Method Article
Isolation of Human BAMBIhighMFGE8high Umbilical Cord-Derived Mesenchymal Stromal Cells
In This Article
Summary
The isolation of BAMBIhighMFGE8high MSCs, one of the three main subgroups constituting heterogeneous human UC-MSCs, is helpful for fully understanding the characteristics and functions of this subtype for its future application to improve clinical efficacy in specific diseases. Here, we present a method for sorting BAMBIhighMFGE8high UC-MSCs.
Abstract
Umbilical cord-derived mesenchymal stromal/stem cells (UC-MSCs) present low immunogenicity and potent immunomodulatory effects for treating various diseases. Human UC-MSCs are a heterogeneous population consisting of three main subpopulations with different cell shapes, proliferation rates, differentiation abilities, and immune regulatory functions. Previously, BAMBIhighMFGE8high UC-MSCs, the first subgroup successfully isolated from UC-MSCs were found to fail to alleviate lupus nephritis. Hence, the function and underlying mechanism of this subgroup in MSC therapy for diseases remains unknown. It is necessary to isolate and further investigate BAMBIhighMFGE8high UC-MSCs in terms of their phenotype, metabolism, and function to completely understand the nature of this MSC subgroup. In this protocol, we describe a detailed method for isolating the BAMBIhighMFGE8high subpopulation from human UC-MSCs. The subpopulation of UC-MSCs is labeled with two surface markers, BAMBI and MFGE8, by flow cytometry sorting. The isolated cells are cultured and verified by flow cytometry analysis. The specific genes expressed in the BAMBIhighMFGE8high UC-MSCs are identified by RT-qPCR. This protocol results in highly efficient and pure cell sorting and describes the marker profiles of the BAMBIhighMFGE8high UC-MSCs.
Introduction
Human mesenchymal stromal/stem cells (MSCs) are somatic progenitors capable of differentiating into osteocytes, adipocytes, chondrocytes, and other cell types1. MSCs were first isolated from bone marrow and are widely derived from the umbilical cord, adipose tissue, and other tissues2. Because UC-MSCs are easily obtained and exhibit low immunogenicity and immunosuppressive effects, they are widely applied in clinical trials to treat various diseases3,4,5. Although MSC therapy shows promising potential for treating diseases, the therapeutic effects are inconsistent across individuals6. However, the reason for MSC therapy instability is still unclear.
Molecular fluctuations, morphology, differentiation capacity, and therapeutic function comprise MSC heterogeneity. Some studies have also postulated that MSCs constitute subpopulations with different functions7,8 and explored MSC heterogeneity via single-cell RNA sequencing (scRNA-seq)9,10. The results revealed that human UC-MSCs have distinct subpopulations with specific transcriptomic features, whereas few studies have successfully isolated so-called MSC subpopulations. We previously dissected human UC-MSCs into three subgroups according to their signatures via scRNA-seq and bioinformatics analysis, in which the BAMBIhighMFGE8high UC-MSC subpopulation was further purified and functionally tested11. However, this subgroup failed to alleviate lupus nephritis. Thus, it is necessary to test the therapeutic effects of BAMBIhighMFGE8high MSCs in other disorders to understand their authentic functions.
This protocol describes methods for isolating the BAMBIhighMFGE8high subgroup from human UC-MSCs via fluorescence-activated cell sorting (FACS) by flow cytometry and the characteristics of the BAMBIhighMFGE8high subgroup.
Protocol
This study was conducted in accordance with the principles set forth under the 1989 Declaration of Helsinki and approved by the Ethics Committee at the Affiliated Drum Tower Hospital of Nanjing University Medical School (approval number: 202019701). Human umbilical cords were obtained from healthy mothers at the Affiliated Drum Tower Hospital of Nanjing University Medical School after natural labor, who gave their informed consent for their use in this work. Primary UC-MSCs were isolated from human umbilical cords as previously reported11.
1. UC-MSC culture and identification before isolation
- Once the MSCs reach 70-80% confluence (P0), wash the cells once with PBS and add 1 mL of 0.25% trypsin-EDTA for 2 min at 37 °C. Then, add 9 mL of complete medium to neutralize the trypsin, transfer the cell suspension to a 15 mL centrifuge tube, and centrifuge at 300 × g for 5 min to collect the cells. Discard the supernatant and add the appropriate complete medium to resuspend the cells. Transfer the cells in each dish to three T75 flasks (P1) and culture in a cell incubator at 37 °C and 5% CO2.
- After passaging the UC-MSCs 2x via trypsinization, at P3, harvest the cells via the same steps described in step 1.1. After centrifugation, discard the supernatant. Resuspend the cells in FACS staining buffer (1x PBS containing 2% FBS) and count them. Add FACS staining buffer to a final concentration of 5-10 × 106 cells/mL and keep the cell suspension on ice.
- Distribute 100 µL per tube of this cell suspension into different 1.5 mL tubes. Add isotype controls and FACS antibodies against CD29, CD73, CD90, CD105, CD14, CD34, CD45, CD79, and HLA-DR (all at 1:200) to the cells at 4 °C for 30 min.
- Wash the cells 2x with the FACS staining buffer and centrifuge at 300 × g for 5 min. Discard the supernatant and resuspend the precipitates in 200 µL of FACS staining buffer for flow cytometry analysis to identify MSC markers.
2. Isolation of BAMBIhigh MFGE8high UC-MSCs by flow cytometry
- Culture UC-MSC to a density of approximately 5-10 × 106 cells when isolating BAMBIhighMFGE8high UC-MSCs. Dissociate the cells with 0.5 mM EDTA for 5 min until they start to have a round morphology, add the complete medium to transfer the cells to a 15 mL conical tube, and pipet the cell suspension up and down several times to prepare a single-cell suspension. Use 10 µL of the cell suspension to count the cells and calculate the total number of cells harvested. Centrifuge the conical tube with the cell suspension at 300 × g for 5 min to collect the cells.
- Resuspend the cells in 1 mL of complete medium to a final concentration of 5-10 × 106 cells/mL and keep the cell suspension on ice.
- Divide the cells into four 1.5 mL microcentrifuge tubes (blank cells only; BAMBI-labeled cells; MFGE8-labeled cells; and both BAMBI- and MFGE8-labeled cells).
- Add primary antibodies at appropriate concentrations (MFGE8 and BAMBI, both at 1:100) to the tubes, mix, and incubate the cells at room temperature for 15 min.
- Wash the labeled cells once with 1x PBS, and centrifuge at 300×g for 5 min. Discard the supernatant. Resuspend the cells in 1 mL of complete medium, add conjugated fluorescent secondary antibodies (goat anti-rabbit IgG H&L Alexa Fluor 488 and Alexa Fluor 647, all at 1: 1,000) to the cells, mix, and incubate the cells at room temperature for 15 min in the dark.
- Wash the labeled cells once with 1x PBS, as described in step 2.5. Resuspend the cells in 500 µL of complete medium, filter through a 70 µm cell strainer to eliminate clumps and debris, and transfer the filtrate into a 15 mL tube for flow cytometry sorting.
- Run the blank cell tube without adding an antibody (negative control) and adjust the forward scatter (FSC) and side scatter (SSC) to gate the scale of the negative population with antibody staining.
- Run the single antibody-labeled cell tubes (i.e., MFGE8 + goat anti-rabbit IgG H&L Alexa Fluor 488, or BAMBI + goat anti-rabbit IgG H&L Alexa Fluor 647) as a gating control to determine where the positivity starts in the plot.
- Run the experimental sample tube(s) to sort and collect the BAMBIhighMFGE8high cell population.
- Plate the sorted BAMBIhighMFGE8high MSCs in a 24-well plate and cultivate the cells in a cell incubator at 37 °C and 5% CO2.
- When the sorted BAMBIhighMFGE8high MSCs have grown for two passages to obtain enough cells, dissociate the cells and perform a post sorting analysis to ensure the purity of the sorted cell populations by flow cytometry, as described in steps 2.1-2.9. Alternatively, examine the purity of the sorted cells with conventional immunofluorescence against human BAMBI and MFGE8 expression if the number of collected cells is too small.
3. Characterization of the BAMBIhigh MFGE8high MSCs
- Grow the sorted BAMBIhighMFGE8high MSCs in a 12-well plate, examine their cell morphology under a microscope, and compare them with that of unsorted MSCs.
NOTE: In general, BAMBIhighMFGE8high cells grow faster than unsorted UC-MSCs. - When the cells are approximately 90% confluent in the 12-well plate, aspirate the cell culture medium, wash the cells once with 1x PBS, and add 500 µL of RNA extraction reagent to the cells. Shake the plate slowly at room temperature (RT) for 5 min.
- Pipette the mixture and transfer it to an RNase- and DNase-free tube. Add 100 µL of chloroform, cap the tube, and shake the mixture vigorously by vortexing for 15 s. Then, allow the tube to stand at RT for 3 min.
- Centrifuge the tube for 15 min at 11,000 × g and 4 °C.
- Transfer approximately 200 µL of the upper aqueous layer into a new tube. Add the same volume of isopropanol and pipette thoroughly up and down several times. Then, allow the mixture to stand at RT for 10 min.
- Centrifuge for 15 min at 11,000 × g and 4 °C. Discard the supernatant.
- Add 500 µL of 75% ethanol to the pellet in the tube and invert the tube gently several times.
- Centrifuge the tube for 5 min at 6,000 × g and 4 °C. Discard the supernatant. Repeat the centrifugation once for 10 s at 6,000 × g, carefully aspirate the remaining fluid via pipette, and dry the tube at RT.
- Add 10 µL of RNase-free water to dissolve the RNA pellet, and measure the RNA concentration using a spectrophotometer.
- Follow the manufacturer's instructions to synthesize first-strand cDNA from up to 1 µg of total RNA.
- Use a qPCR kit to detect and quantify gene expression, according to the manufacturer's instructions.
NOTE: The sequences of the DNA primers used are listed in Table 1.
Results
Figure 1 shows the cell surface marker expression profiles of human UC-MSCs. Cultured MSCs were strongly positive for CD44, CD73, CD90, and CD105 expression and negative for CD14, CD34, CD45, CD79, and HLA-DR expression. The BAMBIhighMFGE8high MSCs were sorted from cultivated human UC-MSCs, and their BAMBI and MFGE8 expression was reanalyzed by flow cytometry after expansion for 3-4 passages (Figure 2). In this process, the frequency of BAMBIhighMFGE8high MSCs fluctuated depending on the donor and dissociation method (Figure 3 and Figure 4). Figure 5 shows a series of highly expressed signature genes in the BAMBIhighMFGE8high MSCs compared with the unsorted MSCs, as determined by RT-qPCR.
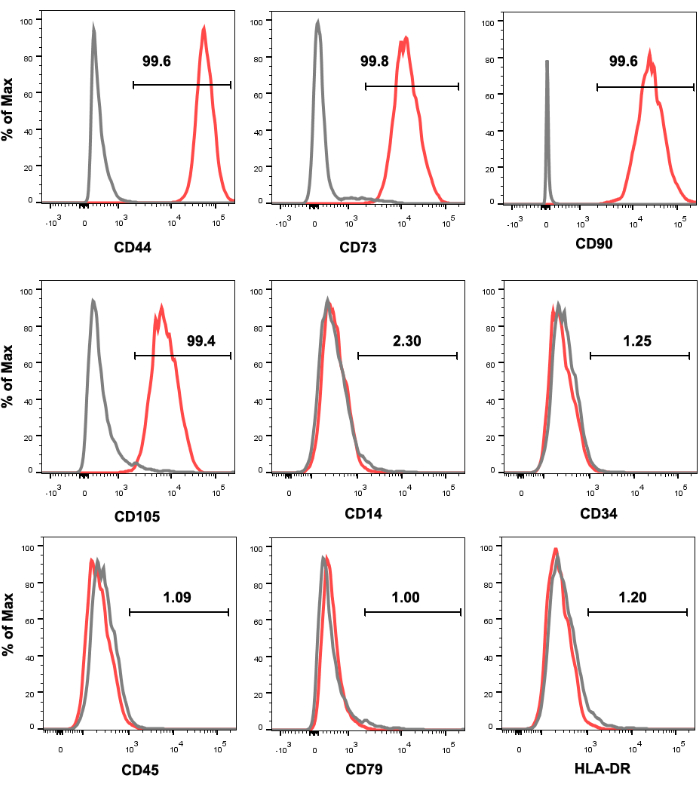
Figure 1: Immunophenotyping of UC-MSCs identified by flow cytometry analysis. UC-MSCs are positive for CD44, CD73, CD90, and CD105 and negative for CD14, CD34, CD45, CD79, and HLA-DR. The black histogram represents the antibody isotype control, and the red histogram represents the antibody signal. Abbreviations: UC-MSCs = umbilical cord-derived mesenchymal stromal/stem cells; HLA-DR = human leukocyte antigen-DR. Please click here to view a larger version of this figure.

Figure 2: Purity of BAMBIhighMFGE8high MSCs after sorting by flow cytometry analysis. The results of flow cytometry analysis of UC-MSCs without antibody staining (upper left) and with MFGE8 and BAMBI staining before (upper middle) and after cell sorting (upper right) are shown. Results of single MFGE8 (lower left) and BAMBI (lower right) staining are also shown. Abbreviations: BAMBI = bone morphogenic protein and activin membrane-bound inhibitor; MFGE8 = milk fat globule epidermal growth factor 8; MSCs = mesenchymal stromal/stem cells. Please click here to view a larger version of this figure.
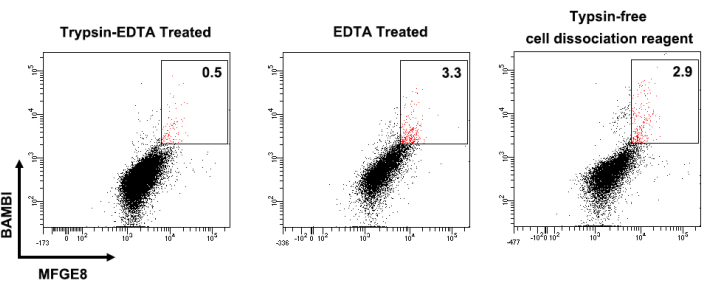
Figure 3: Different frequencies of BAMBIhighMFGE8high MSCs dissociated with different methods. The results of flow cytometry analysis of UC-MSCs from the same donor subjected to trypsin-EDTA dissociation (left), EDTA treatment only (middle), and trypsin-free cell dissociation reagent (right) are shown. Abbreviations: BAMBI = bone morphogenic protein and activin membrane-bound inhibitor; MFGE8 = milk fat globule epidermal growth factor 8; MSCs = mesenchymal stromal/stem cells; TE = trypsin-EDTA. Please click here to view a larger version of this figure.

Figure 4: The frequency of BAMBIhighMFGE8high MSCs from different donors. The frequencies of BAMBIhighMFGE8high MSCs vary among donor samples 1 (left), 2 (middle), and 3 (right). Abbreviations: BAMBI = bone morphogenic protein and activin membrane-bound inhibitor; MFGE8 = milk fat globule epidermal growth factor 8; MSCs = mesenchymal stromal/stem cells. Please click here to view a larger version of this figure.
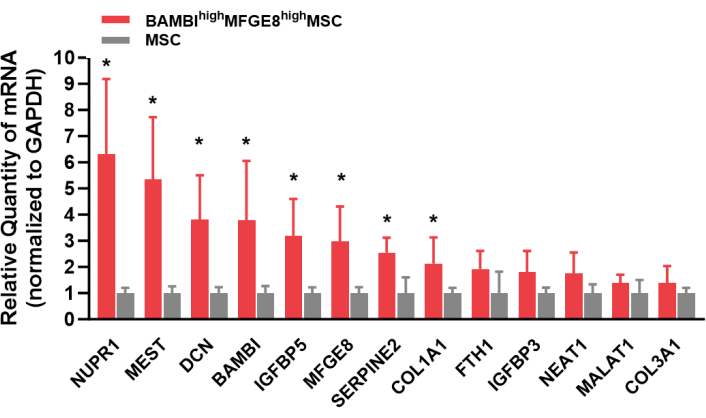
Figure 5: Highly expressed signature genes in the BAMBIhighMFGE8high MSCs examined by RT-qPCR. Abbreviations: BAMBI = bone morphogenic protein and activin membrane-bound inhibitor; MFGE8 = milk fat globule epidermal growth factor 8; MSCs = mesenchymal stromal/stem cells; RT-qPCR = reverse-transcription-quantitative polymerase chain reaction. Student's t-test. *, P<0.05. Please click here to view a larger version of this figure.
| Primer | Sequence Forward (5'-3') | Sequence Reverse (5'-3') |
| BAMBI | CGCCACTCCAGCTACATCTT | CAGTGGGCAGCATCACAGTA |
| COL1A1 | CAAAGAAGGCGGCAAAGGTC | CACGCTGTCCAGCAATACCT |
| COL3A1 | CCTTCGACTTCTCTCCAGCC | TTTCGTGCAACCATCCTCCA |
| DCN | GGCTGGACCGTTTCAACAGA | GATGGCATTGACAGCGGAAG |
| NEAT1 | CACAGGCAGGGGAAATGTCT | TGCTGCGTATGCAAGTCTGA |
| FTH1 | AGCTCTACGCCTCCTACGTT | CCTGAAGGAAGATTCGGCCA |
| IGFBP3 | GCCAGCGCTACAAAGTTGAC | ATGTGTACACCCCTGGGACT |
| IGFBP5 | TCCCCACGTGTGTTCATCTG | AAATGGGATGGACTGAGGCG |
| MALAT1 | TGGGGGAGTTTCGTACTGAG | TCTCCAGGACTTGGCAGTCT |
| MEST | TGGGAGCTCTTGCCTCTGTA | AGAATCGACACTGTGGACCG |
| MFGE8 | TGTCTTCCCCTCGTACACCT | AGAAGGTCACACGCACAGAC |
| SERPINE2 | GTCCTCGTCAACGCAGTGTA | GTCCTCGTCAACGCAGTGTA |
| NUPR1 | CCTTCCCACCAGCAACCAG | GGTAGGAATGGGCCAGGCTA |
| GAPDH | TCAGTGGTGGACCTGACCTG | TGCTGTAGCCAAATTCGTTG |
Table 1: Sequences of the DNA primers used in this protocol.
Discussion
This protocol describes how to isolate and enrich the BAMBIhighMFGE8high subpopulation from human UC-MSCs. The method is important for further study of the morphology, growth, and function of this MSC subgroup. Some steps are vital for the successful isolation and high yield of BAMBIhighMFGE8high cells.
First, the most critical technical aspect to consider is the use of an appropriate cell dissociation solution in the present protocol. Although conventional 0.25% trypsin-EDTA is used to dissociate MSCs for passaging, it is better to utilize an enzyme-free EDTA solution for sorting the BAMBIhighMFGE8high subgroup because trypsin treatment reduces the yield of BAMBIhighMFGE8high MSCs when they are sorted (Figure 3). A possible reason may be that trypsin impairs the distribution of the MFGE8 and BAMBI transmembrane proteins on the cell surface as it does for other proteins12. In contrast, the use of EDTA solution has little effect on the cell surface expression of MFGE8 and BAMBI. Second, the cell sorting protocol describes the parameter settings for the selection of the BAMBIhighMFGE8high MSCs. It is necessary to establish a blank sample and single antibody-labeled samples for accurately gating the exact BAMBIhighMFGE8high MSCs for FACS sorting.
Unlike trypsinization, the detachment of human UC-MSCs by EDTA is recommended for sorting the BAMBIhighMFGE8high MSCs in this protocol. However, other modifications involving mild cell dissociation methods, such as Dispase and Tryple E13,14, may also be applicable for harvesting BAMBIhighMFGE8high MSCs, but need to be verified. Notably, long-term dissociation of UC-MSCs should be avoided since excessive dissociation tends to reduce cell viability. In addition, ROCK inhibitors (e.g., Y-27632 at 10 µM), which prevent cell apoptosis15, can be added to the cell culture medium to increase the survival of sorted BAMBIhighMFGE8high MSCs by reducing their degree of apoptosis. Moreover, re-evaluating the purity of BAMBIhighMFGE8high MSCs in regular expansion after sorting is recommended to ensure that they maintain their primary identities, especially before further functional assay tests are performed.
Although there was no correlation between the BAMBIhighMFGE8high MSC ratio and sex, the ratios of BAMBIhighMFGE8high UC-MSCs were acquired from different donors (Figure 4). Notably, the proportion of the BAMBIhighMFGE8high subpopulation may vary depending on different passages or culture conditions. If the proportion of BAMBIhighMFGE8high cells in the UC-MSC population is extremely small, the present protocol will not be well applicable. Other limitations of this protocol include relatively severe cell damage caused by the FACS sorting method, which easily leads to the cell death of a proportion of BAMBIhighMFGE8high MSCs after cell sorting. Moreover, the current two-step primary and secondary antibody sorting methods take more time and are less efficient for cell sorting. Future applications of BAMBI and MFGE8 antibodies directly conjugated with fluorescence are preferable for increasing the sorting efficiency of BAMBIhighMFGE8high MSCs.
The isolation of each subpopulation of mixed MSCs is indispensable for revealing their authentic functions and underlying mechanisms in treating diseases. Hence, the present method provides a basic and vital tool for pooling and further investigating BAMBIhighMFGE8high UC-MSCs to completely understand their nature. Future optimization of BAMBIhighMFGE8high cell culture conditions will produce a large number of these cells in the industry for stem cell therapy for specific patients in the clinic.
Disclosures
The authors declare that they have no conflicts of interest.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant no. 82271843).
Materials
| Name | Company | Catalog Number | Comments |
| 0.6 mL microcentrifuge tube | Corning | Axygen MCT-060-A | |
| 1.5 mL microcentrifuge tubes | Beijing Labgic Technology | MCT-001-150 | |
| 100 mm cell culture dish | Beijing Labgic Technology | 12311 | |
| 12 well plate | Beijing Labgic Technology | 11210 | |
| 15 mL centrifuge tube | Nanjing Vazyme Material Technology | TCF00115 | |
| 24 well plate | Beijing Labgic Technology | 11310 | |
| 50 mL centrifuge tube | Nanjing Vazyme Material Technology | TCF00150 | |
| 5 mL Round-Bottom Tubes | Corning | FALCON 352003 | |
| 70 μm cell strainer | Falcon | 352350 | Dilution: 1:1000 |
| APC anti-human CD79a (Igα) Antibody | BioLegend | 333505 | 581 Dilution: 1:200 |
| APC/Cyanine7 anti-human CD73 (Ecto-5'-nucleotidase) Antibody | BioLegend | 344022 | G46-6 Dilution: 1:200 |
| APC-Cy7 Mouse IgG1, κ Isotype Control | BD Bioscience | 557873 | MOPC-31C (Isotype Control) Dilution: 1:200 |
| BAMBI antibody | Bioss | bs-12418R | |
| Brilliant Violet 510 anti-mouse/human CD44 Antibody | BioLegend | 103044 | 5E10 Dilution: 1:200 |
| Brilliant Violet 510 Rat IgG2b, κ Isotype Ctrl Antibody | BioLegend | 400646 | MOPC-21 (Isotype Control) Dilution: 1:200 |
| CD105 (Endoglin) Monoclonal Antibody APC | eBioscience | 17-1057-42 | HM47 Dilution: 1:200 |
| Cell Counting Chamber Slides | Shanghai QIUJING | XB-K-25 | |
| Centrifuge | Beijing BAIYANG | BY-320C | |
| ChamQ Universal SYBR qPCR Master Mix | Vazyme | Q711-02 | |
| Chloroform | XILONG Scientific | 13700908 | |
| DMEM/F-12 (1:1) basic (1x) | Gibco | C11330500BT | |
| EDTA (0.5 M), pH 8.0, Rnase free | Invitrogen | AM9260G | Dilution: 1:1000 |
| Ethanol | XILONG Scientific | 12803405 | |
| Fetal Bovine Serum (FBS) | Gibco | 10099-141C | |
| FITC Mouse Anti-Human CD34 | BD Bioscience | 555821 | IM7 Dilution: 1:200 |
| FITC Mouse Anti-Human CD45 | BD Bioscience | 555482 | AD2 Dilution: 1:200 |
| FITC Mouse Anti-Human HLA-DR | BD Bioscience | 555811 | SN6 Dilution: 1:200 |
| Flow Cytometer | BD Bioscience | FACSAria™ III Cell SorterAria | |
| Flowjo | BD Bioscience | V10 | |
| Gentle Cell Dissociation Reagent | STEMCELL Technologies | 100-0485 | |
| Goat Anti-Mouse IgG H&L (Alexa Fluor 488) | abcam | ab150113 | HI30 Dilution: 1:200 |
| Goat Anti-Rabbit IgG H&L (AlexaFluor 594) | abcam | ab150080 | Dilution: 1:1000 |
| HiScript II Q RT SuperMix for qPCR (+gDNA wiper) | Vazyme | R223-01 | |
| Inverted Microscopes | Nikon | ECLIPSE Ts2 | |
| Isopropyl Alcohol | XILONG Scientific | 12802505 | |
| MFGE8 antibody | Biorbyt | orb388429 | Dilution: 1:100 |
| Microcentrifuge | Thermo Fisher Scientific | FRESCO 21 | |
| Mouse IgG1 kappa Isotype Control APC | eBioscience | 17-4714-42 | P3.6.2.8.1 (Isotype Control) Dilution: 1:200 |
| Mouse IgG1 kappa Isotype Control FITC | eBioscience | 11-4714-42 | eBMG2b (Isotype Control) Dilution: 1:200 |
| Mouse IgG2b kappa Isotype Control FITC | eBioscience | 11-4732-42 | RTK4530 (Isotype Control) Dilution: 1:200 |
| PBS (10x) | Sangon Biotech (Shanghai) | E607016-0500 | |
| PE-Cy5 Mouse Anti-Human CD90 | BD Bioscience | 555597 | P3.6.2.8.1 (Isotype Control) Dilution: 1:200 |
| PE-Cy5 Mouse IgG1 κ Isotype Control | BD Bioscience | 550618 | |
| Penicillin-Streptomycin 100x | Cytiva | SV30010 | Dilution: 1:100 |
| Real-Time PCR System | Applied Biosystems byThermo Fisher Scientific | Q6 | |
| RNase-free water | QIAGEN | 129112 | |
| Spectrophotometer | Thermo Fisher Scientific | NanoDrop One(840-317400) | |
| Sterile micropipette tips | Beijing Labgic Technology | Dilution: 1:100 | |
| T75 cell culture flask | Beijing Labgic Technology | 13212A | |
| Thermal Cycler | Applied Biosystems byThermo Fisher Scientific | Veriti | |
| Tri reagent | Sigma Aldrich | T9424 | |
| Typsin-EDTA Solution | Bio-Channel Biotechnology | BC-CE-005 | |
| Water-Jacketed CO2 Incubator | Thermo Fisher Scientific | 3111 |
References
- Liechty, K. W., et al. Human mesenchymal stem cells engraft and demonstrate site-specific differentiation after in utero transplantation in sheep. Nat Med. 6 (11), 1282-1286 (2000).
- Zhou, T., et al. Challenges and advances in clinical applications of mesenchymal stromal cells. J Hematol Oncol. 14 (1), 24 (2021).
- Xie, Z., et al. Tnf-alpha-mediated m(6)a modification of elmo1 triggers directional migration of mesenchymal stem cell in ankylosing spondylitis. Nat Commun. 12 (1), 5373 (2021).
- Mcguire, J. J., et al. Mesenchymal stem cell-derived interleukin-28 drives the selection of apoptosis resistant bone metastatic prostate cancer. Nat Commun. 12 (1), 723 (2021).
- Yuan, X., et al. Mesenchymal stem cell therapy induces flt3l and cd1c(+) dendritic cells in systemic lupus erythematosus patients. Nat Commun. 10 (1), 2498 (2019).
- Wang, D., et al. Umbilical cord mesenchymal stem cell transplantation in active and refractory systemic lupus erythematosus: A multicenter clinical study. Arthritis Res Ther. 16 (2), R79 (2014).
- Ortiz, L. A., et al. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci U S A. 100 (14), 8407-8411 (2003).
- Phinney, D. G. Functional heterogeneity of mesenchymal stem cells: Implications for cell therapy. J Cell Biochem. 113 (9), 2806-2812 (2012).
- Wang, Z., et al. Single-cell transcriptome atlas of human mesenchymal stem cells exploring cellular heterogeneity. Clin Transl Med. 11 (12), e650 (2021).
- Chen, P., et al. Single-cell and spatial transcriptomics decodes wharton's jelly-derived mesenchymal stem cells heterogeneity and a subpopulation with wound repair signatures. Adv Sci (Weinh). 10 (4), e2204786 (2023).
- Chen, H., et al. Dissecting heterogeneity reveals a unique bambi(high) mfge8(high) subpopulation of human uc-mscs. Adv Sci (Weinh). 10 (1), e2202510 (2022).
- Tsuji, K., et al. Effects of different cell-detaching methods on the viability and cell surface antigen expression of synovial mesenchymal stem cells. Cell Transplant. 26 (6), 1089-1102 (2017).
- Lai, T. Y., et al. Different methods of detaching adherent cells and their effects on the cell surface expression of fas receptor and fas ligand. Sci Rep. 12 (1), 5713 (2022).
- Avinash, K., Malaippan, S., Dooraiswamy, J. N. Methods of isolation and characterization of stem cells from different regions of oral cavity using markers: A systematic review. Int J Stem Cells. 10 (1), 12-20 (2017).
- Galvao, I., et al. Rock inhibition drives resolution of acute inflammation by enhancing neutrophil apoptosis. Cells. 8 (9), 964 (2019).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved