Method Article
Reduced-gravity Environment Hardware Demonstrations of a Prototype Miniaturized Flow Cytometer and Companion Microfluidic Mixing Technology
* These authors contributed equally
In This Article
Summary
Spaceflight blood diagnostics need innovation. Few demonstrations have been published illustrating in-flight, reduced-gravity health diagnostic technology. Here we present a method for construction and operation of a parabolic flight test rig for a prototype point-of-care flow-cytometry design, with components and preparation strategies adaptable to other setups.
Abstract
Until recently, astronaut blood samples were collected in-flight, transported to earth on the Space Shuttle, and analyzed in terrestrial laboratories. If humans are to travel beyond low Earth orbit, a transition towards space-ready, point-of-care (POC) testing is required. Such testing needs to be comprehensive, easy to perform in a reduced-gravity environment, and unaffected by the stresses of launch and spaceflight. Countless POC devices have been developed to mimic laboratory scale counterparts, but most have narrow applications and few have demonstrable use in an in-flight, reduced-gravity environment. In fact, demonstrations of biomedical diagnostics in reduced gravity are limited altogether, making component choice and certain logistical challenges difficult to approach when seeking to test new technology. To help fill the void, we are presenting a modular method for the construction and operation of a prototype blood diagnostic device and its associated parabolic flight test rig that meet the standards for flight-testing onboard a parabolic flight, reduced-gravity aircraft. The method first focuses on rig assembly for in-flight, reduced-gravity testing of a flow cytometer and a companion microfluidic mixing chip. Components are adaptable to other designs and some custom components, such as a microvolume sample loader and the micromixer may be of particular interest. The method then shifts focus to flight preparation, by offering guidelines and suggestions to prepare for a successful flight test with regard to user training, development of a standard operating procedure (SOP), and other issues. Finally, in-flight experimental procedures specific to our demonstrations are described.
Introduction
The inadequacy of current space-ready health diagnostics presents a limiting factor to deeper manned space exploration. Diagnostics need to be comprehensive, easy to use in reduced gravity, and relatively unaffected by the stresses of launch and spaceflight (e.g., high g-forces, vibration, radiation, temperature changes, and cabin pressure changes). Developments in point-of-care testing (POCT) may translate to effective spaceflight solutions through the use of smaller patient specimens (e.g., a finger prick), simpler and smaller fluidics (i.e., microfluidics), and reduced electrical power requirements, among other advantages. Flow cytometry is one attractive approach for in-space POC because of the broad utility of the technology, including toward cell counting and biomarker quantification, as well as significant miniaturization potential. Previous space-relevant flow cytometers include the ‘nuclear packing efficiency’ (NPE) instrument that utilized simultaneous arc-lamp induced fluorescence and electronic volume (Coulter volume) measurement 1-4, a relatively small benchtop flow cytometer representing the ‘first generation of real-time flow cytometry data during zero gravity’ 5, a ‘sheathless microflow cytometer’ capable of 4- and 5-part white blood cell (WBC) differential count using pretreated 5 µl whole blood samples 6-9, and a ‘fiber-optic-based’ flow cytometer recently tested onboard in the International Space Station 10.
Evaluating diagnostic technology for potential space applications is typically performed onboard reduced-gravity aircraft that use an approximately parabolic flight trajectory to simulate a chosen level of weightlessness (e.g., zero-gravity, martian-gravity) 11. Evaluation is challenging because flight opportunities are limited, repetitive short windows of microgravity can make it difficult to assess methodologies or processes that normally require uninterrupted periods longer than 20-40 sec, and demonstrations may require additional equipment not easily utilized in-flight 12-15. Furthermore, previous demonstrations of in vitro diagnostic (IVD) technologies used in, or designed for, reduced gravity are limited and much work remains unpublished. In addition to the above flow cytometers, other space-relevant IVD-technologies described in the literature include a whole blood staining device for immunophenotyping applications 16, an automated camera-based cytometer 12, a handheld clinical analyzer for integrated potentiometry, amperometry, and conductometry 12,17, a microfluidic ‘T-sensor’ device for analyte quantitation that relies on diffusion-based mixing and separation 18, and a rotating ‘lab on a CD’ diagnostics platform 19,20. Newcomers to reduced gravity testing may also look to parabolic flight demonstrations unrelated to in vitro diagnostics when attempting to make device evaluation possible (or figuring out what is possible). Demonstrations from other previous medical or biological experimentation with well-documented flight preparation, in-flight strategies, and flight test equipment are included in Table 115, 21-35. These may be informative due to inclusion of manual in-flight tasks, use of specialized equipment, and experimental containment.
| Category | Examples |
| Emergency medical care | Tracheal intubation (laryngoscope-guided, on manikin) 21, cardiac life support (anesthetized pigs) 22 |
| Surgical care | Laparoscopic surgery (video simulated 23, on anesthetized pigs 24,25) |
| Medical imaging or physiology assessment | Ultrasound with lower body negative pressure chamber 26, Doppler flowmeter (head mounted) 27, central venous pressure monitor 28 |
| Specialized biological equipment | Microplate reader (and in-flight glove box) 29, temperature control system for cell cycle experiments 30, microscope (brightfield, phase contrast, and multi-channel fluorescence capable) 15, capillary electrophoresis unit coupled to video microscope 31 |
| Other | Plant harvesting with forceps 32, contained rats 33,34 and fish 35 for observation |
Table 1. Parabolic Flight Demonstration Examples with Well Described Methods/Experiments
To expand on previous examples and provide greater insight into successful in-flight demonstrations, we are presenting a modular and adaptable procedure for construction and operation of a prototype flow cytometer with related microfluidic mixing technology as part of a parabolic flight test rig. The rig enables demonstrations of sample loading, microfluidic mixing, and fluorescent particle detection, and was tested onboard the 2010 NASA Facilitated Access to the Space Environment (FAST) parabolic flights, flown from September 29 to October 1, 2010. These demonstrations pull from the beginning, middle, and end, respectively of a potential device workflow in which fingerstick-sized blood samples are loaded, diluted or mixed with reagents, and analyzed via optical detection. Scaling a flow cytometer into a compact unit requires innovation and careful part selection. Custom and off-the-shelf components are used here, chosen as best early approximations of final component choices, and may be adaptable to the designs of other innovators. Following an outline of prototype component choices, setup is described on a support structure serving as a skeleton for rig assembly. Prototype components are assigned locations, secured, and accompanied by additional components necessary for successful experimentation. Attention then shifts to more abstract procedures involving standard operating procedure (SOP) development, training, and other logistics. Finally, demonstration-specific procedures are described. The strategies described here and the choices of supporting rig components (e.g., microscope, acrylic box, etc.), although implemented here for specific prototype, speak to the general issues and challenges relevant to testing any blood diagnostic equipment in a reduced-gravity environment.
In the 2010 flights, two lunar-gravity (achieving approximately 1/6 earth gravity)and two micro-gravity flights were scheduled across 4 days, although ultimately these were rescheduled across 3 days. Demonstrations were performed onboard a modified privately operated, narrow-body jet airliner 36. Each flight provided 30-40 parabolas, each yielding about 20 sec of high-gravitation (roughly 1.8 g) followed by 20-25 sec of reduced-gravity conditions. After half of the parabolas were executed, the plane paused for a period of about 5-10 min in level flight to enable the plane to turn around and head back toward the landing site while performing the remainder of the parabolas.
Protocol
The human blood samples used in this protocol were collected with IRB approval using minimally invasive protocols (see Acknowledgements).
1. Rig Assembly
- Assemble prototype components (fluidics, optical, control/data acquisition electronics) for a simple flow cytometry system to be used in reduced gravity conditions
- Prepare a pressure system with minimal weight and power needs to drive system fluidics
- Connect a miniaturized air pump to a differential pressure sensor.
- To maintain a constant driving pressure, control pump output using pulse-width-modulation and a duty cycle regulated using a proportional–integral–derivative controller in custom control software (step 1.1.7).
- Assemble a fluid source container that can be loaded without trapping air (see step 3.4)
- Fit a rigid plastic vial (Figure 1A) with a latex diaphragm, firmly securable cap, and inlet air tubing at the vial base (sealed connection using optical adhesive).
- Ensure that the pump pressurizes the vial without air or fluid leaks, compressing the diaphragm to drive fluid flow out of the cap exit tubing.
- Design a fluid waste container to collect waste without building a backpressure that will compromise flow
- Use a vial-glued-within-a-vial design (Figure 1B) for double containment.
- Cap the vials with a secured foam sponge window that traps floating waste but allows air pressure equalization with the cabin environment.
- Make a sample loader for use in reduced gravity
- Machine and assemble a spring-loaded clamp design with guiderails (Figure 1C) such that it reliably clamps a sheath-fitted capillary between two O-rings in the fluid line. Ensure it preserves sample volume when loading, accommodates system priming when a sample is not inserted, and avoids errant bubble introduction.
- Ensure that in the absence of a capillary, the springs press the O-rings together to complete the fluid line and enable priming without leaking (Figure 1D, left).
- Design a micromixer that does not rely on powered mechanical subcomponents to function
- Conceive a two-inlet spiral-vortex micromixer (Figure 1E) that achieves chaotic advection necessary to overcome laminar flow within the microfluidic channels. This design delivers all entering fluid downstream so that one sample run does not affect the next.
- For convenience, fabricate chosen design using the rapid-prototype polydimethylsiloxane (PDMS) method (Figure 1F). Utilize a two-dimensional computer-aided designed photomask printed at 20,000 dpi to fabricate the necessary SU-8 mold in a cleanroom facility 37.
NOTE: Use a modified 23 gauge fit to a vertical drilling mill to drill holes at the inlets, vortex, detection inlet, and detection outlet spots, and a hand magnifier to help aim the needle. Cut out the chips from the PDMS using a razorblade and fit the holes with 0.5” hollow steel pins sticking out of the non-molded back side of the chip. Connect the central spiral exit pin to the detection channel entrance pin using microbore tubing. - Thoroughly clean chip with ethanol and dry molded surface with matte scotch tape. Use an empty syringe to blow ethanol out of the pins. Treat PDMS chip and a pristine cover glass inside plasma cleaner and bond them within 10 sec by applying light pressure, checking immediately by light microscopy that the chip is fully pressed without compromising channel patency.
- Mount a palm-sized miniature optical block to detect individual flowing particles
- The design in Figure 2AB is suitable for two-color epifluorescence laser illumination and detection, and utilizes a PDMS straight-channel (120 by 200 µm) flow cell for convenience.
- Mount block (Figure 2C) using commercially available optomechanical components and align fiber-coupled single photon counting modules.
- Design electronics and software for device control and data acquisition
- For convenience in early prototyping, utilize hand-soldered pieces connected to data acquisition (DAQ) cards (Figure 2D).
- Code and program a custom software (example in Figure 2E) to operate rig devices and synchronize all data.
- Prepare a pressure system with minimal weight and power needs to drive system fluidics
- Additional components (not formally part of prototype)
- Incorporate a 3-dimensional accelerometer (Figure 2D, left) and a flow rate meter (not pictured). An accelerometer is present onboard the aircraft but (likely) cannot be directly synced to other recorded data.
- Electrical power scheme
- A mechanism for quick and complete electronics shutdown (required for safety reasons on reduced-gravity flights)
- Connect a single power strip (with single I/O button) to the aircraft power distribution panel (120 VAC 60 Hz).
- Remove laptop battery and set laptop to operate through power cable alone.
- Power for all devices
- Directly power the laptop (battery removed), a light microscope, and two photon detectors using power strip.
- Power remaining devices via USB DAQ cards connected to the laptop or using batteries.
- A mechanism for quick and complete electronics shutdown (required for safety reasons on reduced-gravity flights)
- Flight-ready rig layout
- Considerations for successful in-flight performance
- Total space available is limited to a smaller area than provided for a similar demonstration on the ground (Figure 3A). Consider total space available and how that space will be divided between experimental rig space (including for components beyond those formally part of the prototype) and user space surrounding the rig. Experimental rigs vary in terms of forward or aft positioning, but this largely does not affect available operational space (or in-flight physics).
- Determine which components are more appropriately accessed at a standing, kneeling, or floor height, as well as considering which components will benefit most from the protection attained within a support structure.
- Rig support structure
- Obtain or construct a vertical equipment rack that meets considered layout needs, contains all components, enables different vertical levels for organization, withstands flight accelerations, and securely attaches to the intended aircraft cabin floor.
- Assign components to levels within the equipment rack (Figure 3B): a top level to place the laptop, a mid-rack level to contain prototype subcomponents and a floor level to contain extra wipes, gloves, and a miscellaneous waste container.
- Conceive additional structures within the rack to accommodate different desired levels. Implement support beams at ‘mid’-height to hold a 2 ft. by 2 ft. microscope breadboard plate for screwing down rig components, and support beams approximately 2 feet higher to support a flight-approved laptop trough.
- Within vertical levels, determine optimal component arrangement, taking into account accessibility limitations incurred due to the presence of other components as well as due to the potential position/orientation of the rig itself onboard a flight (e.g., 4th side of a square rig may be close to aircraft wall, leaving only 3 sides accessible).
NOTE: The leg straps to secure test operators are at a fixed distance from the rig and may not be available on all sides. - Based on these determinations, divide the breadboard plate into 4 quadrants (Figure 3C), placing dedicated locations for electronics and optical block toward the aircraft wall, and the sample loader and microfluidic chip toward the cabin space.
- Considerations for successful in-flight performance
- Prototype securing, containment, and visualization setup
- System electronics
- Design, laser-cut, and assemble a custom acrylic box (Figure 2D) to contain the DAQ cards (strapped down) and hand-soldered boards (screwed to box wall).
- Utilize a swinging door for easy access (secured in-flight with fabric hook-and-loop fastener) and exit holes for USB cables and wires.
- Sample loader
- Fabricate a custom acrylic ‘glove’ box (Figure 4A) with arm access holes to provide a cubic space in which to perform the loader demonstration (Figure 4C) without risking contamination of the flight cabin.
- Feed tubing to and from the loader through small circular holes in the side of the box.
- Micromixer
- Adapt equipment used on the ground. Bolt a stereomicroscope (Figure 4B) to the breadboard plate and fit it with a custom acrylic chip holder, also bolted to the plate.
- Fit a USB CCD camera to the microscope eyepiece and connect it to the laptop (Figure 4D) to save video synchronized with other data (gravity, driving pressure, and flow rate).
- Optical block
- Fabricate a custom opaque acrylic box (Figure 4A, right) to cover the block, shielding it from ambient light and controlling laser hazards.
- Utilize an optical filter ‘window’ to safely check laser function.
- Laptop
- Bolt a flight-approved laptop tray to the support beams within the support structure.
- Use hook-and-loop fastener to secure USB cables along rack architecture.
- System electronics
- In-flight demonstration implementation
- Simple interventions to proceed through demonstrations
- Incorporate additional components that eliminate required manual tubing adjustments in-flight or other actions that require significant dexterity or could risk leaking fluids into the cabin environment.
- Custom-machine and integrate a pressure manifold (Figure 5A) consisting of an aluminum cylinder drilled and tapped to fit a screw-on needle luer adaptor serving as a pressure inlet. Drill smaller holes around the circumference to fit O-rings and microbore tubing as outlets. Use to pressure multiple source vials simultaneously.
- Assemble a panel of three-way solenoid valves (Figure 5B) controlled by tandem MOSFET switches (Figure 5C) wired to a DAQ card. Adapt microbore tubing to fit valve ports. Use to control fluid flow from the different vials.
- Program software to proceed through demonstrations (Figure 6) using single-button interventions (e.g., single click on the laptop).
- Incorporate additional components that eliminate required manual tubing adjustments in-flight or other actions that require significant dexterity or could risk leaking fluids into the cabin environment.
- Backup manual control
- Add slide clamps to rig to enable some manual control over the fluidics, perhaps if tubing unexpectedly needs to be disconnected and reconnected during flight.
- Include sufficient cleanup wipes in the floor rack section in case of leaks in flight.
- Simple interventions to proceed through demonstrations
- Flight disturbance readiness: Ready system for possible sudden jolting forces, vibration, or passenger collision in flight.
- Alignment stabilization
- Apply quick-drying epoxy to aligned components that are easily misadjusted, particularly optical components.
- Apply industrial grade epoxy over the quick-dry epoxy as well as to secure other components as necessary, including the CCD camera attachment to the microscope eyepiece.
- Physical disturbance testing
- Shake rig support structure with all components in place.
- Check individual component functionality after subjecting the rig to the disturbance, particularly aligned optical components.
- Passenger risk management
- Apply foam padding to areas (corners, edges) of the vertical equipment rack structure that could harm a flight passenger that accidentally knocks into the rig (Figure 4C).
- Secure padding with black duct tape.
- Alignment stabilization
2. Demonstration Preparation and Logistics
- In-flight and ground team role assignments
- Assign rig operator(s) to perform both rig setup and all hands-on operations in-flight. Hands-on operators can best visualize when rig setup is complete.
- Assign an SOP reader to read the SOP aloud during training and in-flight. The process of SOP reading during training may identify awkward or ill-timed staging.
- Assign ground support to perform sample preparation and any other preparation tasks not directly involving the rig, minimizing time burdens on rig operators.
- Initial standard operating procedure (SOP) development
- Write all steps to incorporate pre-flight (day before and morning before), in-flight, and post-flight procedures utilizing only equipment and materials that will be available at flight location. A 5 to 10 min block of level plane flight may be available for last minute setup procedures before parabolas begin or at the halfway point as the plane turns around.
- Assign in-flight experimental procedures to dedicated numbers of parabolas, noting that the parabolas will likely be separated partway through to allow the plane to turn around and head back to the landing site, and that another group may request the plane to level out mid-experiment or fewer parabolas may be flown than expected.
- Conceive demonstration procedures to minimize biological hazard risk beyond effective containment, avoiding actual biological specimens when possible. Utilize blue food dye spiked with fluorescent counting beads (Figure 1D) as an alternative to blood during the sample loader demonstration.
- Demonstration training
- Set a training schedule sufficient to fully revise and refine the SOP, as well as generate thorough ground control data to compare with flight data.
- After performing pre-flight SOP, ‘lock’ the rig into a room to simulate the in-flight experience, cutting access to tools or ground materials. For even stricter training, mark off a section of the floor meeting the allocated dimensions that will be available in-flight 32.
- During training, follow SOP exactly, and use a stopwatch to announce 20 to 30 sec parabolas, indicating entrance and exit of reduced gravity, as well as a mid-flight parabola break.
- Incorporate finalized SOPs into actual flight day schedules, dividing ‘pre-flight’ activities between day-of-flight and day-before-flight.
- Train for unexpected in-flight occurrences including sudden forces hitting the rig or the plane suddenly leveling out in the middle of an experiment.
- Test stabilities of samples and reagents when subjected to an extended break (hr or more) between pre-flight procedures and in-flight activity. Note also that temperatures may be significantly higher at flight location.
- Train multiple individuals as primary operators to expertly operate the device in-flight. It is unpredictable who will get sick during the parabolas, and a given user may be unaffected on one flight and become sick on another.
- Ground equipment and supporting materials
- Assemble a toolbox to include backup components and equipment necessary for repairs, including hand tools, soldering equipment, and glue/epoxy among many other items.
- Gather sample and reagent quantities beyond what is intended for use during the scheduled flights in case unexpected flight postponement occurs after a sample or reagent has already been readied for flight.
- Shipping
- Setup shipment necessary to transport the rig, ground equipment (tools, centrifuge, pipets, vortex mixer, others) and perishables (blood cells, reagents). Ensure adequate time to receive, inspect, assemble, and test hardware for the flight campaign.
- Encase rig on all sides except bottom using bubble wrap. Ship rig using a custom wooden crate box, fitted internally with foam pads and shock material.
- Ship supporting ground equipment/tools in a rigid container or chest.
- Ship perishables in 1 in. thick insulated foam box, containing dry ice for items requiring -20 °C storage and freezer cool pack for items requiring 4 °C storage.
- Pre-flight testing
Perform pre-flight testing at the flight location to check functionality of all components several days before the flights.
Flight rigs are weighed and crane loaded onto the aircraft, and likely remain on the aircraft for the duration of the flight week.
3. In-flight Demonstrations
Demonstrations/experiments are divided between two day designations (“Day A” and “Day B” below). Day A is designated for the micromixing demonstration and Day B is designated for the particle detection and sample loading demonstrations.
- Ground sample preparation for micromixer demonstrations (Day A only)
- Dilute 3 ml blue food dye into 12 ml 1x phosphate buffered saline (PBS).
- Dilute 3 ml yellow food dye into 12 ml 1x PBS.
- Strain 15 ml of commercially purified red blood cells.
CAUTION: Because no testing methods can guarantee with 100% certainty the absence of an infectious agent, human derived products should always be handled as biological hazards. - Load sample vials (See step 3.3) for each sample, plus an additional vial containing only saline.
- Ground sample preparation for optical block demonstration
- Combine 60 µl fluorescent counting beads with 14 ml 1x PBS (4.3 beads/µl) with 1% Tween. Load into sample vial.
CAUTION: Handle all chemicals with caution and using personal protective equipment (PPE). - Dilute a 50 µl finger stick whole blood sample 100-fold with 1x PBS and add SYTO 83 dye for [Final] = 5 µM. Lightly vortex to mix. Incubate for >5 min at room temperature.
CAUTION: SYTO 83 dye is dissolved in dimethylsulfoxide (DMSO), which is readily absorbed through the skin. May be irritating to eyes, respiratory system and skin. Handle using PPE. - Centrifuge cell sample (at 2,300 x g for 4 min), pipet off supernatant.
- Wash stained cell sample by adding 1 ml 1x PBS, centrifuging at 2,300 x g for 4 min pipetting off supernatant. Repeat two more times.
- Return volume to 15 ml with 1x PBS for to reach a final 1:500-fold dilution of original commercial stock. Strain cells and load into sample vial.
- Combine 60 µl fluorescent counting beads with 14 ml 1x PBS (4.3 beads/µl) with 1% Tween. Load into sample vial.
- Ground sample preparation for sample loader demonstration (Day B only)
- Prepare capillary consumables for sample loader demonstration by cutting micro-hematocrit capillary tubes into 15 mm segments with a razor blade.
- Prepare sample for loader demonstration: Mix 250 µl stock fluorescent beads with 250 µl undiluted blue food dye (500 beads/µl). Draw 250 µl sample into two 1 ml syringes, each fitted with a blunt tip needle that is taped shut with electrical tape.
- Load fluid source vials
- Apply fresh, powder-free latex diaphragm to vial (cut finger from glove acceptable). Make sure the diaphragm is long enough to extend from the vial floor and fold over the top outer rim. Slide the vial ring over the folded portion.
- Place a temporary slide clamp onto cap outlet tubing that will prevent fluid expulsion during cap insertion.
- Before filling the vial, negatively pressurize the vial with a syringe to expand the diaphragm. Pour fluid to top of vial and insert the cap at an angle such that no air is trapped under the cap during cap placement (some fluid will spill out). Briefly remove slide clamp to prime the outlet tubing and release collapsing pressure exerted by the diaphragm.
- Prepare rig demonstrations
- Connect and check all tubing connections
- Hook source vials into system. Fit vials into a custom acrylic vial holder and secure them with and hook-and-loop fastener.
- Empty any contained waste in vials or bins.
- Check hard drive space and startup custom demonstration software.
- Perform system fluidics priming procedure specific to each demonstration.
- Swap in new batteries to any battery-powered device (e.g., accelerometer).
- Manually shake fluorescent particle samples.
- Run brief pre-flight test experiment.
- Avoid in-flight motion sickness
- Take provided medications (scopolamine and dextroamphetamine, both safe and effective for preventing motion sickness in-flight)
- Heed recommended body positioning strategies in-flight (e.g., lie flat on back during increased gravity, with body straight and head cocked forward, and allow body to float up on its own during transition to reduced gravity). If possible, use several early parabolas to adjust to the gravity changes.
- Retain a plastic vomit bag easily accessible in a front pocket. Vomiting can occur suddenly and without preceding nausea.
- Position rig operators once in-flight, nearing dedicated parabola airspace. Provide enough space to allow rig operators to lie down during high-gravitation intervals and enable access to leg straps. Once parabolas begin, do not apply strong forces on body during reduced gravity as this may send the body up too quickly and somewhat dangerously.
- Perform microfluidic mixer demonstration (Day A only)
- Manually shake blood vial before test run.
- Mix blood and saline in a 1:1 ratio at 1.5, 2, 3, 4, 5, and 6 psi, for at least 2 parabolas each, recording video data synchronized to other readings.
- Inject air into saline inlet to test whether channel architecture will trap a bubble that could prevent optimal mixing.
- Mix blue and yellow food dyes at 1.5, 2, 3, 4, 5, and 6 psi for at least 2 parabolas each, again recording synchronized data.
- Apply slide clamps to system fluidics when finished to prevent further waste production.
- Check data integrity before shutting down electronics in case demo repeat is required.
- Perform optical block and sample loader demonstrations (Day B only)
- Manually shake samples before running.
- Drive fluorescent counting beads through the optical block for 3 parabolas. Flush system with saline for at least 1 parabola between sample types.
- Repeat 3.9.2 for the fluorescent hydrogel particles and WBCs.
- Check data for any missing entities that need to be repeated before moving on to sample loader demonstration.
- Begin recording sample loader demonstration using HD video recorder.
- When the plane enters reduced gravity, use a sample syringe to place a drop of the counting bead dye mixture on a fingertip to simulate a finger prick sample. Use an unrealistically large drop (Figure 1D) to test the limits of keeping a finger prick sample on a finger during reduced gravity.
- Use capillary consumable to pick up sample (about 10 µl) off finger and load into capillary loader.
- Wipe remaining sample off finger using wipes included in box.
- Drive sample into optical system for detection.
- Repeat tests several times using different operators.
- Check data for any missing entities that need to be repeated before shutting down electronics.
- Post-flight shutdown
- Empty and dispose waste properly using biohazard labeled containment receptacles as necessary. Hazardous waste may require shipment out of the aircraft facility.
- Thoroughly flush system, using a 5 ml syringe loaded with water to provide forceful cleaning. Flush valves backwards and forwards through all 3 ports.
- Wipe down any mess using alcohol wipes.
- Reprime system for next demonstration.
Results
Representative results for the micromixer demonstration appear in Figure 7, as viewed by the CCD camera fitted to the stereomicroscope. Mixing can be visually assessed at any point along the spiral, as well as in the Exit channel for experiments involving two sets of fluids: blood/saline and blue/yellow dye. Quantitative analysis of the two-dimensional images can include determination of shade uniformity across the channel width in different regions, as shown in other publications 38-40. See Supplementary Figure 1 for further details. See Supplementary Figure 2 for demonstration of bubble handling by the microfluidic chip.
Results for particle detection in the optical block and sample loader demonstrations appear in Figure 7C and D, respectively. Optical block detection of fluorescently labeled white blood cells (Figure 7C) appears relatively unperturbed by a transition from approximately 1.5 g to nearly zero-g, and continues during the transition back to 1.5 g. The sample loader data demonstrates that a sample was successfully loaded (here under lunar gravity conditions) and reached the optical block for detection (Figure 7D). Quantitative analysis of the data reading utilizes a custom peak counting algorithm to compare counts and signal-to-noise ratio in reduced versus normal and high gravity conditions. See Supplementary Figure 3 for extended traces and example analysis.

Figure 1: Fluidics Subcomponents. (A) The candidate source vial uses a custom-machined aluminum cap fitted with two O-rings along its inserted portion. The cap screws down to the vial ‘ring,’ holding the cap firmly against the upper vial rim. (B) The candidate waste vial cap allows air but not fluid to pass through the cut opening in the top. (C) The candidate sample loader comprises individually machined head, center, and foot pieces, fit to two guiderails. Guiderail spacing facilitates capillary positioning. (D) A collected sample drop from a finger tip is loaded into the fluid line. (E) The candidate spiral-vortex micromixer mixes two solutions through a 3-rotation (‘1’, ‘2’, ‘3’) spiral (inner radii from 1.9 to 0.9 mm) and vortex drain (‘V’, diameter 320 µm). Fluid then passes via microbore tubing to an exit channel (‘E’). Channels are 200 µm wide by 120 µm high. The height of the vortex drain (V) is 1-2 mm before meeting pin. (F) Chip footprint is comparatively smaller than a dime.

Figure 2: Optical and Electronic Subcomponents. (A) Candidate optical block component design includes two lasers (‘Green’ and ‘Red’) plus several beamsplitters (‘BS’), lenses, and photon detectors (‘PD’). (B) A solid modeled design (inset) is machined, anodized, and assembled. Stage (S), flow cell placement site (blue arrow), red laser (red arrow) are labeled. (C) For in-flight testing, the block is fixed using clamps and alignment fixtures, which also hold fiber optics feeding to photon counting modules. (D) Large DAQ boards and hand-soldered electronics are practical solutions before control/acquisition electronics can be reduced to microelectronic equivalents. The optical block (covered in a custom black acrylic box, unlabeled to the left) is visible in the photograph with an accelerometer (‘Acc.’) fixed on top. (E) Example custom software for the micromixer demonstration enables simultaneous device control, readouts, and data storage.

Figure 3: Test Rig Layout. (A) Flight environment may be crowded depending on how many groups are simultaneously running experiments in-flight. (B) Rig components are assembled on a vertical equipment rack divided between 3 levels. Leg straps (red and yellow) are visible in an arc around the rack. (C) The microscope breadboard plate is divided into 4 quadrants for demonstrations and placement of the electronics box.

Figure 4: Containment and Visualization. (A) A custom-fabricated acrylic ‘glove’ box enables the sample loader demonstration in-flight. Inner bins hold samples, capillaries, and waste. (B) A stereomicroscope fitted with a custom-fabricated microfluidic chip holder enables in-flight visualization of the micromixer demonstration. The microscope is modified with an extended neck to make space for the chip holder, which holds two chips simultaneously that can be quickly flipped between using a chip tray fitted with magnets to hold it in one of two positions. (C) A rig operator performs the sample loader demonstration while kneeling in-flight. A second operator operates a video camera to his left. (D) The micromixer is visible on the laptop.
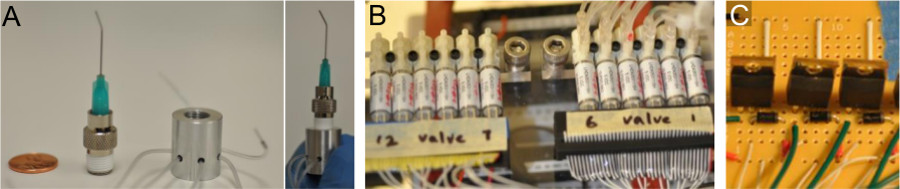
Figure 5: Additional Components to Enable Demonstrations to Operate Via Simple Interventions. (A) The air pressure splitter consists of a partially hollowed and tapped cylinder to which a needle is adapted. Pressure outlets can be selectively clamped to reduce number of outlet ports. (B) The panel of 12 three-way solenoid valves is controlled through the tandem MOSFET circuit in (C).

Figure 6: In-Flight Demonstrations. The three-way solenoid valves have a common port (white arrow tip) that is always connected to either the default OFF port (red) or ON port (green). The switch to ON state is triggered with a 5-volt I/O signal. (A) The sample loader demonstration includes loading a sample and driving the sample to the optical block (OB) for detection. The setup utilizes two valves, one before and one after the loader. During loading, both valves are set to OFF, preventing fluid movement as the loader is utilized. Turning the valves ON opens the fluidics pathway extending from the saline (S) vial to the waste (W) vial, allowing the pump to drive the sample for analysis. (B) The transition from ‘manual’ to ‘1-button’ interventions in the optical block demonstration allows sequential testing of three different sample types — fluorescent counting beads (CB), a proprietary fluorescent hydrogel microparticle (NS), and fluorescently labeled WBCs — without a need to reconfigure tubing connections. Saline is able to flush the system between samples. Spl. = Air pressure splitter.

Figure 7: Representative Results. (A) Blue-yellow dye mixing under micro gravity conditions. (B) Blood-saline mixing under lunar gravity conditions. (C) WBC detection during microgravity flight. Critical performance metrics for the flow cytometry data include the coefficient of variation of the peak intensities, signal-to-noise ratios, peak counting rates, and detection efficiency. (D) Fluorescent counting beads spiked into a loaded sample are detected following demonstration of the loader in lunar gravity.
Supplementary Figure 1: Mixing analysis (blood-saline). (A) Mixing images are converted to grayscale and analyzed in the designated regions (inlet, spirals 1-3, and exit) per the equation σ = <(I - <I>)2>1/2, where σ reflects the degree of mixing, I = grayscale intensity between 0 and 1, and < > is the average across the sample. This method reflects similar determinations in published literature 38-40. For a completely mixed sample, σ equals zero. For an unmixed sample, σ equals 0.4 to 0.5. In practice, complete mixing when the sigma value is less than 0.1. This method, although sufficient for demonstration purposes, is limited because mixing is a 3-dimensional process and therefore requires 3-dimensional assessment (through confocal microscopy or other means) to fully describe the degree of mixing. (B) Blood-saline mixing results obtained in flight are displayed under different gravity conditions. The ‘high’ gravity graph was obtained during a micro gravity flight. Pump driving pressure setting increases from left to right in each graph.
Supplementary Figure 2: Demonstration of bubble handling. Two bubbles, one injected in high gravity and one injected in micro-gravity, are traced over time via video observation. Each bubble effectively clears the microfluidic chip. The performance contrasts with that of other ground-tested mixing geometries with a greater tendency to trap bubbles (data not shown). White arrows indicate air moving through the chip, which is difficult to distinguish from saline in the static images.
Supplementary Figure 3: Extended flow cytometry traces. Fluorescent counting bead (A) and white blood cell (B) detection traces recorded over 3 parabolas are shown. Detection rates (peaks/second) are displayed (white text) during high and low gravity periods as determined via custom software. Other critical metrics (e.g., coefficient of variation of peak intensity, signal-to-noise ratio) can be measured for insight into the effects of gravity on the fluidics and optical detection architecture.
Discussion
The method described here enabled effective demonstration of the major technology components (sample loading, microfluidic mixing, and optical detection) during the 2010 FAST parabolic flights, with comparable results to ground testing. Training and SOP methods described here were particularly effective, and helped to illuminate tools and other ‘crutches’ being relied on for practice demonstrations that would not be available onboard the parabolic flight.
Areas for improvement include containment and layout. Custom acrylic components may not be sufficiently robust for containment purposes. The ‘glove’ box was struck by a passenger in-flight during a gravity transition and subsequently fell apart during a rough plane landing. Tubing connected to the microfluidic chip became unhooked during a blue-yellow dye mixing demonstration, briefly leaking food dye into the cabin environment. This needed to be fixed during a high-g interval, which was particularly difficult because reconnecting microbore tubing requires dexterity and user stability. In terms of layout, placement of the laptop at standing height made it difficult to operate during the high-g intervals. Users may become light-headed when attempting to stand during the high-g phases. A mid-level computer could be a better alternative, but here would have required displacement of prototype subcomponents. Other researchers have included seating in their parabolic flight setups for stabilization of test operators 26, although this requires additional space, which is scarce on parabolic flights.
In addition to providing a greater level of detail regarding preparation and setup compared to previous demonstrations of parabolic flight flow cytometry, this work describes inclusion of potentially significant ‘companion’ technology (i.e., the microfluidic chip for reagent mixing and sample dilution) alongside the cytometer. Sample pre-processing (e.g., fluorescent staining, mixing, incubation), as performed on the ground, may be difficult or hazardous in space, in turn making companion technologies, such as a mixing chip, necessary to achieve the same functions in reduced gravity. In contrast to the present work, previous demonstrations of potentially space-worthy flow cytometers have focused almost entirely on cytometry performance (using samples pre-processed on earth) and without indicated strategies to bridge the gaps in sample pre-processing. The described ‘fiber-optic-based’ flow cytometer, for example, used ground-loaded sample cartridges for immunophenotyping and microbead-based cytokine assays and it is not obvious how the system could be adapted for actual in-flight diagnostics. Some efforts have partially addressed the issue, including development of the whole blood staining device which has seen recent improvements 41. The NASA-tested flow cytometer utilized a pre-staining method potentially usable with the whole blood staining device 5. Still, efforts to develop necessary space-ready companion technology seem to lag sufficiently behind those to develop flow cytometers to keep flow cytometry impractical for diagnostic purposes in space and other resource-limited environments in the near future. More generally, developers of any IVDs for outer space need to consider full workflow adaptation for their technology and should always consider testing of potentially necessary companion technology to take full advantage of limited reduced-gravity flight opportunities.
The described prototype flow cytometer is a starting point for a more sophisticated design, utilizing more advanced fluidics, optics, and electronics. Hydrodynamic flow focusing and additional detection channels (e.g., light scatter, absorption) would improve particle discrimination for applications such as white blood cell differential. Some components will need to be replaced simply because they are convenient in rig-based designs but would be impractical in actual handheld devices (e.g., waste vial, control/acquisition electronics). More advanced electronics would include microelectronics operated using a miniature screen interface and embedded microprocessors to eliminate the laptop and associated DAQ cards.
Disclosures
Eugene Y. Chan, Candice Bae, and Julia Z. Sharpe are inventors on related technology patents filed through the DNA Medicine Institute, a commercial entity.
Acknowledgements
Hardware development was supported by the NASA SBIR Contracts NNX09CA44C and NNX10CA97C. Data analysis for the optical block and sample loader demonstrations was supported by NASA Phase III Contract NNC11CA04C. The human blood collection was performed using NASA IRB Protocol # SA-10-008. Control/acquisition software provided through the National Instruments Medical Device Grant Program. Molds for the microchips were made at the Johns Hopkins microfabrication facility and the Harvard Center for Nanoscale Systems. Otto J. Briner and Luke Jaffe (DNA Medicine Institute) aided in rack assembly during summer 2010. NASA flight video staff provided video footage during flight week. Carlos Barrientos (DNA Medicine Institute) provided photograph and figure assistance. Special thanks to the Facilitated Access to the Space Environment for Technology 2010 Program, the NASA Reduced Gravity Office, the Human Adaptation and Countermeasures Division, NASA Glenn Research Center, ZIN Technologies, and the Human Research Program.
Materials
| Name | Company | Catalog Number | Comments |
| Micro air pump | Smart Products, Inc. | AP-2P02A | Max pressure = 6.76 psi; 1.301” x 0.394” x 0.650”, 0.28 oz (8 g); available direct from Smart Products |
| Differential pressure sensor | Honeywell International, Inc. | ASDX015D44R | Range of 0-15 psi; 0.974" x 0.550" x 0.440", 0.09 oz (2.565 g); suppliers include Digi-Key and Mouser Electronics |
| Rigid plastic vial (small size) | Loritz & Associates, Inc. | 55-05 | Polystyrene; ID 0.81" (20.6 mm), IH 2.06" (52.4 mm); available direct from LA Container Inc.; similar product available from Dynalab Corp. |
| latex examination gloves | dynarex corporation | 2337 | Middle finger used for latex diaphragm in fluid source vial. Other brands (e.g., Aurelia ® Vibrant ™) acceptable. |
| Optical glue | Norland Products | NOA 88 | Low outgassing adhesive; available direct from Norland; Also available from Edmund Optics Inc. |
| 3-way solenoid valves | The LEE Company | LHDA0531115H | Gas valves, but can function with liquid; 1.29" L, 0.28" D. Discontinued product. Similar products available from The LEE Company. |
| Volumetric water flowmeter | OMEGA Engineering inc. | FLR-1602A | Non-contacting flow rate meter strongly preferred. We recommend SENSIRION LG16 OEM Liquid Flow Sensor for flow rates from nl/min up to 5 ml/min. |
| PCD-mini photon detector | Sensl | PCDMini-00100 | For fluorescence detection; available direct from Sensl |
| Accelerometer | Crossbow Technology, Inc. | CXL02LF3 | 3-demensional force detection. Supplied to DMI by NASA. Similar product available from Vernier Software & Technology, LLC. |
| Stereomicroscope | AmScope | SE305R-AZ-E | |
| CCD Camera | Thorlabs | DCU223C | 1,024 x 768 Resolution, Color, USB 2.0; available direct from Thorlabs |
| USB and Trigger Cable (In/Out) for CCD Camera | Thorlabs | CAB-DCU-T1 | Available direct from Thorlabs |
| Microbore tubing | Saint-Gobain Corporation | AAD04103 | Tygon®; ID 0.02", OD 0.06", 500 ft, 0.02" wall. Suppliers: VWR, Thermo Fisher Scientific Inc. |
| Hollow steel pins | New England Small Tube | (Custom) | 0.025" OD, 0.017" ID, 0.500” L, stainless steel tube, type 304, cut, deburred, passivated; enable microbore tubing connections, chip tubing connections |
| Slide clamp | World Precision Instruments, Inc. | 14042 | Available direct from World Precision Instruments |
| Leur adaptor pieces | World Precision Instruments, Inc. | 14011 | Available direct from World Precision Instruments |
| Silicon wafer | Addison Engineering, Inc. | 6" diameter; for SU-8 mold fabrication | |
| Polydimethylsiloxane (PDMS) elastomer curing agent | Dow Corning | 3097358-1004 | Supplier: Global Industrial SLP, LLC |
| Needle (23 gauge), bevel tip | Terumo Medical Corporation | NN-2338R | Ultra thin wall; 23 G x 1.5"; 22 G also usable; suppliers: Careforde, Inc., Port City Medical |
| Dispensing needle (23 gauge), blunt tip | CML Supply | 901-23-100 | 23 G x 1"; available from CML Supply |
| Cover glass | Thermo Fisher Scientific, Inc. | 12-518-105E | Gold Seal™ noncorrosive borosilicate glass; for PDMS chip cover; 24 x 60 mm; available from Thermo Fisher Scientific, Inc. |
| Vacuum pump | Mountain | MTN8407 | For degassing PDMS; supplier: Ryder System, Inc. |
| Vacuum chamber | Thermo Fisher Scientific, Inc. | 5311-0250 | Nalgene™ Transparent Polycarbonate; available from Thermo Fisher Scientific, Inc. |
| Plasma cleaner | Harrick Plasma | PDC-32G | |
| Hand magnifier | Mitutoyo | 183-131 | Use in reverse direction to enable viewing at ~15". |
| Ethanol | CAROLINA | 861283 | For chip cleaning. Dilute to 70% using millipore water. |
| Water purification system | Thermo Fisher Scientific, Inc. | D11901 | Available direct from Thermo Fisher Scientific, Inc. |
| Optomechanical translation mounts | Thorlabs | K6X | 6-Axis Kinematic Optic Mount; discontinued product; new product (K6XS) available direct from Thorlabs |
| Laptop | Hewlett-Packard | VP209AV | HP Pavilion Laptop running Windows 7 |
| Laptop tray (spring loaded) | National Products, INC. | RAM-234-3 | RAM Tough-Tray™. Can accommodate 10 to 16 inch wide laptops. |
| USB splitter | Connectland Technology Limited | 3401167 | |
| USB Data Acquisition Cards (8 analog input, 12 digital I/O) | National Instruments | NI USB-6008 | 12-Bit, 10 kS/s Low-Cost Multifunction DAQ |
| USB Data Acquisition Cards (16 analog input, 32 digital I/O) | National Instruments | NI USB-6216 | 16-Bit, 400 kS/s Isolated M Series MIO DAQ, Bus-Powered |
| Control/acquisition Software | National Instruments | LabVIEW 2009 | Custom coded National Instruments (NI) LabVIEW |
| 3D Solid Modeling Software | Dassault Systèmes SolidWorks Corp. | SolidWorks 2011 | |
| 2D Modeling Software | AUTODESK | AutoCAD LT 2008 | |
| Vertical equipment rack | (NASA provided) | N/A | |
| Solid aluminum optical breadboard | Thorlabs | MB2424 | 24" x 24" x 1/2", 1/4"-20 Taps; available direct from Thorlabs |
| Industrial grade steel and hardener | The J-B Weld Company | J-B Weld Steel Reinforced Epoxy Glue | |
| Micro-hematocrit capillary | Fisher Scientific | 22-362-574 | inner diamter 1.1 to 1.2 mm |
| 1 ml syringes | Henke-Sass, Wolf | 4010.200V0 | NORM-JECT®; supplier: Grainger, Inc. |
| Human red blood cells | Innovative Research | IPLA-WB3 | Tested and found negative by supplier for: HBsAg, HCV, HIV-1, HIV-2, HIV-1Ag or HIV 1-NAT, ALT, and syphilis by FDA-Approved Methods. Because no test methods can guarantee with 100% certainty the absence of an infectious agent, human derived products should be handled as suggested in the U.S. Department of Health and Human Services Manual on BIOSAFETY IN MICROBIOLOGICAL AND BIOMEDICAL LABORATORIES, FOR POTENTIALLY INFECTIOUS HUMAN SERUM OR BLOOD SPECIMENS |
| Phosphate buffered saline concentrate | P5493 | SIGMA | 10x; diluted to 1x |
| Tween | P9416 | SIGMA | TWEEN® 20 |
| Centrifuge | LW Scientific | STRAIGHT8-5K | Swing-Out 8-place Centrifuge. Available through authorized dealers. Other centrifuges available direct from LW Scientific. |
| HD video recorder | Sony | MHS-CM5 | |
| Orange fluorescent nucleic acid stain | Invitrogen | S-11364 | SYTO® 83 Orange Fluorescent Nucleic Acid Stain. Stored in DMSO solvent. Always wear reccommended Personal Protective Equipment. No special handling advice required. |
| Fluorescent counting beads | Invitrogen | MP 36950 | CountBright™ Absolute Counting Beads. Always wear reccommended Personal Protective Equipment. No special handling advice required. |
References
- Thomas, R. A., Krishan, A., Robinson, D. M., Sams, C., Costa, F. NASA/American Cancer Society High-Resolution Flow Cytometry Project-I. Cytometry. 43, 2-11 (2001).
- Wen, J., Krishan, A., Thomas, R. A. NASA/American Cancer Society High-Resolution Flow Cytometry Project - II. Effect of pH and DAPI concentration on dual parametric analysis of DNA/DAPI fluorescence and electronic nuclear volume. Cytometry. 43, 12-15 (2001).
- Krishan, A., Wen, J., Thomas, R. A., Sridhar, K. S., Smith, W. I. NASA/American Cancer Society High-Resolution Flow Cytometry Project - III. Multiparametric analysis of DNA content and electronic nuclear volume in human solid tumors. Cytometry. 43, 16-22 (2001).
- Cram, L. S. Spin-offs from the NASA space program for tumor diagnosis. Cytometry. 43, 1 (2001).
- Crucian, B., Sams, C. Reduced gravity evaluation of potential spaceflight-compatible flow cytometer technology. Cytometry B Clin. Cytom. 66 (1), 1-9 (2005).
- Shi, W., Kasdan, H. L., Fridge, A., Tai, Y. -. C. Four-part differential leukocyte count using μflow cytometer. 2010 IEEE 23rd International Conference on Micro Electro Mechanical Systems. 13 (7), 1019-1022 (2010).
- Tai, Y. -. C., Ho, C. -. M., Kasdan, H. L. . In-Flight Blood Analysis Technology for Astronaut Health Monitoring NASA Human Research Program Investigators’ Workshop. , (2010).
- Shi, W., Guo, L. W., Kasdan, H., Fridge, A., Tai, Y. -. C. Leukocyte 5-part differential count using a microfluidic cytometer. 2011 16th International Solid-State Sensors, Actuators and Microsystems Conference. , 2956-2959 (2011).
- Shi, W., Guo, L., Kasdan, H., Tai, Y. -. C. Four-part leukocyte differential count based on sheathless microflow cytometer and fluorescent dye assay. Lab Chip. 13 (7), 1257-1265 (2013).
- Dubeau-Laramée, G., Rivière, C., Jean, I., Mermut, O., Cohen, L. Y. Microflow1, a sheathless fiber-optic flow cytometry biomedical platform: Demonstration onboard the international space station. Cytometry A. , (2013).
- Crucian, B., Quiriarte, H., Guess, T., Ploutz-Snyder, R., McMonigal, K., Sams, C. A Miniaturized Analyzer Capable of White-Blood-Cell and Differential Analyses During Spaceflight. Lab Medicine. 44 (4), 304-331 (2013).
- Rehnberg, L., Russomano, T., Falcão, F., Campos, F., Everts, S. N. Evaluation of a novel basic life support method in simulated microgravity. Aviat. Space. Environ. Med. 82 (2), 104-110 (2011).
- Pump, B., Videbaek, R., Gabrielsen, A., Norsk, P. Arterial pressure in humans during weightlessness induced by parabolic flights. J. Appl. Physiol. 87 (3), 928-932 (1999).
- Strauch, S. M., Richter, P., Schuster, M., Häder, D. The beating pattern of the flagellum of Euglena gracilis under altered gravity during parabolic flights. J. Plant Physiol. 167 (1), 41-46 (2010).
- Sams, C. F., Crucian, B. E., Clift, V. L., Meinelt, E. M. Development of a whole blood staining device for use during space shuttle flights. Cytometry. 37 (1), 74-80 (1999).
- Smith, S. M., Davis-Street, J. E., Fontenot, T. B., Lane, H. W. Assessment of a portable clinical blood analyzer during space flight. Clin. Chem. 43, 1056-1065 (1997).
- Weigl, B. H., Kriebel, J., Mayes, K. J., Bui, T., Yager, P. Whole Blood Diagnostics in Standard Gravity and Microgravity by Use of Microfluidic Structures (T-Sensors). Microchimica Acta. 131 (1-2), 75-83 (1999).
- . Revolutionizing Medical Technology for Earth and Space. Canadian Space Agency. , (2012).
- Peytavi, R. Microfluidic device for rapid (<15 min) automated microarray hybridization. Clin. Chem. 51, 1836-1844 (2005).
- Groemer, G. E. The feasibility of laryngoscope-guided tracheal intubation in microgravity during parabolic flight: a comparison of two techniques. Anesthesia and analgesia. 101 (5), 1533-1535 (2005).
- Johnston, S. L., Campbell, M. R., Billica, R. D., Gilmore, S. M. Cardiopulmonary resuscitation in microgravity: efficacy in the swine during parabolic flight. Aviat. Space Environ. Med. 75 (6), 546-550 (2004).
- Panait, L., Broderick, T., Rafiq, A., Speich, J., Doarn, C. R., Merrell, R. C. Measurement of laparoscopic skills in microgravity anticipates the space surgeon. Am. J. Surg. 188 (5), 549-552 (2004).
- Kirkpatrick, A. W. Intraperitoneal gas insufflation will be required for laparoscopic visualization in space: a comparison of laparoscopic techniques in weightlessness. J. Am. Coll. Surg. 209 (2), 233-241 (2009).
- Campbell, M. R. Endoscopic surgery in weightlessness: the investigation of basic principles for surgery in space. Surg. Endosc. 15 (12), 1413-1418 (2001).
- Caiani, E. G., Sugeng, L., Weinert, L., Capderou, A., Lang, R. M., Vaïda, P. Objective evaluation of changes in left ventricular and atrial volumes during parabolic flight using real-time three-dimensional echocardiography. J. Appl. Physiol. 101 (2), 460-468 (2006).
- Ansari, R., Manuel, F. K., Geiser, M., Moret, F., Messer, R. K., King, J. F., Suh, K. I., Manns, F., S derberg, P. G., Ho, A. Measurement of choroidal blood flow in zero gravity. Ophthalmic technologies XII : 19-20 January 2002, San Jose, USA. , 177-184 (2002).
- Foldager, N. Central venous pressure in humans during microgravity. J. Appl. Physiol. 81 (1), 408-412 (1996).
- Hausmann, N. Cytosolic calcium, hydrogen peroxide and related gene expression and protein modulation in Arabidopsis thaliana cell cultures respond immediately to altered gravitation: parabolic flight data. Plant Biol. (Stuttg). 16 (1), 120-128 (2014).
- Thiel, C. S. Rapid alterations of cell cycle control proteins in human T lymphocytes in microgravity). Cell Commun. Signal. 10 (1), 1 (2012).
- Tsuda, T., Kitagawa, S., Yamamoto, Y. Estimation of electrophoretic mobilities of red blood cells in 1-G and microgravity using a miniature capillary electrophoresis unit. Electrophoresis. 23, 2035-2039 (2002).
- Paul, A. -. L., Manak, M. S., Mayfield, J. D., Reyes, M. F., Gurley, W. B., Ferl, R. J. Parabolic flight induces changes in gene expression patterns in Arabidopsis thaliana. Astrobiology. 11 (8), 743-758 (2011).
- Zeredo, J. L., Toda, K., Matsuura, M., Kumei, Y. Behavioral responses to partial-gravity conditions in rats. Neurosci. Lett. 529 (2), 108-111 (2012).
- Taube, J. S., Stackman, R. W., Calton, J. L., Oman, C. M. Rat head direction cell responses in zero-gravity parabolic flight. J. Neurophysiol. 92 (5), 2887-2897 (2004).
- Hilbig, R. Effects of altered gravity on the swimming behaviour of fish. Adv. Space Res. 30 (4), 835-841 (2002).
- Yang, J., Qi, L., Chen, Y., Ma, H. Design and Fabrication of a Three Dimensional Spiral Micromixer. Chinese J. Chem. 31, 209-214 (2013).
- Zhang, K. Realization of planar mixing by chaotic velocity in microfluidics. Microelectron. Eng. 88, 959-963 (2011).
- Liu, R. H. Passive mixing in a three-dimensional serpentine microchannel. J. Microelectromechanical Syst. 9, 190-197 (2000).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved