Method Article
Sodium Taurocholate Induced Severe Acute Pancreatitis in C57BL/6 Mice
In This Article
Summary
Animal models for severe acute pancreatitis enable the study of pathophysiological changes at the initial stage, facilitating observation of the evolution of inflammatory events. Here we provide a protocol for the induction of severe acute biliary pancreatitis by retrograde infusion of sodium taurocholate into the pancreatic duct of anesthetized C57BL/6 mice.
Abstract
Biliary acute pancreatitis induction by sodium taurocholate infusion has been widely used by the scientific community due to the representation of the human clinical condition and reproduction of inflammatory events corresponding to the onset of clinical biliary pancreatitis. The severity of pancreatic damage can be assessed by measuring the concentration, speed, and volume of the infused bile acid. This study provides an updated checklist of the materials and methods used in the protocol reproduction and shows the main results from this acute pancreatitis (AP) model. Most of the previous publications have limited themselves to reproducing this model in rats. We have applied this method in mice, which provides additional advantages (i.e., the availability of an arsenal of reagents and antibodies for these animals along with the possibility of working with genetically modified strains of mice) that may be relevant to the study. For acute pancreatitis induction in mice, we present a systematic protocol, with a defined dose of 2.5% sodium taurocholate at an infusion speed 10 µL/min for 3 min in C57BL/6 mice that reaches its maximal level of severity within 12 h of induction and highlight results with outcomes that validate the method. With practice and technique, the total estimated time, from the induction of anesthesia to the completion of the infusion, is 25 min per animal.
Introduction
In humans, the presence of gallstones is the most common cause of pancreatitis due to the obstruction of the terminal portion of the choledochal, interrupting the flow of pancreatic secretions and causing an intense inflammatory process in the pancreas, with an increase in the concentration of digestive enzymes in the serum and inflammatory mediators1,2.
Two different theories have been proposed to explain the development of acute pancreatitis (AP). The "common channel" theory suggests that the stones present in the gallbladder obstruct the distal common bile duct system, allowing bile secretion to flow retrograde into the pancreatic duct. The second theory (the "duct obstruction" theory) suggests that the obstruction of the pancreatic duct by excess gallstones causes a blockage in the flow of pancreatic secretion to the duodenum, causing ductal hypertension3. Although the mechanisms that lead to acute biliary pancreatitis are not fully understood, the outcome is an intense inflammatory process. Digestive enzyme eruption and pancreas self-digestion lead to histopathological changes, an increase in inflammatory cytokines (IL-1β, IL-6, TNF-α) in ascitic fluid and serum, and an increase in acute phase proteins4,5,6.
Severe acute pancreatitis is a condition that deserves clinical attention due to the involvement of multiple organs and a high mortality risk. Animal models for the reproduction of acute pancreatitis (AP) are important as these explain the pathophysiological mechanisms of the disease and help in monitoring the evolution of inflammatory events, starting from the initial stages of the disease. This is usually not possible in the clinics2,7. In addition, access to pancreatic tissues is easy in preclinical studies, favoring the elucidation of changes linked to clinical conditions8 along with the possibility of working with isogenic species, eliminating undesirable variables, and mirroring clinical similarity with the outcomes observed in the human condition9.
Biliary and non-biliary models for the induction of acute pancreatitis in rats and mice species have been frequently studied in the scientific literature. Non-biliary methods of induction include administration of supramaximal stimulating doses of the cholecystokinin secretagogue or its analog cerulein10; administration of almost lethal doses of L-arginine; or administration of a choline-deficient diet supplemented with ethionine11. Although these methods are easy to reproduce and result in pancreatic inflammation, they do not replicate the mechanisms that in theory trigger AP (i.e., the reflux of bile secretion into the pancreatic duct). The technique that addresses the biliary model is based on the retrograde infusion of bile acids into the pancreatic duct and requires well-trained researchers to carry out this protocol. Several studies have been published using this method in rats (apparently for technical reasons since these experiments involve surgical procedures)12,13. However, the approach in mice may offer more interesting outcomes in the study of inflammation3,14,15. In this study, we will show a checklist of the steps to be followed for the reproduction of severe acute pancreatitis by infusion of sodium taurocholate in C57BL/6 anesthetized mice.
For works that involve the need for experiments with antibodies and analysis of the gene and protein expression, the use of mice is preferable because of the greater arsenal of materials for these animals and the possibility of working with isogenic and knockout species, among others that can be used relevant to studies16. Mice C57BL/6 is an inbred strain of mice originally developed for the study of antitumor activity and immunology. This strain is increasingly being preferred by researchers for being isogenic, allowing for a greater reproducibility of results, which may imply the use of a smaller number of animals in an experiment and less variability of results between the same group17,18.
Perides et al. (2010)14 published a protocol for AP induction in mice by sodium taurocholate infusion. Here we update this model using a higher sodium taurocholate concentration (2.5%) in C57BL/6 mice, with a defined volume and speed of infusion (Figure 1). The maximal level of severity is reached within 12 h of induction in mice. The elevation of the concentration of IL-6 both in the serum and in the peritoneal cavity is correlated with the progression of AP. With practice, the total estimated time from the induction of anesthesia to the completion of the infusion, is 25 min per animal. It is essential that a trained researcher conducts this experiment. To ensure that the solution is properly injected into the common bile duct, perform several pilot training sessions using methylene blue instead of sodium taurocholate.
Protocol
This protocol was approved by the Ethics Committee for the use of animals of the USP Medicine School, Num. Project: 1343/2019-CEUA: FMUSP. For this protocol, C57BL/6 mice, aged 6 weeks, weighing 20 ± 2 g were used (n = 9/group).
1. Laparotomy
- Anesthetize animals with xylazine (10 mg/kg) and ketamine solution (80 mg/kg), subcutaneously (0.1 mL/10g of body weight) using a 1 mL syringe and 13x0.45mm needle 26G ½. Check for sufficient anesthesia depth by pinching the toe. Control body temperature using heated pads. Ensure that all surgical materials are sterile.
- Clean the abdominal area with 5% povidone-iodine solution and use a trimmer to remove hair between the chest and lower abdomen (approximately 2 cm2). Clean the surgical area with 70% alcohol.
- Immobilize the animal on the surgical board using surgical tape. Use scissors to cut 5 mm of the skin horizontally, on the upper part of the abdomen and 1 cm below the xiphoid process. Repeat the cut on the peritoneum. This will result in a laparotomy with minimal exposure of the cavity.
2. Locating and exposing the pancreas
- With the aid of a retractor, pull the liver towards the mouse's head, ~1 cm from the intestine.
- Locate the region of the pancreas that will be injected with sodium taurocholate (pancreas head). Locate the duodenum with reference to the liver- below the liver, on the right side (on the left as the mouse is viewed). The duodenum is the first part of the small intestine and is connected to the final portion of the stomach.
- With the aid of forceps, lift the liver towards the animal head, and gently pull the small intestine portion. Fix the two lateral ends of the small intestine with a 6-0 polypropylene suture to better view the distal portion of the common bile duct.
3. Severe acute pancreatitis induction
- Temporarily occlude the proximal common bile duct with a microvessel clip to prevent retrograde infusion from leaking into the liver. The common bile duct can be seen on the liver side of the duodenum and its junction with the duodenum will appear white. Expose the organ out of the abdominal cavity.
- Puncture the periampullary region (whitish part of the small intestine's wall) to access the common bile duct with a 0.4 mm needle connected to a 0.54 mm polyethylene tube.
- Make a temporary occlusion of the distal common bile duct with 8-0 suture to prevent the sodium taurocholate solution from leaking into the duodenum.
- Start the infusion pump and program a 2.5% sodium taurocholate solution (diluted in 0.9% saline) infusion at a constant speed of 10 µL/10 g body weight for 3 min.
- After the infusion, remove the microvessel clip, the temporary 8-0 suture, and the injection needle from the bile pancreatic duct to reconstitute the physiological flow of the bile.
- At the end, suture the abdomen with 6-0 nonabsorbent monofilament polypropylene suture. The time between the laparotomy and the end suture should be a maximum of 30 min (see Figure 1).
- After the surgery, house the animals in polyethylene boxes lined with wood shavings and water and food ad libitum.
- Treat control mice in the same manner as the experimental mice but ensure that the infusate consists of saline only. Perform the surgical procedure and the infusion of saline solution (10 mL/min, for 3 min) in a control group (SHAM) to eliminate the inflammatory bias caused by surgery and cannulation.
- Use tramadol 12.5 mg/kg subcutaneously every 8 hours, starting after post-surgical recovery.
4. Methods for analysis
- At 12 h after AP induction, anesthetize the animals with xylazine (10 mg/kg) and ketamine (80 mg/kg) to collect approximately 250 µL of blood via the orbital plexus.
- Gently hold the skin on the back, promoting a slight protrusion of the eyeball, and position it with the eye facing upwards.
- Instill a drop of eye ointment containing local anesthetic in the animal's eye.
- Position the end of the capillary tube in the medial corner of the eye and insert it gently under the eyeball, with an angle of ~30°-45°. Rotate the capillary tube until blood flow begins. Remember that it is not necessary to use force for the procedure.
- Once the collection is over, ensure homeostasis by keeping the eyelids closed by light compression with the gauze. Discard the capillary tube in the sharps container19.
- Centrifuge the serum (700 x g, 15 min) and stock the supernatant for amylase and IL-6 dosing (step 4.7 and 4.8).
- Euthanize mice by CO2 asphyxiation.
- Use a 27 G needle to inject 4 mL of ice-cold 1x PBS into the peritoneal cavity. Tened the abdominal skin and ensure that the needle is pushed slowly in the peritoneum to not puncture any organs. After the injection, gently massage the peritoneum for 10 s to remove cells adhered to the peritoneum.
- Using scissors and tweezers, make a small cut (0.5 cm) on the inner skin and musculature to expose the abdominal cavity. Insert a bulb pipette in the peritoneum and collect the fluid. Be careful not to aspirate fatty tissue or other organs.
- Collect as much fluid as possible and deposit the collected cell suspension in tubes kept on ice. Discard the bulb pipette in the sharps container20. Centrifuge the peritoneal fluid (250 x g, 5 min) and stock the supernatant for IL-6 dosing (step 4.9).
- Collect the pancreas region adjacent to the duodenum (<5mm).
- Process the pancreas by fixing in 10% formalin and incorporate it into paraffin.
- Stain the slides with hematoxylin and eosin for histopathological analyzes under light microscopy. Use Schmidt's protocol21 (pancreatic edema, acinar cell, injury/necrosis, pancreatic inflammation) to assess the extent of AP.
- Measure amylase (U/dL) using commercially available kits according to the manufacturer's recommendations.
- Measure IL-6 by Luminex assays using commercial kits according to the manufacturer's recommendations.
- Store the serum and peritoneal fluid supernatant obtained in the steps 4.1 and 4.4 in a freezer at -80 °C if needed.
Results
Pancreatitis severity was scored between 0-3 according to the Schmidt's scale21 where zero corresponds to the absence, 1 corresponds to a mild presence (<25%), 2 corresponds to a moderate presence (between 25 and 50%) and 3 corresponds to an intense presence (> 50%) (Table 1). The measurements performed were plasma amylase activity, pancreatic edema, acinar cell, injury/necrosis, pancreatic inflammation (by histology analysis of H&E-stained sections) and IL-6 cytokine concentration in the serum and PerC fluid. After 12 h of severe AP, the APTA group showed an increase in the serum amylase concentration (6194 ± 336.7 U/dL), compared to the sham group (3845 ± 135.7 U/dL). At the same time, the APTA group showed increased IL-6 cytokine concentration in the serum and PerC fluid (Figure 2). Figure 3 shows a representative hematoxylin-eosin staining of sham and APTA group.

Figure 1: Schematics of severe acute pancreatitis induction by 2.5% sodium taurocholate in C57BL/6 mice. (A) gallbladder; (B) common bile duct; (C) pancreatic duct; (D) portal vein; (E) microvessel clip; (F) puncture site (needle attached to a polyethylene tube and connected to infusion pump); (G) temporary needle fixation in the common bile duct. Please click here to view a larger version of this figure.
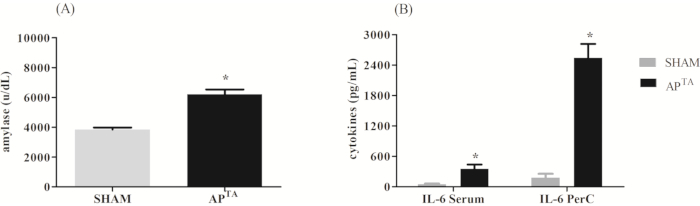
Figure 2: Representative results after 12 h of severe acute pancreatitis. (A) Animal's serum amylase concentration (U/dL). (B) IL-6 cytokine concentration in serum and PerC fluid. Differences between groups were assessed by unpaired t-test analysis * p <0.05 if APTA ≠ sham (n=9/ group). Please click here to view a larger version of this figure.
| Interstitial edema | Inflammatory infiltration | Parenchyma necrosis | Parenchyma hemorrhage | |
| SHAM | 1±0* | 0.0 | 0.0 | 0.0 |
| APTA | 3±0* | 3±0 | 3±0 | 3±0 |
| *P<0,05 if SHAM≠APTA. |
Table 1: Histological changes in pancreatic tissue after 12 h of severe AP. The pancreas was processed and analyzed according to the Schmidt's scale21. The results were expressed as mean ± SEM and differences between groups were assessed by the Student t test. * p <0.05 if APTA ≠ SHAM; (n=9/group).

Figure 3: Representativehematoxylin-eosin staining in pancreatic tissue after 12 h of severe AP. Histological changes in (A) SHAM and (B) APTA pancreatic tissue (Hematoxylin-Eosin staining- 40x magnification). Please click here to view a larger version of this figure.
Discussion
The method of inducing acute pancreatitis by retrograde sodium taurocholate infusion has already been shown in rats22,23,24. Three similar works, published in 2008, 2010 and 2015, served as a reference for the protocol3,14,15. In this work, we list all the critical steps for reproducing this method in C57BL/6 mice and some possibilities for validating it.
A critical step in this test is blocking the bile duct at the level of the hilum with a microvessel clip (step 3.1) to avoid sodium taurocholate reflux into the liver. This step requires much attention, since the portal vein is next to the duct (Figure 1D), so care must be taken to not block it together. The needle should be inserted only into the most distal duct portion. If it is introduced deeply into the duct, it can cause a rupture with the acid overflowing to the glandular parenchyma and/or to other ducts14. Check that the polyethylene tube has air inside to prevent the obstruction of the common bile duct.
This model requires an incision in the mouse abdomen. Inserting the cannula through the pancreatic duct orifice requires experience but that can be achieved with training15,25. It is important to highlight that the severity of pancreatitis in this model is proportionally dependent on the concentration, volume of the infusion, pressure of the infusion, and the time of AP induction. Thus, a constant infusion machine with controlled volume and pressure must be used.
For this study, we standardized a concentration of 2.5%, with an infusion speed of 10 µL/min, for 3 min and constant pressure. At 12 h of AP, increased inflammatory parameters and necrosis of the pancreatic tissues were observed, with animals dying within 16 h after AP induction.
Although the main causes of AP are alcohol consumption or gallstones, these models are not experimentally reproducible26. Currently, the most used protocol for AP induction in mice involves 7 intraperitoneal injections of cerulein (50 µg/kg body weight) at 1 h intervals27. Cerulein has been used to induce a mild or moderate acute pancreatitis as well. The variability in this model limits its use in studying the destructive effects of the disease, which confer clinical morbidity and mortality10. Models that trigger high mortality in a short time are relevant for the study of severe AP (necrotizing), as they can evaluate the effectiveness of new drugs or interventions. Among these models are hemorrhagic AP induction in young female rodents (between 4 and 6 weeks) by a choline-deficient diet28 and L-arginine (e.g., 3 x 3 g/kg or 2 x 4 g/kg) based acute pancreatitis induction in mice but the proper dosing of L-arginine to induce AP should be tested by each laboratory and in each mouse strain29. In 2015, a study showed that an intra-ductal taurocholate infusion followed by distal common bile duct ligation leads to a severe, necrotic model of pancreatitis in mice3. However, this model is not useful for testing drug efficacy and interventions due to its irreversible condition.
The outcomes found in this study correlate with recent literature such as the elevation of serum amylase concentration and IL-6 being associated with disease progression. It is possible that in the future, the measurement of proteins such as TNF-α, IL-1β and myeloperoxidase will be a clinical prognostic parameter for severe acute pancreatitis30,31,32.
In conclusion, the protocol used in the present study to induce AP in mice by the infusion of sodium taurocholate directly into the common bile duct results in severe acute pancreatitis with necrosis of pancreatic tissue that can be observed even at 12 h after the induction with elevation of IL-6 cytokine in the serum and peritoneal fluid and with high lethality (100% mortality in 16 h, data not shown).
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors thanks to Post Graduation Program in Medical Clinic of University of São Paulo; Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and University of São Paulo Medical School (FMUSP).
Materials
| Name | Company | Catalog Number | Comments |
| 0.4 mm needle | INTRAG MEDICAL TECH | 90183210 | 30G |
| 0.54 mm polyethylene tube | Tygon | 730010 | - |
| Styrofoam block | - | - | - |
| masking tape for mounting the mouse | Missner | 1236 | - |
| Infusion pump scheduled to 10µL / min. | Havard aparatus-Peristaltic Pump Series | MA1 55-7766 | Model 66 Small Peristaltic |
| Scissors and forceps | |||
| Antiseptic providine iodine | Pfizer | 12086OR | antisepsis |
| 70% ethanol | SIGMA | 459836 | Mix 700 mL 100% ethanol with 300 mL dH2O |
| Razor blade | Lord | bdk9a1ghk6 | For trichotomy |
| Sodium taurocholate | Sigma-Aldrich | 86339- 1G | CAS NUMBER- 345909-26-4 |
| microvessel clip | Medicon Surgical | 56.87.35 | Approximator, opening 4.0 mm, closing pressure 30 - 40 g |
| 6-0 prolene | Bioline | 5162 | Suture line |
| Ketamin NP (cloridrato de dextrocetamina) 50mg/mL | Cristália | ||
| Xilazine 2% | Syntec | ||
| Sterile saline solution (0.9% (wt/vol) saline) | Farmace | 105851 | |
| Methyl Blue | Sigma-Aldrich Chemicals | M5528 | |
| MILLIPLEX MAP Mouse Cytokine/Chemokine Magnetic Bead Panel - Immunology Multiplex Assay | MERCK | MCYTOMAG-70K | Simultaneously analyze multiple cytokine and chemokine biomarkers with Bead-Based Multiplex Assays using the Luminex technology, in mouse serum, plasma and cell culture samples. |
| Amylase Assay | Labtest | 11 | |
| Desmarres retractor 13-mm width | ROBOZ | RS-6672 |
References
- Li, X., et al. Significantly different clinical features between hypertriglyceridemia and biliary acute pancreatitis: A retrospective study of 730 patients from a tertiary center. BMC Gastroenterology. 18 (1), 1-8 (2018).
- Rechreche, H., Abbes, A., Iovanna, J. L. Induction of antioxidant mechanisms in lung during experimental pancreatitis in rats. Indian Journal of Experimental Biology. 58 (5), 297-305 (2020).
- T, L., et al. Intraductal infusion of taurocholate followed by distal common bile duct ligation leads to a severe necrotic model of pancreatitis in mice. Pancreas. 44 (3), (2015).
- Botoi, G., Andercou, A. Interleukin 17-prognostic marker of severe acute pancreatitis. Chirurgia. 104 (4), 431-438 (2009).
- Li, D., Li, J., Wang, L., Zhang, Q. Association between IL-1beta, IL-8, and IL-10 polymorphisms and risk of acute pancreatitis. Genetics and Molecular Research. 14 (2), 6635-6641 (2015).
- Feng, C., et al. Effect of peritoneal lavage with ulinastatin on the expression of NF-kappaB and TNF-alpha in multiple organs of rats with severe acute pancreatitis. Experimental and Therapeutic Medicine. 10 (6), 2029-2034 (2015).
- Fang, D. Z., et al. Effects of sildenafil on inflammatory injury of the lung in sodium taurocholate-induced severe acute pancreatitis rats. International Immunopharmacology. 80, (2020).
- Ceranowicz, P., Cieszkowski, J., Warzecha, Z., Dembinski, A. Experimental models of acute pancreatitis. Postępy Higieny i Medycyny Doświadczalnej(Online). 69, 264-269 (2015).
- Wan, M. H., et al. Review of experimental animal models of biliary acute pancreatitis and recent advances in basic research. HPB (Oxford). 14 (2), 73-81 (2012).
- Mayerle, J., Sendler, M., Lerch, M. M. Secretagogue (Caerulein) induced pancreatitis in rodents. Pancreapedia: The Exocrine Pancreas Knowledge Base. (1), (2013).
- Wang, N., et al. Resveratrol protects against L-arginine-induced acute necrotizing pancreatitis in mice by enhancing SIRT1-mediated deacetylation of p53 and heat shock factor 1. International Journal of Molecular Medicine. 40 (2), 427-437 (2017).
- Ma, Z. H., et al. Effect of resveratrol on peritoneal macrophages in rats with severe acute pancreatitis. Inflammation Research. 54 (12), 522-527 (2005).
- Souza, L. J., et al. Anti-inflammatory effects of peritoneal lavage in acute pancreatitis. Pancreas. 39 (8), 1180-1184 (2010).
- Perides, G., Acker, G. J. v., Laukkarinen, J. M., Steer, M. L. Experimental acute biliary pancreatitis induced by retrograde infusion of bile acids into the mouse pancreatic duct. Nature Protocols. 5 (2), 335-341 (2010).
- Wittel, U. A., et al. Taurocholate-induced pancreatitis: a model of severe necrotizing pancreatitis in mice. Pancreas. 36 (2), 9-21 (2008).
- Tao, L., Reese, T. A. Making mouse models that reflect human immune responses. Trends Immunology. 38 (3), 181-193 (2017).
- Vandamme, T. F. Use of rodents as models of human diseases. Journal of Pharmacy and Bioallied Science. 6 (1), 2-9 (2014).
- Song, H. K., Hwang, D. Y. Use of C57BL/6N mice on the variety of immunological researches. Laboratory Animal Research. 33 (2), 119-123 (2017).
- Bogdanske, J. J., Stelle, S. H. -. V., Riley, M. V., Schiffman, B. M. . Suturing Principles and Techniques in Laboratory Animal Surgery. 1st edition. (1), (2010).
- Ray, A., Dittel, B. N. Isolation of mouse peritoneal cavity cells. Journal of Visualized Experiments. (35), e1488 (2010).
- Schmidt, J., et al. A better model of acute pancreatitis for evaluating therapy. Annals of Surgery. 215 (1), 44-56 (1992).
- Liu, D. L., et al. Resveratrol improves the therapeutic efficacy of bone marrow-derived mesenchymal stem cells in rats with severe acute pancreatitis. International Immunopharmacology. 80, 106128 (2020).
- Yang, X. F., et al. Chaiqin chengqi decoction alleviates severe acute pancreatitis associated acute kidney injury by inhibiting endoplasmic reticulum stress and subsequent apoptosis. Biomedicine & Pharmacotherapy. 125 (12), 110024 (2020).
- Yang, X. F., et al. Chaiqin chengqi decoction alleviates severe acute pancreatitis associated acute kidney injury by inhibiting endoplasmic reticulum stress and subsequent apoptosis. Biomedicine & Pharmacotherapy. 125, 110024 (2020).
- Venglovecz, V., Z, R., Hegyi, P. The effects of bile acids on pancreatic ductal cells. Pancreapedia: The Exocrine Pancreas Knowledge Base. (1), (2019).
- Roberts, S. E., Akbari, A., Thorne, K., Atkinson, M., Evans, P. A. The incidence of acute pancreatitis: impact of social deprivation, alcohol consumption, seasonal and demographic factors. Alimentary Pharmacology and Therapeutics. 38 (5), 539-548 (2013).
- Lerch, M. M., Gorelick, F. S. Models of acute and chronic pancreatitis. Gastroenterology. 144 (6), 1180-1193 (2013).
- Nakamura, K., Fukatsu, K., Sasayama, A., Yamaji, T. An immune-modulating formula comprising whey peptides and fermented milk improves inflammation-related remote organ injuries in diet-induced acute pancreatitis in mice. Biosci Microbiota Food Health. 37 (1), 1-8 (2018).
- Kui, B., et al. New insights into the methodolgy of L-Arginine-induced acute pancreatitis. PLoS One. 10 (2), 011758 (2015).
- Xue, J., et al. Alternatively activated macrophages promote pancreatic fibrosis in chronic pancreatitis. Nature Communication. 6, 7158 (2015).
- Lesina, M., Wormann, S. M., Neuhofer, P., Song, L., Algul, H. Interleukin-6 in inflammatory and malignant diseases of the pancreas. Seminars in Immunology. 26 (1), 80-87 (2014).
- Rao, S. A., Kunte, A. R. Interleukin-6: An early predictive marker for severity of acute pancreatitis. Indian Journal of Critical Care Medicine. 21 (7), 424-428 (2017).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved