Efficiency of Liquid-liquid Extraction
Przegląd
Source: Kerry M. Dooley and Michael G. Benton, Department of Chemical Engineering, Louisiana State University, Baton Rouge, LA
Liquid-liquid extraction (LLE) is a separation technique used instead of distillation when either: (a) the relative volatilities of the compounds to be separated are very similar; (b) one or more of the mixture components are temperature sensitive even near ambient conditions; (c) the distillation would require a very low pressure or a very high distillate/feed ratio.1The driving force for mass transfer is the difference in solubility of one material (the solute) in two other immiscible or partially miscible streams (the feed and the solvent). The feed and solvent streams are mixed and then separated, allowing the solute to transfer from the feed to the solvent. Normally, this process is repeated in successive stages using counter-current flow. The solute-rich solvent is called the extract as it leaves, and the solute-depleted feed is the raffinate. When there is a reasonable density difference between the feed and solvent streams, extraction can be accomplished using a vertical column, although in other cases a series of mixing and settling tanks may be used.
In this experiment, the operational goal is to extract isopropanol (IPA, ~10 - 15 wt. %, the solute) from a mixture of C8-to-C10 hydrocarbons using pure water as solvent. A York-Scheibel type (vertical mixers and coalescers, one each per physical stage) extraction column is available. Like most extractors, the overall efficiency (number theoretical stages/physical stages) of this column is quite low, especially in comparison to many distillation columns. The low efficiencies arise from both slow mass transfer (two liquid resistances instead of one as in distillation) and often also from maldistribution of the phases. The effect of agitator speed on both the solute recovery in the extract and the overall column efficiency will be evaluated.
Zasady
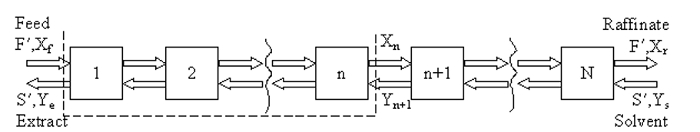
Either the a) McCabe-Thiele method, or b) a process simulator (e.g., ASPEN Plus, HYSYS, ChemSep) may be used to estimate the number of equilibrium (theoretical) stages. The McCabe-Thiele method is employed on a solvent-free basis, meaning both the solubilities of the solvent in the extract and of the diluent compound in the raffinate are neglected. A stagewise representation of counter-current liquid-liquid extraction is shown in Figure 1, where F' is the molar flow rate of the feed (approximately constant), S' is the molar flow rate of extract (approximately constant), Xf is the mole fraction of solute in feed, Ys is mole fraction of solute in the solvent, Ye is mole fraction of solute in solvent in extract stream, and Xr is mole fraction of solute in diluent in the raffinate stream.
Figure 1: Stagewise representation of the extraction process.
At steady state, a material balance on the solute between the feed end of the column and any stage, n (dotted outline above) leads to the operating line:
 (1)
(1)
In particular, the equation is satisfied at both ends of the column, so the points (Xf, Ye) and (Xr, Ys) lie on the line.The equilibrium data in the Appendix can be used in conjunction with this equation (either graphically or numerically) to step through the column.
The process simulators can do more rigorous stage-to-stage calculations, but still assuming equilibrium stages. Either the NRTL or UNIQUAC methods (both sets of parameters in the Appendix) can be used to model the equilibrium relationship. Note that the big advantage of the simulators is that they DO tell you how much solvent winds up in the extract and how much diluent winds up in the raffinate. They also can give the exit temperatures for an adiabatic column, or the heat duty needed to keep the column isothermal.
A York-Scheibel apparatus is shown in Figure 2. Feed can be introduced at the bottom (11 stages) or at the middle of the column (6 stages).

Figure 2: York-Scheibel liquid-liquid extraction apparatus.
The extraction unit consists of a 2" I.D. Pyrex column, with 11 extraction stages, each consisting of a one-inch mixing section and a four-inch wire mesh packing (coalescing) section. The column is mechanically agitated by paddlewheel-type (Rushton turbine) agitators. A variable speed motor, with a control knob and digital readout on the control panel, controls the speed of the agitator. Rotameters on the feed and solvent inlets are used to measure those flow rates. Flow rates of the extract and raffinate can be measured with a graduated cylinder and stopwatch.
The following equations relate the rotameter readings to volumetric flow rates (the flows can also be checked with a graduated cylinder):
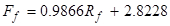 (2)
(2)
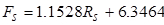 (3)
(3)
where Ff is the feed flow rate (~10 wt. % IPA) in mL/min,Rf is the feed rotameter reading,Fs is the solvent flow rate in ml/min, andRs is the solvent rotameter reading.
Procedura
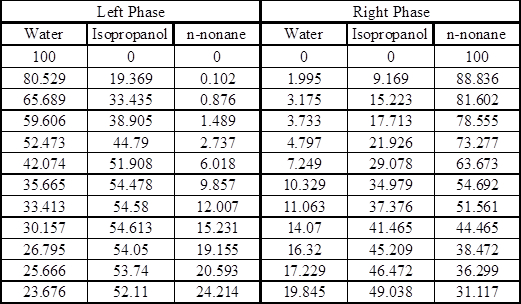
In this experiment, the properties of n-nonane are a good approximation to those of the hydrocarbon mixture for equilibrium data purposes. The ternary system water/isopropanol/n-nonane exhibits Type I equilibrium behavior (there is some composition range over which phase splitting will not take place) at room temperature. The equilibrium data for this system can be found in the Appendix.
1. Operating the York-Scheibel Column
- Fill the extractor with hydrocarbon mixture /IPA feed (if necessary) and bleed air from the feed line. Turn off the feed flow.
- Start the mixer and keep the agitator speed constant.
- Open the solvent, feed, extract, and raffinate ball valves; and start the flow of solvent (water) into the column.
- If no interface is present between the solvent entrance and the raffinate exit, let the dispersed phase rise and form the upper interface.
- When the upper interface forms, (re-)start the feed flow.
- Control the interface level by adjusting the height of the inverted U on the extract line from the bottom of the tower.The upper interface level adjustment (inverted U) is sensitive. Movements of a fraction of an inch are often sufficient.
- Periodically check the raffinate stream for steady state using gas chromatography. The gas chromotagraph will separate and quantify the components in the sample.
- Use a hydrometer to measure the specific gravity of the extract stream and determine the composition. (This also can help confirm that steady state has been reached.) The extract stream composition vs. specific gravity tables can be found in Perry's Handbook.3 This data can be used to interpolate for weight percent of IPA.
- Use a hydrometer to measure the specific gravity of the feed and raffinate for use in subsequent calculations.
2. Shutdown Procedure
- Once the experiments are complete, turn off the agitator and main power switch.
- Close the feed and solvent ball valves, leaving the raffinate and extract ball valves open.
Wyniki
Figures 3 and 4 show results when both the agitation and feed flow rates were varied over a wide range. The overall efficiency and recovery increase before becoming asymptotic, which is fairly typical of liquid-liquid extractors that are not at or near flooding. At near flooding conditions, the overall efficiency and recovery are expected to sharply decrease. Note that, unlike distillation, flooding can take place in liquid-liquid extraction at either high solvent or high feed rates (or ratios).1 In this experiment, the lighter organic phase is also the dispersed (droplet) phase, so at high feed rates it is expected that the droplets coalescence prior to flooding, leading to lower rates of mass transfer and, therefore, lower recoveries and efficiencies. At high solvent rates the droplets should remain small, so it is expected that the recovery and efficiency remain high until very near the flooding point.
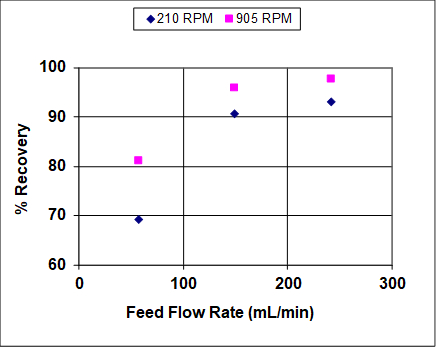
Figure 3: Percent recovery of IPA from hydrocarbon mixture into water, for a York-Scheibel column, 11 stages, 16 - 18 mol% IPA in Isopar E (feed), S/F (molar) = 1.5.
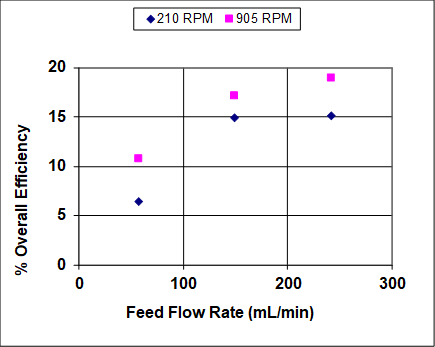
Figure 4: Percent overall stage efficiency for IPA extraction using a York-Scheibel column, 11 stages, 16 - 18 mol% IPA in hydrocarbon mixture (feed), S/F (molar) = 1.5.
As seen from Figures 3 and 4, increasing the agitation rate increases both the recovery and overall efficiency. This is because with greater power input the droplets of the dispersed phase are smaller - the observed dependence is roughly inverse with respect to agitator speed.4 The "a" parameter (interfacial area/total volume) that appears in mass transfer correlations and fundamental mass flux equations can be written as follows for uniform-size spherical droplets:
a = 6 ε/d (4)
where ε is the volume fraction of the dispersed phase. While ε can increase with an increase in either phase's superficial velocity, its changes are usually less marked than the change of diameter with respect to speed. So (usually) the more speed, the more interfacial area, leading to faster mass transfer.
The exception to the above discussion is at very high speeds, which were not reached in Figs. 3 and 4, where the two phases are so well-mixed that if the interfacial tension between them is low, emulsification will take place. The formation of an emulsion negatively impacts recovery and efficiency because the phases can no longer separate cleanly to move up or down to the next physical stage.Emulsification is a problem in many liquid-liquid extractions and where it cannot be limited a series of mixer and settler vessels in series is often preferred to column-type designs such as sieve trays or York-Scheibel units.
Wniosek i Podsumowanie
Liquid-liquid extraction (LLE) is an alternative to distillation which relies upon solvent-feed immiscibility (or slight miscibility) and favorable solute partition coefficients to attain high solute recoveries in a solvent phase at as low a solvent/feed ratio as practical. Although the range of flows (the "turndown") over which LLE will be effective is often limited, and while stage efficiencies are low such that phase equilibrium is not attained, certain mixtures just cannot be separated using other methods in a continuous countercurrent process. Mathematical analysis of the equilibrium operation of such extractors follows a familiar McCabe-Thiele-type procedure (although reflux is often lacking, so only one operating line). The non-equilibrium ("rate-based") analysis of LLEs is complex and depends strongly on the relative velocity between the two phases (the "slip velocity"), bubble size, and dispersed phase fraction, all of which can be observed but are difficult to predict.
To perfectly describe the hydraulics and mass transfer of a typical LLE is beyond the capability of even the most sophisticated process simulators, at present. Therefore, design of industrial units still relies on scale-up from pilot-plant-type units, such as that which was tested in this experiment. Normally the engineer attempts to duplicate key descriptors such as the "a" parameter, solvent/feed ratio, total agitator power input/volume, feed location and number of physical stages to keep the stage efficiency and recovery constant during scale-up. Even so, scale-up is an inexact science, and impurities, which alter the interfacial tension, can greatly impact the performance of real systems. The more factors that are held constant, the more likely the scale-up will be successful.
There are many different LLE contactors: a series of mixers - settler vessels, structured packings similar to those used in absorbers and distillation columns, sieve tray columns, rotating disk contactors (similar to the York-Scheibel, but with baffles instead of mesh), Kuhni contactors (a combination of rotating disk and sieve trays), and Podbielniak contactors ("Pods"), where the flow is radial and centrifugal force is used to enhance liquid phase separation.5
A classic example of industrial LLE is the separation of acetic acid from water using ethyl ether or ethyl acetate;6 it is preferred over distillation at lower acetic acid concentrations. Possibly the biggest volume LLE process is that of propane deasphalting, which is used to refine lubricating oils in refineries at near-supercritical conditions.1 However, most applications are found in the production of specialty chemicals and in pharmaceutical industries, ranging from citric acid extraction from fermentation broth to purification of antibiotics and protein purifications.1In these cases, a wide variety of oxygenated organic solvents or two-phase aqueous systems (with one phase being mostly water and the other aqueous dissolved salts and polymers) are utilized. For the latter, a typical polymer system is poly(ethylene glycol)/dextran with NaCl and Na2SO4 as salts. Applications include red blood cell separation and extraction of the phophofructokinase enzyme from S. cerevisiae.7
Appendix – Equilibrium Data8
Experimental Tie Lines in Mole Percent at 25 °C
Specific Model Parameters in Kelvin
| UNIQUAC | NRTL (a = 0.2) | ||||
| I | J | AIJ | AJI | AIJ | AJI |
| 1 | 2 | -186.05 | 104.6 | 814.26 | -468.11 |
| 1 | 3 | 361.91 | 621.82 | 3151 | 1367.4 |
| 2 | 3 | -126.43 | 311.7 | 581.79 | -25.91 |
R1 = 0.92 R2 = 2.7792 R3 = 6.523
Q1 = 1.4 Q2 = 2.508 Q3 = 5.476
| Mean Deviation between Calculate and Experimental Concentrations in Mol. % | |
| UNIQUAC (specific parameters) | 1.4 |
| NRTL (specific parameters) | 0.54 |
| UNIQUAC (default parameters) | 1.68 |
Odniesienia
- T.C. Frank, L. Dahuron, B.S. Holden, W.D. Prince, A.F. Seibert and L.C. Wilson, Ch. 15 of “Chemical Engineers Handbook, 8th Edition”, R.H. Perry and D.W. Green, Eds., McGraw-Hill, New York, 2008.
- W.L. McCabe, J.C. Smith, and P. Harriott, “Unit Operations of Chemical Engineering”, 7th Ed., McGraw-Hill, New York, 2005, Ch. 23; C.J. Geankoplis, “Transport Processes and Unit Operations”, 3rd Ed., Prentice-Hall, Englewood Cliffs, 1993, Ch. 12; R.K. Sinnott, “Coulson and Richardson’s Chemical Engineering Vol. 6 – Chemical Engineering Design (4th ed.): http://app.knovel.com/hotlink/toc/id:kpCRCEVCE2/coulson-richardsons-chemical/coulson-richardsons-chemical
- B.E. Poling, G.H. Thomson, D.G. Friend, R.L. Rowley and W.V. Wilding, Ch.2 of “Chemical Engineers Handbook, 8th Edition”, R.H. Perry and D.W. Green, Eds., McGraw-Hill, New York, 2008.
- J.C. Godfrey, R. Reeve and F.I.N. Obi, Chem. Eng. Prog. Dec. 1989. pp. 61-69; I. Alatiqi, G. Aly, F. Mjalli and C.J. Mumford, Canad. J. Chem. Eng., 73, 523-533 (1995).
- http://www.pharmaceuticalonline.com/doc/podbielniak-contactor-a-unique-liquid-liquid-0003 (accessed 12/19/16).
- C.J. King, Ch. 18.5 of “Handbook of Solvent Extraction”, T.C. Lo, M.H.I Baird and C. Hanson, Eds., Wiley, New York, 1983.
- “Methods in Enzymology, Vol. 228, Aqueous Two-Phase Systems,” H. Walter and G. Johannson, Eds., Academic, San Diego, 1994.
- A.I. Vorobeva and M.Kh. Karapetyants, Zh. Fiz. Khim., 41, 1984 (1967). Fits to data from: J. Gmehling, and U. Onken, "Vapor-liquid equilibrium data collection", Dechema, 1977.
Tagi
Przejdź do...
Filmy z tej kolekcji:

Now Playing
Efficiency of Liquid-liquid Extraction
Chemical Engineering
48.4K Wyświetleń

Testing the Heat Transfer Efficiency of a Finned-tube Heat Exchanger
Chemical Engineering
17.9K Wyświetleń

Using a Tray Dryer to Investigate Convective and Conductive Heat Transfer
Chemical Engineering
43.9K Wyświetleń

Viscosity of Propylene Glycol Solutions
Chemical Engineering
32.7K Wyświetleń

Porosimetry of a Silica Alumina Powder
Chemical Engineering
9.6K Wyświetleń

Demonstration of the Power Law Model Through Extrusion
Chemical Engineering
10.0K Wyświetleń

Gas Absorber
Chemical Engineering
36.6K Wyświetleń

Vapor-liquid Equilibrium
Chemical Engineering
88.6K Wyświetleń

The Effect of Reflux Ratio on Tray Distillation Efficiency
Chemical Engineering
77.6K Wyświetleń

Liquid Phase Reactor: Sucrose Inversion
Chemical Engineering
9.7K Wyświetleń

Crystallization of Salicylic Acid via Chemical Modification
Chemical Engineering
24.2K Wyświetleń

Single and Two-phase Flow in a Packed Bed Reactor
Chemical Engineering
18.9K Wyświetleń

Kinetics of Addition Polymerization to Polydimethylsiloxane
Chemical Engineering
16.1K Wyświetleń

Catalytic Reactor: Hydrogenation of Ethylene
Chemical Engineering
30.3K Wyświetleń

Evaluating the Heat Transfer of a Spin-and-Chill
Chemical Engineering
7.4K Wyświetleń
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone