Method Article
A Non-Degradative Extraction Method for Molecular Structure Characterization of Bacterial Glycogen Particles
* These authors contributed equally
In This Article
Summary
Bacterial glycogen structure is greatly impacted by extraction methods which may result in molecular degradation and/or biased sampling. It is essential to develop methods to minimize these problems. Here, four extraction methods have been compared using size distribution and chain length distribution as key criteria for minimizing extraction artifacts.
Abstract
Currently, there exist a variety of glycogen extraction methods, which either damage glycogen spatial structure or only partially extract glycogen, leading to the biased characterization of glycogen fine molecular structure. To understand the dynamic changes of glycogen structures and the versatile functions of glycogen particles in bacteria, it is essential to isolate glycogen with minimal degradation. In this study, a mild glycogen isolation method is demonstrated by using cold-water (CW) precipitation via sugar density gradient ultra-centrifugation (SDGU-CW). The traditional trichloroacetic acid (TCA) method and potassium hydroxide (KOH) method were also performed for comparison. A commonly used lab strain, Escherichia coli BL21(DE3), was used as a model organism in this study for demonstration purposes. After extracting glycogen particles using different methods, their structures were analyzed and compared through size exclusion chromatography (SEC) for particle size distribution and fluorophore-assisted capillary electrophoresis (FACE) for linear chain length distributions. The analysis confirmed that glycogen extracted via SDGU-CW had minimal degradation.
Introduction
Glycogen is a highly branched polysaccharide that consists of glucosyl residues and also a small but significant amount of proteins, in which all glucosyl residues are linked together via α-1,4-glycosidic bonds in linear chains and α-1,6-glycosidic bonds at branching points1. The structure of glycogen particles is generally divided into three hierarchies: 1) short-chain oligomers, 2) spherical β particles (~20 nm in diameter), and 3) large rosette-shaped α particles aggregated together by β particles, the diameter of which ranges roughly up to 300 nm. Recently, it has been found that glycogen α particles have two structural states in eukaryotes, i.e., a fragile state and a stable state. Here, fragility means the dissociation of larger α particles into smaller β particles in the presence of a chaotropic agent like DMSO2. Further analyses found that glycogen α particles in the diabetic liver are consistently fragile3 and the fragile α particles degrade much faster than stable α particles4. Thus, glycogen structural fragility may exacerbate hyperglycemic conditions in diabetes2,4, which makes fragile α-particle a potential pathological biomarker of diabetes at a molecular level. However, the existence of glycogen α particles in prokaryotes is only sporadically reported5, and there is no report of the two different structural states of glycogen α particles in bacteria.
In order to understand the physiological functions of bacterial glycogen particles, it is essential to determine the fine structure of glycogen molecules, which requires glycogen isolation with maximal yield and minimal degradation1. So far, various techniques have been developed for glycogen extraction, including but not limited to hot water extraction, trichloroacetic acid (TCA) extraction, and hot alkaline (potassium hydroxide, KOH) extraction6. In addition, another method that is commonly used for eukaryotic glycogen isolation, the sugar density gradient ultra-centrifugation (SDGU) method, was also reported for bacterial glycogen isolation in Selenomonas ruminantium and Fibrobacter succinogenes7,8. Although the pros and cons of these methods have been widely discussed in eukaryotic studies9,10, there are rarely comparative studies of glycogen fine structures isolated via different extraction methods in bacteria from the perspective of glycogen particle structures.
In this study, this issue has been addressed by using Escherichia coli BL21(DE3) as the model organism. A total of four glycogen extraction methods were compared, namely, TCA-precipitated hot water extraction (TCA-HW), TCA-precipitated cold-water extraction (TCA-CW), hot 30% KOH solution extraction (KOH-HW), and cold-water extraction using sucrose density gradient ultracentrifugation (SDGU-CW). Glycogen particle size distribution was then measured via size exclusion chromatography (SEC) while chain-length distribution was detected via fluorophore-assisted carbohydrate electrophoresis (FACE), both of which were used for assessing the quality of extraction methods. In addition, the stability and fragility of bacterial glycogen α particles were also compared among the various extraction methods by comparing particle size distribution before and after treating with the commonly used chaotropic agent, dimethyl sulfoxide (DMSO). The detailed procedures for glycogen extraction and structural characterization are presented below. In summary, the SDGU-CW method has the best overall effect in terms of glycogen structural integrity and is, therefore, recommended for bacterial glycogen extraction in future relevant studies.
Protocol
1. Bacteria culture and collection
- Resuscitate E. coli BL21(DE3) from bacterial glycerol stock (-80 °C) by inoculating sterile LB agar plate (10 g/L tryptone,5 g/L yeast extract, 10 g/L NaCl, and 15 g/L agar). Put the plate into a standard incubator and cultivate overnight at 37 °C.
- Pick up a single colony and inoculate it into a 10 mL sterile LB liquid medium (10 g/L tryptone,5 g/L yeast extract, and 10 g/L NaCl). Mix well via vortexing and culture overnight at 37 °C with shaking at 220 rpm.
NOTE: Unless otherwise specified, all liquid culture conditions were 37 °C with a 220-rpm shaking rate. - Transfer 1 mL of the overnight E. coli culture into 100 mL of sterile LB liquid medium and culture for 5 h. Transfer 50 mL to 1 L of 1x M9 minimal medium (3 g/L KH2PO4, 0.5 g/L NaCl, 6.78 g/L Na2HPO4, and 1 g/L NH4Cl) containing 0.8% D-(+)-glucose. Mix well, and culture for 20 h.
NOTE: 1x M9 minimal medium and glucose were sterilized separately and then mixed when cooling down to room temperature aseptically. - After culturing for 20 h, centrifuge bacterial solution at 6,000 x g for 15 min at 4 °C. Discard the supernatant. Store the cell pellet at -80 °C overnight and then freeze-dry the pellet.
- Seal and store the lyophilized bacterial powder in a refrigerator (-20 °C) for later use.
2. Glycogen extraction
- Trichloroacetic acid precipitated hot water extraction (TCA-HW)
- Precisely weigh 500 mg of freeze-dried E. coli BL21 (DE3) powder and resuspend it in 20 mL of ice-cold 0.05 M triethanolamine (TEA) buffer. Use an ultrasonic cell crusher (25% energy, 4 °C) to disrupt bacterial cells for 3 min (30 s working cycles and 2 s intervals). Ensure that there are no obvious pellets in the solution.
NOTE: Cell disruption was carried out on the ice. Adjust the pH of TEA buffer to pH 7 by HCl addition and store at room temperature. - Transfer all the bacterial homogenate to two 10.4 mL ultracentrifuge tubes (10 mL/ tube). Fill the tubes with deionized water to the top, and centrifuge at 104,000 x g in an ultracentrifuge at 4 °Cfor 90 min. Discard the supernatant.
- Add 2 mL of deionized water to resuspend the pellet in each tube. Transfer suspension to a 50 mL centrifuge tube and add deionized water to a final volume of 20 mL. Heat and boil for 5 min to denature all proteins.
- Centrifuge the suspension for 10 min at 18,000 x g and retain the supernatant (S1). Treat the precipitate the same way as in step 2.1.3. Pool the new supernatant (S2) with S1.
- Add 50% TCA (0.1 volume) to the supernatant (S1+S2) and place the mixture on ice for 10 min to precipitate macromolecules such as DNA, RNA, proteins, etc. Centrifuge the mixture at 18,000 x g for 10 min and mix the supernatant with 1.5 volume of absolute ethanol.
NOTE: The concentration of TCA is 50% v/v. For storage, keep it away from light. - Precipitate glycogen on ice for 20 min and centrifuge at 18,000 x g for 10 min. Pour out supernatant.
- Dissolve the pellet in 5 mL of ddH2O, and then add 5 mL of ice-cold absolute ethanol. Incubate the solution overnight at 4 °C and centrifuge at 18,000 x g for 10 min. Discard the supernatant and keep the pellet.
- Repeat the "wash and precipitation" steps as in step 2.1.7 two more times.
- Finally, dissolve the precipitated glycogen in 400 µL of ddH2O in a 2 mL tube, pre-freeze at -80 °C,and freeze-dry to obtain dry glycogen powder.
- Precisely weigh 500 mg of freeze-dried E. coli BL21 (DE3) powder and resuspend it in 20 mL of ice-cold 0.05 M triethanolamine (TEA) buffer. Use an ultrasonic cell crusher (25% energy, 4 °C) to disrupt bacterial cells for 3 min (30 s working cycles and 2 s intervals). Ensure that there are no obvious pellets in the solution.
- Trichloroacetic acid precipitated cold water extraction (TCA-CW)
NOTE: TCA-CW method is the same as the TCA-HW method except that, after discarding the supernatant, treat the pellet without boiling (see step 2.1.3) and resuspend it in 20 mL of ddH2O containing 1 mg/mL protease inhibitor cocktail.- To prepare a 1 mg/mL protease inhibitor cocktail solution, dissolve 50 mg of protease inhibitor cocktail in 1 mL of deionized water and dilute at a ratio of 50:1 (deionized water: protease inhibitor cocktail solution).
- Cold water extraction using sucrose density gradient ultracentrifugation (SDGU-CW)
- Dissolve and homogenize 1 g of freeze-dried E. coli powder in 4 mL of glycogen extraction buffer (GEB) with a 1 mg/mL protease inhibitor cocktail.
NOTE: GEB consists of 50 mM Tris, 150 mM NaCl, 2 mM EDTA, 50 mM NaF, and 5 mM sodium pyrophosphate. GEB needs to be adjusted to pH 8 with HCl. - Use an ultrasonic cell crusher (25% energy, 4 °C) to disrupt bacterial cells for 3 min (8 s working cycles and 9 s intervals). Ensure that the entire process is carried out on the ice. After sonification, transfer the bacterial homogenate to a centrifuge tube, fill it with GEB to a final volume of 10 mL, and vortex the tube to mix.
NOTE: Do not homogenize for too long a time as heat generated may degrade glycogen. - Centrifuge at 6,000 x g for 10 min at 4 °C. Transfer the supernatant to a 10.4 mL ultracentrifuge tube, fill the tube to the top with GEB, and then centrifuge at 360,000 x g for 2 h at 4 °C.
NOTE: Make sure that the tube is filled up and no air bubbles exist. - Discard the supernatant after centrifugation and resuspend the precipitate with 2 mL of deionized water.
- In a new ultracentrifuge tube, slowly layer 4 mL of 75% sucrose solution with 4 mL of 37.5% sucrose solution. Then, layer the suspension obtained in step 2.3.4 on top of the sucrose solution, and top up with deionized water (see Figure 1).
NOTE: The sucrose concentration is 75% [v/v] and 37.5% [v/v]. Be careful when making the sucrose density gradient and ensure that there is an observable layering between the two sucrose solutions.Also, ensure that there are no air bubbles in the ultracentrifuge tube. - Centrifuge at 360,000 x g for 2.5 h at 4 °C and discard the supernatant. Dissolve the pellet in 200 µL of deionized water. Add 800 µL of absolute ethanol for glycogen precipitation.
- Precipitate glycogen at -20 °C overnight. Centrifuge at 4,000 x g for 10 min at 4 °C and discard the supernatant.
- Dissolve the resulting pellet in 400 µL of ddH2O in a 2 mL tube, pre-freeze at -80 °C,and freeze-dry to obtain dry glycogen powder. Preserve dry glycogen powder at 4 °C for structural analysis.
NOTE: The detailed procedure is illustrated in Figure 1.
- Dissolve and homogenize 1 g of freeze-dried E. coli powder in 4 mL of glycogen extraction buffer (GEB) with a 1 mg/mL protease inhibitor cocktail.
- Hot 30% potassium hydroxide solution extraction (KOH-HW)
- Boil freeze-dried E. coli powder (50 mg) in 1 mL of 30% [w/v] KOH for 1 h.
- Add 67% [v/v] ethanol containing 15 mM LiCl for precipitation at -20 °C for at least 1 h. Centrifuge the samples at 16,000 x g at 4 °C for 20 min.
NOTE: 67% [v/v] ethanol with 15 mM LiCl contains 0.6358 g LiCl, 67 mL of absolute ethanol and 33 mL of deionized water. - Redissolve the pellets in 1 mL of ddH2O and heat for 10 min at 95 °C with intermittent agitation.
- Repeat the ethanol precipitation step three more times as described in step 2.4.2. Redissolve the final pellet in 400 µL of ddH2O in a 2 mL tube, pre-freeze at -80 °C, and freeze-dry to obtain dry glycogen powder.
3. Glycogen structure determination
- Transmission Electron Microscopy (TEM)
- Resuspend glycogen powder in 50 mM Tris-buffered saline (pH 7) with a final concentration of 1 mg/mL.
- Make a 10-fold dilution of the suspension and apply the diluted suspension onto the glow-discharge 400-mesh copper grid.
- After 2 min, draw the excess sample off the grid using a filter paper and stain the grid with 2-3 drops of 1% uranyl acetate.
- Use a transmission electron microscope operating at 75 kV to examine the preparations.
- Size Exclusion Chromatography (SEC)
- Preparation of mobile phase: prepare 0.02% (w/w) sodium azide and 50 mM sodium nitrate solutions in deionized water, and filter through a 0.45 µm filter membrane. Use the ultrasonic oscillating water bath to sonicate the solution for more than 15 min to remove air bubbles.
- Sample preparation: Use the mobile phase to dissolve glycogen powder so that the final concentration is 1 mg/mL. Incubate the solution at 80 °C overnight in a thermomixer. Centrifuge the dissolved sample at 6,000 x g for 10 min at room temperature and transfer the supernatant to a standard SEC vial.
- Preparation of DMSO-treated glycogen samples
- Dissolve 1 mg of glycogen powder in 300 µL of DMSO and incubate it at 80 °C overnight in a thermomixer. Add 4x volume of ethanol to precipitate glycogen and centrifuge the solution at 6,000 x g.
- Wash the pellet twice with ethanol, dissolve the pellet in ddH2O and lyophilize.
- Analyze the freeze-dried glycogen powder via SEC by preparing samples as described above.
- Use SEC system with pre-columns, and 1000 and 10000 columns for the analysis of glycogen particle size distribution. Keep the columns at 80 °C and the flow rate at 0.3 mL/min.
- Fluorophore-Assisted Carbohydrate Electrophoresis (FACE)
- Glycogen debranching
NOTE: When using FACE to detect chain length distribution (CLD), all samples to be tested need to undergo the pretreatment of debranching.- Add 0.5 mg of glycogen powder, 90 µL of hot water (90 °C), 1.5 µL of NaN3 solution, 3.5 µL of isoamylase (200 U/mL), and 8 µL of acetic acid-sodium acetate buffer (pH 3.5) to a test tube.
NOTE: Isoamylase is added to specifically cut the branched-chain of the sample to be tested. Isoamylase is a debranching enzyme that can specifically destroy the α-(1-6) bond without destroying the α-(1-4) bond, so it can specifically cleave the glycogen side chain without destroying the structure of the main chain. - Incubate the mixture at 37 °C for 3 h in a thermomixer. After making the pH neutral with 8 µL of 0.1 M NaOH solution, incubate the mixture at 80 °C for 1 h in a thermomixer.
- Add 4x volume of absolute ethanol to the mixture to precipitate glycogen. Then, centrifuge at 6,000 x g for 10 min at room temperature. Wash the pellet twice with ethanol, dissolve the pellet into ddH2O and lyophilize.
- Add 0.5 mg of glycogen powder, 90 µL of hot water (90 °C), 1.5 µL of NaN3 solution, 3.5 µL of isoamylase (200 U/mL), and 8 µL of acetic acid-sodium acetate buffer (pH 3.5) to a test tube.
- Preparation of fluorescent solution
- Centrifuge the APTS (8-Aminopyrene-1,3,6-trisulfonic acid trisodium salt) reagent bottle (each bottle contains 5 mg APTS) at 4,000 x g for 2 min. Add 50 µL of 15% acetic acid solution to dissolve the powder, mix well, and centrifuge at 4,000 x g for 2 min at room temperature to obtain 0.2 M APTS acetic acid solution.
NOTE: Keep the APTS solution in a refrigerator at -20 °C.It must be used completely within two weeks once opened. Otherwise, it will be inactivated. APTS is a commonly used negatively charged dye that binds to the reducing end of the glycogen chain. Since the chains with different degrees of polymerization (DP) all carry only one negative charge, FACE can separate them based on their different mass-to-charge ratios. In this way, signals of different DP values are detected by the fluorescence detector.
- Centrifuge the APTS (8-Aminopyrene-1,3,6-trisulfonic acid trisodium salt) reagent bottle (each bottle contains 5 mg APTS) at 4,000 x g for 2 min. Add 50 µL of 15% acetic acid solution to dissolve the powder, mix well, and centrifuge at 4,000 x g for 2 min at room temperature to obtain 0.2 M APTS acetic acid solution.
- Transfer the debranched glycogen to a test tube and add 1.5 µL of APTS solution and 1.5 µL of sodium cyanoborohydride solution. Incubate at 60 °Cfor 1.5 h in the dark. Add 80 µL of deionized water, centrifuge at 4,000 x g for 10 min at room temperature and keep the supernatant.
- Introduce the sample into the capillary electrophoresis system by injecting for 3 s at 0.5 psi (3.4 kPa above atmospheric pressure).
NOTE: Fluorescently labeled linear glucans were separated through applied voltage of 30 kV and an approximate current of 14 mA at 25 °C. Peak areas gave relative amounts of glucans with different masses (degree of polymerization (DP) of glucans in adjacent peaks differed by 1 DP). The sample temperature was kept at 18 °C.
- Glycogen debranching
Results
Size distribution of glycogen particles
A series of studies have shown that glycogen α particles in the diabetic liver are fragile and easily broken apart in the hydrogen bond disruptor DMSO11,12,13,14. The present study tested how particle size and structural stability changed for bacterial glycogen extracted through four different methods. All glycogen samples from the four methods were treated with water (blue curves) and DMSO (red curves), respectively. The weight distributions, w(logRh), are given in Figure 2. Water-treated glycogen extracted via TCA-HW (Figure 2A, blue curves) and TCA-CW (Figure 2B, blue curves) is dominated by smaller particles with peaks at Rh ~ 20 nm. On the other hand, water-treated glycogen extracted via KOH-HW (Figure 2C, blue curves) and SDGU-CW (Figure 2D, blue curves) exhibits larger particle sizes with peaks at Rh ~ 40 nm thus indicating that glycogen α particles are present in the bacteria. Whereas both KOH-HW and SDGU-CW methods extract glycogen α particles, TCA-HW, and TCA-CW methods either degrade larger α particles into β particles or only extract smaller β particles.
In terms of glycogen stability and fragility, DMSO treatment did not alter weight distributions of glycogen particles extracted through TCA-HW (Figure 2A, red curves), TCA-CW (Figure 2B, red curves), and KOH-HW (Figure 2C, red curves). However, glycogen extracted via the KOH-HW method mainly consisted of stable α particles while TCA methods mainly generated β particles. As for glycogen extracted from SDGU-CW, a large change of α-particle and β-particle compositions was observed after DMSO treatment. It is inferred from Figure 2D that a fraction of the α particles (blue curves) degraded into β particles (red curves), leading to the observed plateau region and thereby suggesting that there is a co-existence of both stable and fragile α particles in bacterial glycogen.
In order to support the morphological structures of glycogen particles extracted from SDGU-CW, representative TEM pictures of glycogen are shown in Figure 3. The morphology of raw-state glycogen particles from E. coli is similar to that from healthy mouse liver15, showing the presence of rosette-shaped α particles and few β particles, which confirms that glycogen α particles can be extracted from E. coli via mild extraction methods.
Chain-length distributions
The chain length distributions (CLDs) of glycogen particles measured by FACE are shown in Figure 4. Average chain length (ACL) is the reciprocal of branching percentage and can be calculated using the formula Σ (DP Percentage x Number of DP). According to the results, glycogen from SDGU-CW (Figure 4D) and TCA-CW (Figure 4B) had the highest ACLs (~14 DP), indicating that CW extraction had the least CLD damage. For TCA-HW extracted glycogen, chains were partially degraded due to the brief boiling step, as seen from a slight reduction of ACL (Figure 4A). Finally, the CLD for glycogen from KOH-HW shifted toward smaller DP values and the ACL decreased by more than 2 DPs (Figure 4C), which indicated that boiling in a strongly alkaline medium could damage the primary structure of glycogen particles.

Figure 1: A schematic illustration of bacterial glycogen extraction through the SDGU-CW method. Please click here to view a larger version of this figure.

Figure 2: SEC weight distributions [w(log Rh) (a.u)] for E. coli BL21(DE3) glycogen particles extracted through four methods. (A) TCA-HW, (B) TCA-CW, (C) KOH-HW, and (D) SDGU-CW. Size distribution curves are shown for glycogen particles treated with water (blue color) and DMSO (red color). All SEC analyses were performed in duplicate. W1: duplicate 1 treated with water. D1: duplicate 1 treated with DMSO. W2: duplicate 2 treated with water. D2: duplicate 2 treated with DMSO. This figure has been reproduced from reference 1. Please click here to view a larger version of this figure.
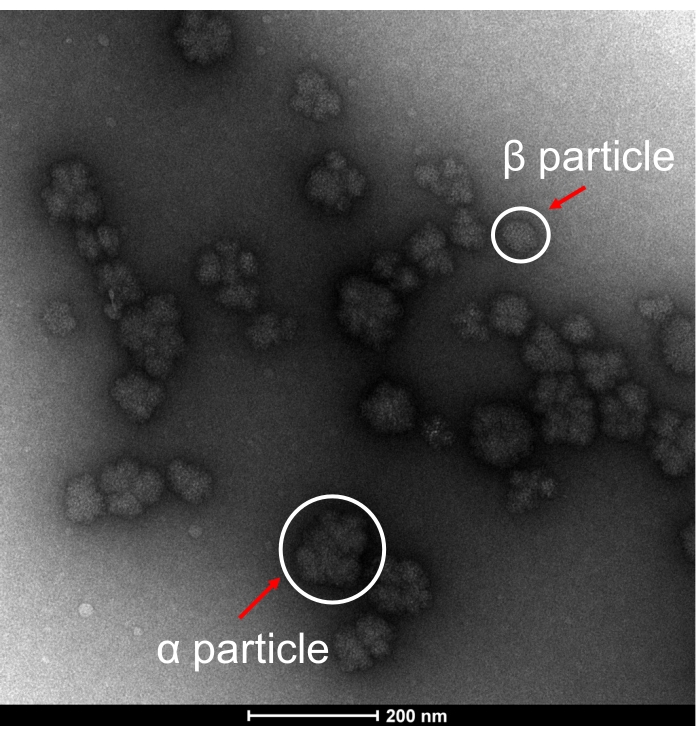
Figure 3: Representative TEM image. TEM image of bacterial α glycogen particles and β glycogen particles extracted through cold-water extraction using sucrose density gradient ultra-centrifugation (SDGU-CW) method. Representative particles are denoted with red arrows. Please click here to view a larger version of this figure.

Figure 4: Chain length distributions and average chain lengths of E. coli glycogen extracted through four different methods. (A) TCA-HW, (B) TCA-CW, (C) KOH-HW, and (D) SDGU-CW. Three independent extractions were performed for each method and the average chain length distributions, together with standard error means, were presented. This figure has been adapted from data published in reference 1. Please click here to view a larger version of this figure.
Discussion
Glycogen is an important energy reserve that has been identified in many bacteria16. To dissect the physiological functions of glycogen particles, it is essential to have a better understanding of the fine structure of glycogen molecules. So far, a variety of methods have been developed to extract glycogen from bacterial culture. However, different size distributions of glycogen particles have been observed from different extraction methods, which suggests damaged glycogen structure. Thus, it is necessary to compare and standardize extraction procedures in order to make sure that glycogen structures from different studies are comparable. In this study, a detailed protocol is presented describing four commonly used methods for glycogen extraction from E. coli liquid culture, which are then evaluated through structural characterizations of glycogen particles.
As for size distribution, glycogen particles from both TCA-CW and TCA-HW show a weight distribution towards smaller particle size with peaks at Rh ≈ 20 nm (β particles). Thus, these two methods are not suitable to extract glycogen particles for structural characterization. In fact, it might be part of the reason why only β particles were thought to exist in bacteria since TCA methods have been widely used for bacterial glycogen study. On the other hand, glycogen particles from both KOH-HW and SDGU-CW methods have dominantly larger particle sizes with the highest peaks at Rh ≈ 40 nm (α particles). Thus, KOH-HW and SDGU-CW methods are better than TCA-CW and TCA-HW methods. However, since only stable α particles can be extracted via the KOH-HW method, it indicates that fragile α particles are disrupted by the harsh conditions used in this method.
In terms of chain length distributions (CLD), glycogen from SDGU-CW and TCA-CW have longer chains, which confirms that cold-water extraction results in minimal CLD damage. Chain lengths of glycogen particles extracted via TCA-HW were partially reduced due to the brief boiling step in the extraction procedure, leading to a decrement of average chain length (ACL). With the KOH-HW method, CLD reveals shorter chains of glycogen, and the ACL decreases by more than 2 DPs owing to long-term boiling in alkaline solutions. Thus, based on this primary structure analysis of glycogen particles, it is confirmed that cold water extraction, being much milder than hot water extraction, can extract glycogen particles with longer chains and hence less degradation.
In summary, minimal degradation is essential to study the properties of the native glycogen. Minimal degradation of glycogen particles is indicated when size distribution analysis shows larger molecules and chain-length distribution shows the greatest number of longer chains. Since cold water extraction via sucrose density gradient ultra-centrifugation achieves the best overall effect from the perspective of glycogen structural integrity, this method is recommended for bacterial glycogen extraction in future relevant studies.
Disclosures
The authors have no conflicts of interest.
Acknowledgements
We are greatly thankful to Professor Robert G. Gilbert from the University of Queensland and Yangzhou University who provided insights and expertise that greatly assisted the completion of this study. We acknowledge the financial support of the National Natural Science Foundation of China (No. 31900022, No. 32171281), Natural Science Foundation of Jiangsu Province (No. BK20180997), Young Science and Technology Innovation Team of Xuzhou Medical University (No. TD202001), and Jiangsu Qinglan Project (2020).
Materials
| Name | Company | Catalog Number | Comments |
| Equipment | |||
| Agilent 1260 infinity SEC system | Agilent | 1260 infinity II | Particle size distribution |
| Analytical column | PSS | 10-1000 | - |
| Centrifuge | Eppendorf | 5420 | - |
| Filter membrane | Cambio | Km-0220 | - |
| Fluorescence-assisted capillary electrophoresis system | Beckman Coulter | - | Chain length distribution |
| Freeze dryer | Xinzhi | SCIENTZ-10N | Lyophilization of bacteria and glycogen |
| Freezer | Thermo Fisher | Forma 900 | Sample storage |
| Guard column | PSS | SUPPERMA | - |
| Incubator | Thermo Fisher | PR505750R-CN | - |
| Low-speed large-capacity centrifuge | Hexi | HR/T20MM | Sample centrifugation |
| Multiskan FC microplate reader | Thermo Fisher | 1410101 | - |
| Optima XPN ultracentrifuge | Beckman | XPN-100/90/80 | For glycogen |
| Oscillator | Xinbao | SHZ-82 | - |
| PA-800 Plus System | Beckman Coulter | A66528 | - |
| pH meter | Mettler Toledo | FE28 -TRIS | - |
| Refractive index detector | Wyatt | Optilab T-rEX | - |
| Refrigerator | Haier | BCD-406WDPD | - |
| Thermomixer | Shanghai Jingxin | JXH-100 | Sample incubation |
| Transmission electron microscope | Hitachi Corporation | H-7000 | Glycogen particle morphology |
| Ultracentrifuge tube | Beckman | 355651 | - |
| Ultrasonic cell crusher | Ningbo Xinzhi | Scientz-IID | Bacteria disruptor |
| Ultrasonic oscillating water bath | Jietuo | JT-1027HTD | - |
| Vortex mixer | Tiangen | OSE-VX-01 | - |
| Water system | Merck Millipore | H2O-MM-UV-T | Deionized water |
| Material | |||
| 8-Aminopyrene-1,3,6-Trisulfonic Acid Trisodium Salt | Sigma-Aldrich | 196504-57-1 | - |
| Absolute ethanol | Guoyao | 10009228 | - |
| Agar powder | Solarbio | A1890 | - |
| Alpha-amylase | Megazyme | E-BLAAM-40ML | - |
| Amyloglucosidase | Megazyme | E-AMGDF-40ML | - |
| cOmplete Mini | Roche | 4693159001 | - |
| D-(+)Glucose | Sigma-Aldrich | G8270-1kg | - |
| D-Glucose Assay Kit (GOPOD Format) | Megazyme | K-GLUC | Glycogen quantification |
| Dimethyl sulfoxide | Vicmed | Vic147 | Chaotropic agent |
| E. coli BL21(DE3) | Tiangen | CB105-02 | - |
| Ethylene diamine tetra-acetic acid | Vicmed | Vic1488 | - |
| Glacial acetic acid | Guoyao | 10000218 | - |
| Glycerol | Guoyao | 10010618 | Bacterial storage |
| Hydrochloric acid | Guoyao | 10011008 | - |
| Hydroxymethyl aminomethane | Sigma-Aldrich | V900483-500g | - |
| Isoamylase | MegaZyme | 9067-73-6 | Glycogen debranch |
| Lithium chloride | Sigma-Aldrich | 62476-100g | - |
| M9, Minimal Salts, 5× | Sigma-Aldrich | M6030-1kg | Bacterial culture |
| Potassium hydroxide | Guoyao | 10017008 | - |
| Pullulan standard | PSS | - | - |
| Sodium acetate trihydrate | Guoyao | 10018718 | - |
| Sodium azide | Sigma-Aldrich | 26628-22-8 | - |
| Sodium chloride | Guoyao | 10019318 | Bacterial culture |
| Sodium cyanoborohydride | Huaweiruike | hws001297 | - |
| Sodium diphosphate | Sigma-Aldrich | 71515-250g | - |
| Sodium Fluoride | Macklin | S817988-250g | - |
| Sodium hydroxide | Guoyao | 10019762 | - |
| Sodium nitrate | Guoyao | 10019928 | - |
| Sodium pyrophosphate | Sigma-Aldrich | V900195-500g | - |
| Sucrose | Guoyao | 10021463 | - |
| Trichloroacetic acid | Guoyao | 40091961 | - |
| Tryptone | Oxoid | LP0042 | Bacterial culture |
| Yeast Extract | Oxoid | LP0021 | Bacterial culture |
References
- Wang, L., et al. Molecular structure of glycogen in Escherichia coli. Biomacromolecules. 20 (7), 2821-2829 (2019).
- Deng, B., et al. Molecular structure of glycogen in diabetic liver. Glycoconjugate Journal. 32 (3-4), 113-118 (2015).
- Hu, Z., et al. Diurnal changes of glycogen molecular structure in healthy and diabetic mice. Carbohydrate Polymers. 185, 145-152 (2018).
- Nawaz, A., Zhang, P., Li, E., Gilbert, R. G., Sullivan, M. A. The importance of glycogen molecular structure for blood glucose control. iScience. 24 (1), 101953(2021).
- Rashid, A. M., et al. Assembly of α-glucan by GlgE and GlgB in mycobacteria and streptomycetes. Biochemistry. 55 (23), 3270-3284 (2016).
- Wang, L., et al. Recent progress in the structure of glycogen serving as a durable energy reserve in bacteria. World Journal of Microbiology and Biotechnology. 36 (1), 14(2020).
- Kamio, Y., Terawaki, Y., Nakajima, T., Matsuda, K. Structure of glycogen produced by Selenomonas ruminantium. Agricultural and Biological Chemistry. 45 (1), 209-216 (1981).
- Gong, J., Forsberg, C. W. Separation of outer and cytoplasmic membranes of Fibrobacter succinogenes and membrane and glycogen granule locations of glycanases and cellobiase. Journal of Bacteriology. 175 (21), 6810-6821 (1993).
- Mojibi, N. Comparison of methods to assay liver glycogen fractions: the effects of starvation. Journal of Clinical and Diagnostic Research. 11 (3), (2017).
- Orrell, S. A., Bueding, E. A comparison of products obtained by various procedures used for the extraction of glycogen. The Journal of Biological Chemistry. 239, 4021-4026 (1964).
- Tan, X., et al. Proteomic investigation of the binding agent between liver glycogen beta particles. ACS Omega. 3 (4), 3640-3645 (2018).
- Hu, Z., et al. Diurnal changes of glycogen molecular structure in healthy and diabetic mice. Carbohydrate Polymers. 185, 145-152 (2018).
- Sullivan, M. A., Harcourt, B. E., Xu, P., Forbes, J. M., Gilbert, R. G. Impairment of liver glycogen storage in the db/db animal model of type 2 diabetes: a potential target for future therapeutics. Current Drug Targets. 16 (10), 1088-1093 (2015).
- Deng, B., et al. Molecular structure of glycogen in diabetic liver. Glycoconjugate Journal. 32 (3-4), 113-118 (2015).
- Sullivan, M. A., et al. Molecular structural differences between type-2-diabetic and healthy glycogen. Biomacromolecules. 12 (6), 1983-1986 (2011).
- Wang, L., et al. Systematic analysis of metabolic pathway distributions of bacterial energy reserves. G3 Genes|Genomes|Genetics. 9 (8), 2489-2496 (2019).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved