparation of 1% bovine serum albumin (BSA)
- Weigh 0.5 g of BSA, pour it into a 50 mL centrifuge tube, and then add 50 mL of PBS into the tube.
- Let it roll for 1 h at room temperature on a roller to dissolve.
- Store 1% BSA at 4 °C for up to two weeks.
2. Coating of a 6-well plate
- Autoclave stainless-steel module, PDMS ring, and tweezers before use. Perform all the subsequent procedures in a BSC hood and observe aseptic techniques to ensure sterility.
- Place the PDMS ring in a 6-well using tweezers. Use the external rim of the PDMS ring to align the PDMS ring concentrically with the well.
NOTE: Only non-tissue culture treated well plates should be used. - Place the stainless-steel module on top of the PDMS ring using tweezers.
- Insert the tips of the internal retaining ring pliers into the grip holes of the retaining ring, squeeze the holder to reduce the diameter of the retaining ring. Fit it into the 6-well, press it firmly on the stainless-steel module and release the pliers to secure the PDMS ring in the well.
- Add 1 mL of 5 µg/mL fibronectin into the center or the edge of the well (depending on the region of interest) through the opening of PDMS ring and stainless-steel module.
- Swirl the plate to ensure that the fibronectin solution covers all the region of interest.
- Incubate for 30 min at 37 °C in a humidified incubator under 95% air/5% CO2.
- Remove the fibronectin solution from the well and wash twice with PBS. Completely remove the PBS from the well.
- Remove the retaining ring, stainless-steel module, and PDMS ring from the well.
- Add 1.5 mL of 1% Pluronic F-127 into the center or the edge of the well (uncoated surface) and incubate for 1 h at room temperature to passivate the uncoated surface.
- Remove the Pluronic F-127 solution from the well and wash three times with PBS.
- Use the coated well immediately or store it at 4 °C for up to two weeks with a layer of PBS in the coated well.
3. Seeding of HUVECs
- Use HUVECs below passage 5 in the experiment.
- Remove all culture medium and wash cells once with PBS.
- Add 3 mL of 0.05% trypsin and incubate for 3 min at 37 °C in a humidified incubator under 95% air/5% CO2. Gently tap the flask to dislodge the cells.
NOTE: This protocol has been tested using HUVECs and the concentration of trypsin and its incubation time may be different for other type of ECs. - Transfer the solution to a 15 mL centrifuge tube and neutralize the trypsin using 6 mL of culture medium (e.g., Lonza EGM-2) prewarmed to 37 °C.
- Centrifuge at 200 x g for 5 min. Remove the neutralized trypsin solution and resuspend cells with 1 mL of prewarmed culture medium.
- Count the cells using a haemocytometer and seed 180k cells into a coated 6-well plate in 1.5 mL of prewarmed culture medium.
- Shake the well plate laterally to ensure that the cells distribute evenly in the well.
- Leave it in a 37 °C humidified incubator under 95% air/5% CO2 overnight.
- Remove the unattached cells and culture medium and replace with 2mL of prewarmed culture medium.
NOTE: many unattached cells floating in the medium are expected.
4. Shear stress application using an orbital shaker
- HUVECs should reach confluence after 3 days of growth. Replace the medium with 1.9 mL of prewarmed culture medium (to achieve a height of 2 mm).
- Place the plate on the platform of an orbital shaker in a humidified incubator under 95% air/5% CO2 and swirl it at 150 rpm for 3 days.
NOTE: Wipe down the exterior surface of the orbital shaker using 70% ethanol before placing it into the incubator. - (Optional) After 2 days of shear, cytokines can be added to the culture medium to investigate the interplay between cytokines and shear stress. After treatment, shear the cells for another day. In this study, TNF-α was used to activate the cells.
- Perform analyses after 3 days of shear stress application.
5. Staining and imaging of cells
- After 3 days of shear, remove the plate from incubator and wash cells twice with PBS.
- Fix the cells by adding 1.5 mL of 4% PFA into the well and incubate for 10 min at room temperature.
- Remove the 4% PFA from the well and wash twice with PBS.
- Permeabilize the cells by adding 0.1% Triton-X into the well and incubate for 5 min at room temperature.
- Remove the 0.1% Triton-X solution from the well and add 1.5 mL of 1% BSA into the well for blocking. Incubate the cells with 1% BSA for 1 h at room temperature.
- Dilute rabbit anti-human ZO-1 antibody at a 1:200 dilution in 1% BSA. Add 1.5 mL of diluted antibody into the well and incubate it with the cells overnight at 4 °C.
- After overnight incubation, remove the diluted antibody and wash cells three times with PBS.
- Dilute Alexa Fluor 488-labelled goat anti-rabbit IgG secondary antibody at a 1:300 dilution in PBS. Add 1.5 mL of diluted secondary antibody into the well and incubate it with the cells for 1 h at room temperature.
- Remove the diluted secondary antibody and wash cells twice with PBS.
- Dilute DRAQ5 at a dilution of 1:1000 in PBS. Add 1.5 mL of diluted DRAQ5 into the well and incubate it with the cells for 15 min at room temperature to stain the cell nuclei.
- Remove the diluted DRAQ5 and wash three times with PBS.
- Perform a tile scan from the edge to the center of the well with a confocal microscope.
6. Quantification of shape index and cells number
- Post process the images using MATLAB R2016a.
- Read the LIF file from confocal microscope into MATLAB and convert the merged tile scan to a binary image, then threshold the image by area and intensity to distinguish nuclei from background.
- Subdivide the binary tile scan into 1 mm radial segments.
- Fit an ellipse to each individual nucleus.
- Count the number of ellipses within each radial segment to give a cell number.
- Define the shape index = as SI = 4π x Area / Perimeter2. Calculate the shape index for each ellipse18.
Results
Adhesion of HUVECs to regions of the well plate not coated with fibronectin was abrogated by Pluronic F-127 passivation; growth was confined to the region coated with fibronectin even after 72 h of culture, with and without shear stress application (Figure 4A, Figure 4C). Without the Pluronic F-127 passivation, HUVECs attached to the surface without fibronectin and had proliferated further by 72 h of culture (Figure 4B, Figure 4D).
Alignment and elongation of HUVECs are evident at the edge of a swirling well, which has HMUF, while the cells at the center of the well, which has LMMF, exhibited a cobblestone morphology and no alignment (Figure 5A, Figure 5B). Elongation of HUVECs was quantified as shape index: 4π x Area/Perimeter2. A shape index of 1 indicates a circle, whereas a value of 0 indicates a line. Shape index decreased with radial distance from the center, and there was no significant difference between segmented and full wells. TNF-α treatment increased elongation of HUVECs compared to untreated controls (Figure 5C). HMUF also increased the number of HUVECs per mm2 compared to LMMF under both conditions. The number of HUVECs increased gradually with distance along the radius. No significant difference was observed in the number of HUVECs grown in segmented and full wells (Figure 6).
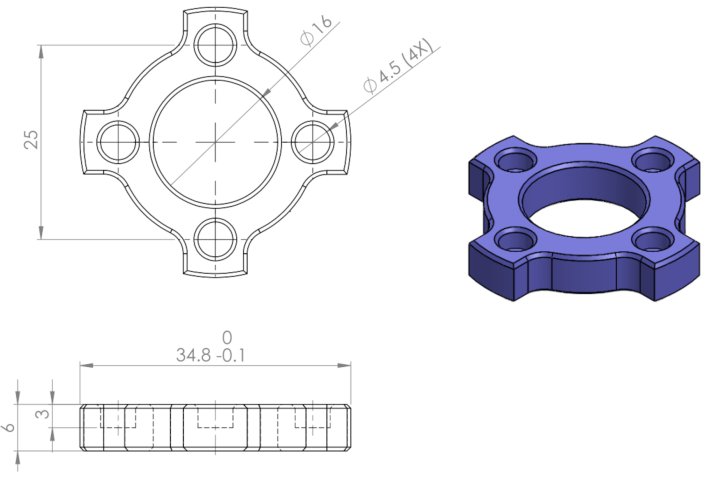
Figure 1 Engineering drawing of stainless-steel module. Please click here to view a larger version of this figure.
Dimensions are in mm.

Figure 2 Engineering drawing of PDMS mold. Please click here to view a larger version of this figure.
Dimensions are in mm.

Figure 3 Engineering drawing of PDMS ring used to segment the wells. Please click here to view a larger version of this figure.
Dimensions are in mm. From Ghim et al.13.

Figure 4 Microscope images showing that Pluronic F-127 prevented human umbilical vein endothelial cells (HUVECs) adhesion to the region without fibronectin coating. Please click here to view a larger version of this figure.
No HUVECs were attached to the part of the well surface that had not been pre-treated with fibronectin prior to passivation with Pluronic F-127, after 24 h (A) and 72 h (C) of growth. Without Pluronic F-127 passivation, HUVECs were attached to the surface without fibronectin 24 h after seeding (B) and had proliferated further by 72 h (D). (Scale bar = 500 µm). From Ghim et al.13.

Figure 5 The morphology of sheared HUVECs in a segmented or full well. Please click here to view a larger version of this figure.
Nuclear (red) stain shows the morphology of sheared HUVECs (A) in the center and (B) at the edge of a full well (scale bar = 100 µm). A and B also show cell outlines, delineated by immunostaining of ZO-1 (green). Note the alignment and elongation of cells at the edge but not at the center (C) No significant difference in nuclear shape index, indicating roundness, between HUVECs grown in full wells and segmented wells was seen for untreated or TNF-α treated HUVEC. Cells were more elongated near the edge of the well. A tendency for greater elongation in TNF-α-treated HUVECs was not consistently significant across locations. (Two-way ANOVA and Bonferroni's post hoc test; n = 3). This figure has been modified from Ghim et al.13

Figure 6 Number of HUVECs per mm2 increased with radial distance in a swirling well plate. Please click here to view a larger version of this figure.
No significant difference was observed between full and segmented wells in the density of (A) untreated and (B) TNF-α-treated HUVECs at different radial locations. In both cases, there were more cells per unit area at the edge than the center of the well. (Two-way ANOVA and Bonferroni's post hoc test; n = 3). This figure has been modified from Ghim et al13.
Discussion
The swirling-well method is capable of generating complex flow profiles in a single well - Low Magnitude Multidirectional Flow (LMMF) in the center and High Magnitude Uniaxial Flow (HMUF) at the edge of the well. However, shear stress-mediated secretions of soluble mediator will be mixed in the swirling medium and affect cells in the whole well, potentially masking the true effect of a particular shear stress profile on the cells.
The coating method demonstrated here overcomes this issue by restricting the growth of cells to a specific region of the well. Cells typically attach to hydrophilic surfaces rather than hydrophobic ones. For this reason, polystyrene culture ware is pre-treated with plasma oxidation. Alternatively, hydrophobic surfaces can be coated with extracellular matrix proteins such as fibronectin, as demonstrated in this protocol; non-fibronectin coated regions were passivated with Pluronic F-127 to prevent any residual adhesion to the hydrophobic surface.
This protocol is dependent on the accuracy of the printed mold. Depending on the 3D printer, there may be variation in the exact dimensions of the mold. This will affect the final PDMS construct, which will in turn result in the cells adhering in an incorrect location within the well. The cells would therefore experience a shear stress profile other than the one modelled by CFD. Another drawback to using a 3D printer is that the mold may not be flat, due to warping during the printing. This will result in the final PDMS construct allowing Pluronic F-127 to leak underneath, preventing cells from adhering in the desired locations. Therefore it is crucial to check for leaks and measure the dimension of the PDMS construct before use.
This method is simple yet effective in allowing the application of a specific type of shear stress (HMUF or LMMF) to cells. It is also convenient to set up as most of the consumables, reagents, and equipment are commercially available. Using this method not only allows the examination or harvesting of cells exposed to well-defined flows but allows the collection of medium conditioned by those cells. The method provides a new avenue investigating endothelial mechanobiology.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors gratefully acknowledge a British Heart Foundation project grant (to PDW), a National Medical Research Council Singapore TAAP and DYNAMO Grant (to XW, NMRC/OFLCG/004/2018, NMRC/OFLCG/001/2017), an A*STAR Graduate Scholarship (to KTP), and a British Heart Foundation Center of Research Excellence studentship (to MA).
Materials
| Name | Company | Catalog Number | Comments |
| Cell and Media | |||
| Endothelial Growth Medium (EGM-2) | Lonza | cc-3162 | |
| Human Umbilical Vein Endothelial Cells | NA | NA | Isolated from cords obtained from donors with uncomplicated labour at the Hammersmith Hospital |
| Reagents and Materials | |||
| Alexa Fuor 488-labelled goat anti-rabbit IgG | Thermofisher Scientific | A11008 | |
| Bovine Serum Albumin | Sigma-Aldrich | A9418-50G | |
| Falcon 6 Well Clear Flat Bottom Not Treated | Scientific Laboratory Supplies Ltd | 351146 | |
| Fibronectin from Bovine Plasma | Sigma-Aldrich | F1141-5MG | |
| Paraformaldehyde | Sigma-Aldrich | 158127-500G | |
| Phosphate-Buffered Saline | Sigma-Aldrich | D8537-6X500ML | |
| Pluronic F-127 | Sigma-Aldrich | P2443 | |
| Recombinant Human TNF-a | Peprotech | 300-01A | |
| RS PRO 2.85 mm Black PLA 3D Printer Filament, 1 kg | RS | 832-0264 | |
| Stainless Steel 316 | Metal Supermarket | NA | |
| Sylgard184 Silicone Elastomer kit | Farnell | 101697 | |
| Triton X-100 | Sigma-Aldrich | X100-100ML | |
| Trypsin-EDTA solution | Sigma-Aldrich | T4049-100ML | |
| Zonula Occludens-1 (ZO-1) antibody | Cell Signaling Technology | 13663 | |
| DRAQ5 (5mM) | Bio Status | DR50200 | |
| Equipments | |||
| Grant Orbital Shaker PSU-10i | Scientific Laboratory Supplies Ltd | SHA7930 | |
| Leica TCS SP5 Confocal Microscope | Leica | NA | |
| Retaining Ring Pliers | Misumi | RTWP32-58 | |
| Retaining Rings/Internal/C-Type | Misumi | RTWS35 | |
| Ultimaker 2+3-D printer | Ultimaker | NA | |
| Softwares | |||
| Cura 2.6.2 | Ultimaker | NA | |
| MATLAB | The MathWorks | NA | |
| Solidworks 2016 | Dassault Systemes | NA |
References
- Hahn, C., Schwartz, M. A. Mechanotransduction in vascular physiology and atherogenesis. Nature Reviews Molecular Cell Biology. 10 (1), 53-62 (2009).
- Wang, C., Baker, B. M., Chen, C. S., Schwartz, M. A. Endothelial Cell Sensing of Flow Direction. Arteriosclerosis, Thrombosis, and Vascular Biology. 33 (9), 2130-2136 (2013).
- Tzima, E., et al. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature. 437, 426-431 (2005).
- Potter, C. M. F., Schobesberger, S., Lundberg, M. H., Weinberg, P. D., Mitchell, J. A., Gorelik, J. Shape and compliance of endothelial cells after shear stress in vitro or from different aortic regions: Scanning ion conductance microscopy study. PLoS ONE. 7 (2), 1-5 (2012).
- Asakura, T., Karino, T. Flow Patterns and Spatial Distribution of Atherosclerotic Lesions in Human. Circulation Research. 66 (4), 1045-1067 (1990).
- Bond, A. R., Iftikhar, S., Bharath, A. A., Weinberg, P. D. Morphological evidence for a change in the pattern of aortic wall shear stress with age. Arteriosclerosis, Thrombosis, and Vascular Biology. 31 (3), 543-550 (2011).
- Giddens, D. P., Zarins, C. K., Glagov, S. The role of fluid mechanics in the localization and detection of atherosclerosis. Journal of biomechanical engineering. 115, 588-594 (1993).
- Schnittler, H. J., Franke, R. P., Akbay, U., Mrowietz, C., Drenckhahn, D. Improved in vitro rheological system for studying the effect of fluid shear stress on cultured cells. The American journal of physiology. 265, 289-298 (1993).
- Levesque, M. J., Nerem, R. M. The elongation and orientation of cultured endothelial cells in response to shear stress. Journal of biomechanical engineering. 107 (4), 341-347 (1985).
- Chiu, J., et al. Analysis of the effect of disturbed flow on monocytic adhesion to endothelial cells. Journal of Biomechanics. 36 (12), 1883-1895 (2003).
- Venugopal Menon, N., et al. A tunable microfluidic 3D stenosis model to study leukocyte-endothelial interactions in atherosclerosis. APL Bioengineering. 2 (1), 016103 (2018).
- Warboys, C. M., Ghim, M., Weinberg, P. D. Understanding mechanobiology in cultured endothelium: A review of the orbital shaker method. Atherosclerosis. 285, 170-177 (2019).
- Ghim, M., Pang, K. T., Arshad, M., Wang, X., Weinberg, P. D. A novel method for segmenting growth of cells in sheared endothelial culture reveals the secretion of an anti-inflammatory mediator. Journal of Biological Engineering. 12 (1), 15 (2018).
- Sage, H., Pritzl, P., Bornstein, P. Secretory phenotypes of endothelial cells in culture: comparison of aortic, venous, capillary, and corneal endothelium. Arteriosclerosis. 1 (6), 427-442 (1981).
- Tunica, D. G., et al. Proteomic analysis of the secretome of human umbilical vein endothelial cells using a combination of free-flow electrophoresis and nanoflow LC-MS/MS. Proteomics. 9, 4991-4996 (2009).
- Griffoni, C., et al. Modification of proteins secreted by endothelial cells during modeled low gravity exposure. Journal of Cellular Biochemistry. 112, 265-272 (2011).
- Ghim, M., et al. Visualization of three pathways for macromolecule transport across cultured endothelium and their modification by flow. American Journal of Physiology-Heart and Circulatory Physiology. 313 (5), 959-973 (2017).
- Levesque, M. J., Liepsch, D., Moravec, S., Nerem, R. M. Correlation of endothelial cell shape and wall shear stress in a stenosed dog aorta. Arteriosclerosis: An Official Journal of the American Heart Association, Inc. 6 (2), 220-229 (1986).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved