Method Article
Design, Fabrication, and Administration of the Hand Active Sensation Test (HASTe)
In This Article
Summary
The Hand Active Sensation Test (HASTe) is a valid and reliable measure of haptic performance, which has been used successfully to identify impaired haptic touch in individuals with stroke. The purpose of this paper is to describe the design, fabrication and administration of the HASTe.
Abstract
The concept of personalizing neurologic rehabilitation, based on individual impairments, has experienced a recent surge. In parallel, the number of outcome measures of upper extremity motor performance has grown. However, clinicians and researchers lack practical, quantitative measures of the hand’s natural role as a receptor of the environment. The Hand Active Sensation Test (HASTe), developed by Williams and colleagues in 2006, is a valid and reliable measure of haptic performance. Though not available commercially, the HASTe can be fabricated from inexpensive materials, and it has been used successfully to identify impairments in haptic touch in individuals with stroke. (Williams, 2006). This paper presents the methods of design and fabrication of the HASTe testing kit, as well as a visual screen to be used during administration, and instructions for the tests administration and scoring.
Introduction
Imagine the feel of a loved one’s hand. It is calloused or smooth? Strong or delicate? Warm or cool? Do you hold it in a handshake or with fingers intertwined? If you have evoked a memory, it is likely grounded in your ability to use movement of your hands to solicit somatosensory information, which is called haptic touch, or active sensation. Characteristics that can be determined with haptic touch include size, shape, weight, texture, surface compliance, and temperature. Haptic receptors include proprioceptors, which are found in the skin, muscles, tendons, and joints, as well as cutaneous receptors, both of which are activated during manual exploration of objects. Different hand actions aid in the determination of object characteristics. For example, repeatedly performing a lateral rubbing motion across an object may expose texture properties, or lifting the object in the hand may reveal object weight. Therefore, both manual dexterity and somatosensation are vital to haptic performance and the human experience.
Following stroke, both somatosensory and motor impairments contribute to diminished haptic performance. 1 Poststroke motor impairments are common and well defined, with approximately 70% of stroke survivors experiencing some level of paresis. 1 Somatosensory impairments after stroke are also common, occurring in 47-89% 2-4 of individuals. Researchers agree that both cutaneous and proprioceptive impairments are common after stroke. For a thorough characterization of the consequences of stroke on somatosensory domains the reader is referred to the works by of Carey et al., 2 Connell et al., 5 and Sullivan. 4
Somatosensory impairments contribute to diminished rehabilitation outcomes, 6 upper limb coordination, 7 function and quality of life.8 However, quantification of somatosensory impairments, especially active sensation, is lacking in clinical practice in part due to the fact that somatosensory loss is less apparent and more difficult to quantify than motor impairments.9 Measures which have been developed to quantify active sensation include the Byl-Chyney-Boczai Sensory Discriminator (BCB), 10 the stereognosis component of the Revised Nottingham Sensory Assessment, 11 the Functional Tactile Object Recognition Tests (fTORT), 12 the Manual Form Perception Test (a subset of the Sensory Information and Praxis Test), 13 and the Haptic Object Recognition Test (HORT). 14 Although these measures are available, a recent systematic review, which sought to describe how somatosensory impairments in the arm and hand relate to upper limb problems following stroke, concluded that clinicians and researchers currently lack valid and reliable tests of somatosensation. 15 Therefore, addressing the availability of clinically useful and parametrically sound measures of haptic performance is essential.
The Hand Active Sensation Test (HASTe) is an 18-item match-to-sample test of weight and texture discrimination, originally published by Williams and colleagues in 2006. 16 The HASTe is a measure of haptic touch and is sensitive to haptic impairments in individuals with stroke (indicated by fewer than 13 correct matches). As the HASTe seeks to measure haptic perception, as minimum criteria, individuals need the abilities to grasp and lift with the hand and arm and follow the test instructions. In the International Classification of Functioning, Disability and Health (ICF) model, 17 the HASTe is considered an activity level measurement. The HASTe takes between 15-30 min to administer per hand tested. Advantages of the HASTe include that it is inexpensive, total of material prices for 2015 estimated at $100, and easy to construct and that it’s 18-point scale provides greater resolution regarding performance than more common dichotomous characterization of “intact” or “impaired”.
The purpose of this paper is to describe the design, fabrication and administration of the HASTe. While it is possible to infer the test set-up from the original HASTe publication, this paper provides detailed methods for fabricating a visual screen and a HASTe test kit, both to be used during testing. The equipment required for assembly, as well as a detailed list of all required materials is listed in the Table of Materials. A single sheet with instructions for administering and scoring the HASTe is also provided as the following:
APPENDIX 1: HASTe Administration Instructions and Score Sheet Participant ID#_______ Date_______
Set Up: Seat the test participant at a table (table ~29 inches high and chair seat ~18 inches high) with, initially, the dominant or less impaired upper extremity resting on the table and placed under the assembled visual screen. During the test, keep all objects in numerical order to maintain organization.
Demonstration Trials: Administer two demonstration trials prior to scoring. Provide the participant with objects A and B, and instruct him/her to compare the objects’ weights. Then, provide the participant with objects A and C, and instruct him/her to compare the objects’ textures. Offer feedback only for the demonstration trials.
Scored Trials: There will be 18 trials scored per extremity, with a maximum of 5 min allowed for each. Instruct the participant to manually explore the specified test object (identified “test” in the tables below) with the dominant or less impaired hand, then explore each of the three possible object matches (numbered otherwise within each trial box below) with this same hand. The possible matches will vary by either weight or texture, but never by both within one trial. Instruct the participant to find the match of the original object and tell him/her they can touch each object as many times as needed to determine an answer. Do not inform the participant as to which object property (texture or weight) he or she is matching within a trial, and do not assist the participant with manual exploration of the objects. Indicate the participant’s final answer for each trial in the tables below by circling the number of that object. Test the more impaired upper extremity after the participant completes the test with the less impaired upper extremity. Using the tables below, score the test by determining the number of correct matches from a total of 18 trials per hand (one box per trial below).
Verbal Instructions to Participant: “Use one hand to manually explore the test object first. Then, explore each of the three possible matches, which vary by either weight or texture but never by both within one trial. Find the match. You can touch each object as many times as you need to determine your answer. There will be 18 trials. You have a maximum of 5 min for each trial.”

Protocol
1. Design and Fabrication of Visual Screen and HASTe (See "Table of Materials" for All Required Equipment and Materials):
- Assemble Visual Screen (Figure 1):
- Cut four pieces of 0.5-inch PVC pipe (material a) to 10 inches (these form the base of the screen). Cut three pieces of 0.5-inch PVC pipe to 20 inches (these form the upright portion of the screen).
- Insert two pieces of 10-inch pipe into both PVC Tee’s (parallel openings) (material b). Insert two pieces of 20-inch pipe into each of the remaining holes of the PVC Tee’s (perpendicular to the 10-inch pieces). Insert PVC elbows (material c) on each of the open ends of the 20-inch pipes.
- Insert each end of the third 20-inch PVC pipe into each of the open PVC elbows. Do not glue PVC joints if easier folding/storage of visual screen is desired. Glue PVC joints for a permanent visual screen, if desired. Drape pillow case (material d) over top pipe. Secure with binder clips (material e) or safety pins.

Figure 1: Set-up of the HASTe. Participants should be seated at a table. The visual screen between the participant and the test objects. The objects for one trial are removed from the test kit and offered to the participant in the following order: test object first, then each of the three possible matches, in the order indicated in Appendix 1: Administration Instructions and Score Sheet. Please click here to view a larger version of this figure.
- Assemble HASTe Testing Kit (includes 9 test objects, a duplicate set of 9 sample objects, and 3 example objects) (Figure 2)
- Cut 21 pieces of 1.5-inch diameter PVC pipe (material f) into 4-inch lengths. Cut cork (material g) into 21 strips, each 4 x 7 inches. Overlay each cork over PVC to ensure no overlap at seam or ends of pipe.
- Trim excess cork flush to edge. Coat each of the 21 pieces of pipe (one-by-one) with all purpose cement (material h) and wrap with cork.
- Measure and cut six 4 x 7-inch pieces of self-adhesive laminate (material i) and six 4 x 7-inch pieces of brown paper (material j). Trim excess. Wrap six pieces with self-adhesive laminate.
- Coat six pieces with all purpose cement and wrap with brown paper wrapping. Coat one piece with all purpose cement and wrap with sandpaper (material k). Coat two pieces with all purpose cement and wrap with glossy cardstock (material l).
- Measure and lay out eight pieces of clay (material m), each weighing 2.2 ounces. Measure and lay out six pieces of clay, each weighing 3.2 ounces. Measure and lay out seven pieces of clay, each weighing 4.2 ounces.
- Mold two 2.2-ounce, 3.2-ounce, and 4.2-ounce pieces of clay into a solid block to fill the diameter of the pipe. Insert each into the center of a pipe covered in brown paper wrapping. Repeat step with pipes covered with cork and self-adhesive laminate.
- Mold two 2.2-ounce pieces of clay into a solid block to fill the diameter of the pipe. Insert one into the center of a pipe covered in glossy cardstock and one into the pipe covered in sand paper.
- Mold one 4.2-ounce piece of clay into a solid block to fill the diameter of the pipe. Insert into the center of a pipe covered in glossy cardstock .
- Fill each pipe to capacity with closed-cell packing foam (material n). Weigh components to ensure accuracy of weight to 0.1 ounce.
- Coat each end cap (material o) with all purpose cement and firmly insert into both ends of each pipe.
- Label each object with weight (on the bottom) and number (on the top) as to be visible to the examiner.
- Cover test objects 1T, 2T and 3T with cork, then brown paper, and weigh to 6, 7, and 8 ounces, respectively. Cover test objects 4T, 5T, and 6T only and weigh to 6, 7, and 8 ounces, respectively. Cover test objects 7T, 8T, and 9T with cork, then self-adhesive laminate, and weigh to 6, 7, and 8 ounces, respectively.
- Label sample objects 1-9 (exact duplicates of the test objects).
- Label the three example objects A, B, and C. Cover example objects A and B with glossy card stock (weighing 6 and 8 ounces, respectively). Cover example object C in sand paper (weighing 6 ounces). (Example objects are not shown in Figure 2.)
- Sore and transport all test items in a plastic storage box (material p).

Figure 2: Organization of the HASTe Test Objects. The test objects are ordered 1-9 and marked with a ‘T’. The nine sample objects, which participants match to the test objects by weight or texture, are also numbered 1-9 and ordered, as shown.
2. Administration of HASTe:
- Seat participants at a standard height table (~29 inch) and chair (~18 inch). Adjust the heights such that the participant can rest and move the test arm comfortably on the table. Place the test arm under a visual screen, as shown in Figure 3, to prevent participant from seeing the test arm, the objects, or the examiner.
Note: Test the less impaired upper extremity first, if the participant being tested has a more impaired upper extremity. - Instruct participant to grasp and lift the example objects to freely determine differences between weight and texture. Provide feedback for the two demonstration trials only.
- Administer two demonstration trials prior to scoring. Have participant compare objects A and B to illustrate differences in weight. Have participant compare objects A and C to illustrate differences in texture.
- Do not offer feedback on the patient’s performance during trials of the scored test. Do not assist the patient with manual exploration of the objects. Organize test materials for all 18 trials by keeping objects in numerical order. (Figure 2)
- Provide verbal instructions to the participant: “Use one hand to manually explore the test object first. Then, explore each of the three possible matches, which vary by either weight or texture but never both within one trial. Find the match. Touch each object as many times as needed to determine the answer. There will be 18 trials. With a maximum of 5 min for each trial.”
- Repeat test instructions during test administration at the discretion of the examiner or upon request of the participant. Do not inform participant as to which object property (weight or texture) they are matching within a trial.
- Slide objects to and away from the participant’s hand, per the participant’s request, if his or her elbow or shoulder movement prevents them from moving between objects (Figure 3).
- Have the participant indicate their choice verbally or by pointing to their choice upon finding the match. Administer the next trial. Do not have participant describe, in any manner, the object properties or explain why they chose the match that they did.
- Score the test by determining the number of correct matches from a total of 18 trials per hand. Use Appendix 1: Administration Instructions and Score Sheet and the overall organization shown in Figure 2 in order to reduce examiner error.
- Analyze and interpret HASTe scores as a measure of haptic performance with the following considerations.
Note: The intervals between ranks on the HASTe, an ordinal scale, may not be consistent and may not be known. However, like many clinical measures, it may be meaningful and appropriate to apply statistical inference as though the data were interval, as shown in Figure 4.

Figure 3: Offering the HASTe Objects to the Participant. The objects can be placed in the participant’s hand, but the examiner should avoid assisting the participant with object manipulation.
Results
Williams and colleagues published the original study, which established the reliability and validity of the HASTe, in 2006. Based on that study, individuals scoring fewer than 13 correct matches on the HASTe are considered to have impaired haptic performance, while those scoring between 13 and 18 are considered to have unimpaired haptic performance. 16 When an individual scores 6 or fewer correct matches, it is considered a chance score, based on the odds in an 18 trial/3 item match to sample design.
Test-retest reliability for the HASTe was strong across all groups (ICC3,1 = 0.77, Pearson r = 0.78). Internal consistency was also strong for the HASTe with a Cronbach coefficient of α = 0.82. The mean accuracy score for the control group was 14.86 ± 1.53 and 8.46 ± 3.51 for stroke survivors (P <0.001). Participants in both groups scored significantly higher (P <0.001) on the 9 texture trials (6.36 ± 2.50) than the 9 weight trials (5.30 ± 2.31). Sensitivity was 0.857 and specificity was 1.0. 16
A second study (Borstad AL, unpublished data, 2015), which used the HASTe with 12 individuals with stroke and 12 age, gender and handedness matched control participants, found participants in both groups exhibited a wide range of haptic performance. Post-stroke participant scores on the HASTe ranged from 4 to 15 for the paretic hand and 8 to 16 for the non-paretic hand. Control participant scores ranged from 8 to 16 for the right hand and from 8 to 17 for the left hand. Between group differences in this sample were not statistically significant for either the paretic and matched hand or the non-paretic and matched hand (Table 1). Nine post-stroke participants (75%) had impaired haptic performance in their right hand (indicated by a score of fewer than 13 correct). In 8 of the 9 post-stroke participants with haptic performance considered impaired, when the contralesional hand was impaired, the ipsilesional hand was also impaired. This relationship was explored using a Pearson correlation coefficient. In this sample, there was a good correlation of HASTe scores between hands (r = 0.70, p <0.001), but no relationship between HASTe scores and age. (Figure 4) Participants in this study provided written informed consent and the institutional biomedical review board approved the study.
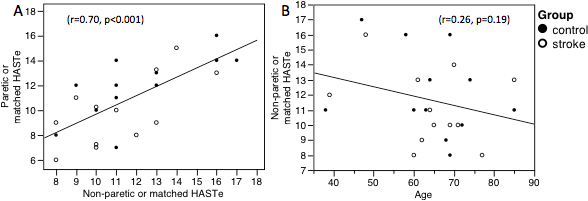
Figure 4: HASTe Scores Correlate with the Opposite Hand but not Age. (Borstad AL, unpublished data, 2015) (A) The number of correct matches for the paretic hand and the matched hand of the control participants showed a good correlation with the other hands (Non-paretic or matched). (B) To examine the relationship between age and performance on the HASTe, the non-paretic and matched hands of the control particpants were used in a Pearson correlation with age. This relationship was not found to be significant.
| Male | Right Hand Dominant | Age, Years | Right hand was Paretic | Chronicity, Months | Paretic or matched HASTe | Non-paretic or matched HASTe | |
| Poststroke (n = 12) | 5 | 11 | 64.3(12.2) | 5 | 12.3(21.2) | 9.8 (2.8) | 11.2(2.5) |
| Controls (n = 12) | 5 | 11 | 63.9(12.4) | -- | -- | 11.9(2.6) | 12.2(2.9) |
| Two-tailed t-test | p = 0.07 | p = 0.37 | |||||
| Mean(SD) | |||||||
Table 1: Participant Description and Average HASTe scores (Borstad AL, unpublished data, 2015).
Discussion
Evidence suggests that similar to motor recovery following stroke, somatosensory recovery requires task specific training. 18,19,20 Therefore, if we aim to improve the hands performance as a haptic receptor, haptic impairments must be identified. 15 The opportunity to quantify haptic ability in the clinic and laboratory has been limited, to some extent, based on the availability of valid and reliable outcome measures. The large number of stroke survivors with somatosensory impairments warrants the need for valid and reliable clinical outcome measures, which identify impairments and inform individualized rehabilitation programs. The HASTe is such a measure.
While the HASTe is not appropriate for individuals with severe upper extremity motor impairments, it is useful to evaluate individuals with moderate to mild motor impairments. These are the same individuals, research suggests, who have good potential to resume meaningful upper limb use after stroke. Measuring haptic ability using a reliable and valid 18-point scale affords the opportunity to identify impairments (fewer than 13 of 18 correct matches) and to quantify improvements, thought to be a change of greater than 3 matches. 16 The HASTe may be appropriate to use in both research and clinical settings. The match to sample design minimizes the opportunity for tester bias. It can be fabricated with inexpensive, common materials. Critical steps in fabrication include accurate measurement of the cork, self-adhesive laminate, and brown paper coverings to ensure that the seams and edges meet but do not overlap (protocol step 1.2.2). Material overlap would result in inconsistencies between objects and premature wear of the objects. A second critical step in the fabrication process is accurately measuring object contents to 0.1 ounce and using packing foam to ensure that materials do not shift in the cylinder during handling (protocol step 1.2.9).
It is possible that the greatest threat to HASTe validity is if the participants do not understand or remember the test instructions throughout the administration of the test. In the second study, no example objects were provided to the participants, which may have resulted in greater variability in healthy participant scores. We suggest this may have resulted in confusion regarding test instructions. This is useful to illustrate the importance of using example objects to clarify the study paradigm. While participants should not be instructed as to which object property (weight or texture) they are matching to within each trial, it is critical that they understand that, within each trial, they will only match based on one property, as one property is kept constant within every trial. For further clarification, these instructions can be repeated during the test at the discretion of the examiner or the request of the participant (protocol step 2.6).
The HASTe is unique when compared to other similar measures. The Stereognosis component of the Revised Nottingham Sensory Assessment, 11 the Byl-Chyney-Boczai Sensory Discriminator (BCB), 10 and the Functional Tactile Object Recognition Tests fTORT, 12 while valid and reliable for stroke, all require visual or verbal matching to a comparison item. Thus, these measures may be confounded by cognitive or language impairments. The manual form perception test, a subset of the Sensory information and praxis test, 13 and the Haptic Object Recognition Test (HORT) 14 involve form matching but do not require texture or weight discrimination, undeniably important aspects of haptic performance. Neither the manual form perception test nor the HORT have been validated in the population with stroke.
The principle limitation of the HASTe is the administration time, which averages between 15 and 20 min per hand. A second limitation is that severe motor impairments preclude participation, as participants must be able to grasp and lift the test objects. Finally, the HASTe measures only two aspects of haptic touch, weight and texture discrimination. The HASTe has several advantages. First, the HASTe’s 18-point scale provides more information about haptic performance than dichotomous descriptions of somatosensory performance such as “intact” and “impaired” routinely used in the clinic. Second, the test is inexpensive and relatively easy to construct. Third, the administration methods limit the possibility of tester bias. Finally, the test has good test-retest reliability (ICC .77), diagnostic accuracy (area under the ROC curve mean = 0.92), sensitivity (0.857) and specificity (1.0). The HASTe may be sensitive to subtle changes in haptic performance of the ipsilesional hand, yet performance does not appear to be affected by age. In the future it may be useful to validate the HASTe for use with other populations of individuals with sensorimotor impairments such as peripheral nerve injury or spinal cord injury.
A final word is to remind the reader that the HASTe, like other standardized measures of human performance, will provide the most meaningful data if the protocol for fabrication and administration of the test are followed precisely.
Disclosures
The authors declare they have no competing financial interests.
Acknowledgements
This work was supported in part by OSU’s CCTS Program through TL1TR001069 award to Dr. Borstad. We would like to acknowledge Amelia Siles, DPT, NCS for her valuable measurement insights and Sarah Alexander for her assistance with editing the final draft of this manuscript.
Materials
| Name | Company | Catalog Number | Comments |
| Equipment Needed for Assembly | |||
| Tape measure | To measure lengths of materials | ||
| PVC saw | To cut PVC pieces to appropriate lengths given below | ||
| Scissors | To cut paper, cork and laminating material | ||
| Scale accurate to 0.1 ounce | To determine exact weight of test objects | ||
| Sharpie Permanent Marker | To label test item number and weight | ||
| Visual Screen Materials | Company | Catalog Number | |
| 0.5-inch PVC Pipe (Sch. 40 Plain-End Pipe) | Home Depot | 530048 | 104 inches total, will be cut into four 10-inch and three 20-inch pieces |
| 0.5-inch PVC Tee (Sch. 40 SxSxS Tee) | Home Depot | 406005RMC | Two pieces |
| PVC Elbow (Lasco 0.5-inch Dia 90 degree PVC Sch 40 Side Outlet Elbow) | Home Depot | 413005RMC | Two pieces |
| Pillowcase | One to be hung from the PVC frame as a visual shield | ||
| ACCO Binder Clips, Medium | amazon.com | 72050 | Two to hold the pillowcase to the PVC frame |
| Testing Kit Materials | |||
| 1.5-inch PVC Sch. 40 DWV Plain End Pipe | Home Depot | 531111 | 85 inches total, will be cut into 21 4-inch pieces |
| Quartet Cork Roll, 1/16-inch thick | amazon.com | NA | 1 roll, 24x48 inches, will be cut into 42 4x7-inch pieces to cover all test and example items |
| Oatey all purpose cement for CPVC and PVC | Home Depot | 308213 | 8 ounce can, to use to adhere cork, paper and end caps to PVC test items |
| Avery Self-Adhesive Laminating Roll | amazon.com | 73610 | One 24x600-inch roll, will be cut into six 4x7-inches pieces to cover cork on six test objects |
| Brown Builder's Paper | Home Depot | 35140 | One roll, will be cut into six 4x7-inch pieces to cover cork on six test objects |
| 3M Pro Grade 9 Sandpaper | Home Depot | 25060P-G | One piece 4x7 inches, to use to cover one example item |
| Ranger Glossy Cardstock | amazon.com | NA | One 8.5x11-inch, 10-Pack, white, will be cut into two 4x7-inch pieces to cover example items |
| Marblex-durable self modeling clay in moist form | amaco.com/shop/ | X-242 | One 5-pound package, used to achieve correct weights of test objects |
| Medium density polyethylene packing foam | amazon.com | NA | One foam sheet, 220 poly, charcoal, 2x24x18 inches, to fill the remaining space in test objects after clay has been inserted |
| Knock Out Plug for 1.5-inch PVC | Home Depot | 85000 | 42 caps to seal the ends of the 21 test items |
| Sterilite 6-quart plastic storage box | Home Depot | 16428960 | One to store/transport test objects |
References
- Nakayama, H., Jgtgensen, H., Stig, K., Raaschou, H. O., Olsen, T. S. Recovery of upper extremity function in stroke patients: the Copenhagen Stroke Study. Age (SD). 74, 12 (1994).
- Carey, L. M., Matyas, T. A. Frequency of discriminative sensory loss in the hand after stroke in a rehabilitation setting. Journal of Rehabilitation Medicine. 43, 257-263 (2011).
- Winward, C. E., Halligan, P. W., Wade, D. T. Somatosensory recovery: A longitudinal study of the first 6 months after unilateral stroke. Disability & Rehabilitation. 29, 293-299 (2007).
- Sullivan, J. E., Hedman, L. D. Sensory Dysfunction Following Stroke: Incidence, Significance, Examination, and Intervention. Topics in Stroke Rehabilitation. 15, 200-217 (2008).
- Connell, L. A., Lincoln, N. B., Radford, K. A. Somatosensory impairment after stroke: frequency of different deficits and their recovery. Clinical Rehabilitation. 22, 758 (2008).
- Winward, C. E., Halligan, P. W., Wade, D. T. Current practice and clinical relevance of somatosensory assessment after stroke. Clinical rehabilitation. 13, 48-55 (1999).
- Torre, K., et al. Somatosensory-related limitations for bimanual coordination after stroke. Neurorehabilitation and neural repair. , (2013).
- Nichols-Larsen, D. S., Clark, P. C., Zeringue, A., Greenspan, A., Blanton, S. Factors influencing stroke survivors' quality of life during subacute recovery. Stroke. 36, 1480-1484 (2005).
- Borstad, A. L., Nichols-Larsen, D. S. Assessing and treating Higher-level Somatosensory Impairments Post Stroke. Topics in Stroke Rehabilitation. 21, 290-295 (2014).
- Byl, N., Leano, J., Cheney, L. K. The Byl-Cheney-Boczai Sensory Discriminator: reliability, validity, and responsiveness for testing stereognosis. Journal of Hand Therapy. 15, 315-330 (2002).
- Lincoln, N. B., Jackson, J. M., Adams, S. A. Reliability and revision of the Nottingham Sensory Assessment for stroke patients. Physiotherapy. 84, 358-365 (1998).
- Carey, L. M., Nankervis, , et al. . , (2006).
- Ayres, A. J. . Sensory integration and praxis test (SIPT). , (1989).
- Kalisch, T., Tegenthoff, M., Dinse, H. R. Improvement of sensorimotor functions in old age by passive sensory stimulation. Clinical Interventions in Aging. 3, 673 (2008).
- Meyer, S., Kattunen, A. H., Thijs, V., Feys, H., Verheyden, G. How do somatosensory deficits in the arm and hand relate to upper limb impairment, activity, and participation problems after stroke? A systematic Review. Physical Therapy. 94, (2014).
- Williams, P. S., Basso, D. M., Case-Smith, J., Nichols-Larsen, D. S. Development of the Hand Active Sensation Test: reliability and validity. Arch. Phys. Med. Rehabil. 87, 1471-1477 (2006).
- . . International Classification of Functioning, Disability and Health (ICF). , (2001).
- McDonnell, M. N., Hillier, S. L., Miles, T. S., Thompson, P. D., Ridding, M. C. Influence of combined afferent stimulation and task-specific training following stroke: a pilot randomized controlled trial. Neurorehabilitation and neural repair. 21, 435-443 (2007).
- Byl, N. N., Pitsch, E. A., Abrams, G. M. Functional outcomes can vary by dose: learning-based sensorimotor training for patients stable poststroke. Neurorehabilitation and neural repair. 22, 494 (2008).
- Carey, L., Macdonell, R., Matyas, T. A. SENSe: Study of the Effectiveness of Neurorehabilitation on Sensation A Randomized Controlled Trial. Neurorehabilitation and neural repair. 25, 304-313 (2011).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved