Method Article
Acquisition and Semi-Automated Analysis of Respiratory Muscle Surface Electromyography
W tym Artykule
Podsumowanie
Here, we describe a protocol to record and analyze respiratory electromyography (EMG) signals. It includes the anatomic references for placing the EMG electrodes over several respiratory muscles, removing electrocardiographic noise from the EMG signals, and acquiring the EMG root mean square (RMS) and onset timing of activity.
Streszczenie
Evaluating respiratory drive presents challenges due to the obtrusiveness and impracticality of current methods like functional magnetic resonance imaging (fMRI). Electromyography (EMG) offers a surrogate measure of respiratory drive to the muscles, allowing the determination of both the magnitude and timing of muscle activation. The magnitude reflects the level of muscle activation, while the timing indicates the onset and offset of muscle activity relative to specific events, such as inspiratory flow and activation of other muscles. These metrics are critical for understanding respiratory coordination and control, especially under varying loads or in the presence of respiratory pathophysiology. This study outlines a protocol for acquiring and analyzing respiratory muscle EMG signals in healthy adults and patients with respiratory health conditions. Ethical approval was obtained for the studies, which included participant preparation, electrode placement, signal acquisition, preprocessing, and postprocessing. Key steps involve cleaning the skin, locating muscles via palpation and ultrasound, and applying electrodes to minimize electrocardiography (ECG) contamination. Data is acquired at a high sampling rate and gain, with synchronized ECG and respiratory flow recordings. Preprocessing includes filtering and transforming the EMG signal, while postprocessing involves calculating onset and offset differences relative to the inspiratory flow. Representative data from a healthy male participant performing incremental inspiratory threshold loading (ITL) illustrate the protocol's application. Results showed earlier activation and prolonged duration of extradiaphragmatic muscles under higher loads, correlating with increased EMG magnitude. This protocol facilitates a detailed assessment of respiratory muscle activation, providing insights into both normal and pathophysiologic motor control strategies.
Wprowadzenie
Respiratory drive (i.e., the output of respiratory centers to respiratory muscles) is challenging to evaluate due to the obtrusive, often impractical nature of evaluative methods such as functional magnetic resonance imaging (fMRI). Moreover, the small size of the respiratory centers located in the brain stem is difficult to localize and is sensitive to alterations by physiologic noise1,2. Measurements of respiratory drive are important because of their association with important clinical outcomes such as dyspnea, an indication of respiratory distress. Electromyography (EMG) is a surrogate of respiratory drive to the respiratory muscles3. Respiratory muscle EMG allows the determination of muscle activity and its intensity by way of the root mean square (RMS) of the EMG signal. Additionally, the timing of muscle activation can be assessed by identifying the onset and offset of their activity (EMG, onset and EMG, offset, respectively)1,2,3,4,5,6,7,8,9,10,11.
The magnitude of the EMG signal refers to the electrical potential generated by muscle cells when they contract, indicating their level of muscle activation12. The magnitude of the EMG signal can vary depending on factors such as the intensity of muscle contraction, the number of motor units recruited, the electrode placement, the movement of muscle and subcutaneous tissues, and the specific characteristics of the muscle being measured12.
The timing of the EMG signal refers to when the electrical activity occurs relative to a specific event or action (e.g., relative to inspiratory flow for breathing)13. The onset timing indicates when muscle activation begins, while the offset timing indicates when muscle activity decreases, ceases, or is in the relaxation phase13. Timing among the activation of several respiratory muscles will facilitate an understanding of coordination and control mechanisms during breathing. Assessing the consistency or variability of timing patterns over time or in individuals can help identify physiologic and pathophysiologic motor control strategies associated with acute or chronic ventilatory failure.
Both the magnitude and timing of the respiratory muscle EMG have been associated with important clinical outcomes12,13,14. The diaphragm generates the majority of ventilation at rest15. When the respiratory demand increases, such as during exercise or increased inspiratory loading associated with lung diseases (e.g., chronic obstructive pulmonary disease, interstitial lung disease, or acute respiratory distress syndrome), extradiaphragmatic respiratory muscles boost ventilation, which can augment or offset diaphragm contractile requirements15. Thus, in addition to the increasing magnitude of diaphragm EMG, the magnitude of extradiaphragmatic muscle EMG will also increase.
Activation of extradiaphragmatic respiratory muscles can protect the diaphragm from developing fatigue16. However, early activation (onset) and prolonged activation have been associated with acute and chronic ventilatory failure14,17,18. The objective here is to describe a protocol to acquire and analyze both the timing and magnitude of respiratory muscle EMG signals in both healthy adults and patients with suspected or confirmed respiratory pathophysiology. This protocol includes previously validated steps from data acquisition to quantify the timing and magnitude of EMG activity13,19.
Protokół
Studies employing this technique have received ethical approval from the University of Toronto and St. Michael's Hospital located in Toronto, Canada, and the University Hospital Gasthuisberg, Leuven, Belgium. One specific protocol is described here. General discussion about several alternative surface EMG (sEMG) approaches have been proposed for the respiratory muscles and are reported elsewhere12.
1. Participant preparation and placement of sEMG electrodes
- To ensure adequate visualization, ask males not to wear a shirt and females to wear a sports bra or singlet. Use a hospital gown with a front opening to provide adequate access and maintain modesty.
- If the participant has long hair, have it tied back and pinned out of place so the scalene and sternocleidomastoid can be evaluated.
- Position the participant in sitting or half-lying position.
- If excess chest or neck hair is found, shave the area for electrode placement for sEMG.
- To reduce skin impedance, clean the skin of oil and dead skin.
- Do this by rubbing with an alcohol wipe and allowing the alcohol to evaporate (i.e., air dry) before the electrode is applied.
- If the skin is lighter, it may appear slightly red, but more importantly, ensure no obvious dirt, oil, or dry skin is apparent where electrodes will be placed. However, avoid excessive rubbing to prevent skin damage. Avoid application of electrodes to areas of broken skin or other skin lesions.
- Locate muscles of interest by landmarking, palpation and/or ultrasound.
NOTE: Ultrasound may be useful in landmarking the costal diaphragm20. Figure 1 shows examples of locations where electrodes can be placed for sEMG of respiratory muscles. - Place sEMG electrodes on the right side of the thorax, farther from the heart, to decrease the amplitude of the ECG signal and minimize its contamination.
- Apply paired EMG electrodes with a 2 cm inter-electrode distance at the center of the muscle belly along the longitudinal alignment of muscle fibers.
- For the costal diaphragm/intercostals, landmark the anterior axillary line and midclavicular line and place the paired electrodes vertically between these two lines at the level of the seventh or eighth intercostal space.
- For the scalene, landmark the posterior triangle of the neck and place the paired electrodes along the longitudinal axis of the muscle at the level of the cricoid process.
- For the parasternal intercostals, landmark the second intercostal space 1-2 cm lateral to the right side of the sternum and place the paired electrodes along the longitudinal axis of the muscle.
- For the sternocleidomastoid, landmark the suprasternal notch and mastoid process. Accentuate the right sternocleidomastoid muscle belly by placing the operator's hand on the left side of the participant's chin and asking the participant to gently perform isometric left rotation against the hand. Place the paired electrodes at the midpoint of the muscle belly along its longitudinal axis.
- Some EMG systems may require a ground sensor. If required, place the ground sensor on a bony structure close to respiratory muscles (e.g., clavicle, C7 cervical spinous process).
- Attach EMG sensor clips to EMG electrodes. Be sure that wires from EMG sensors from two different muscles (even if wireless) do not overlap and contaminate or provide crosstalk between the two muscles.
NOTE: Wires from the same sensor can overlap, but wires from two different sensors should not. - Apply further fixation of EMG electrodes and sensors by using double-sided tapes that secure the underside of the sensor to the skin.
- Apply medical-grade hypoallergenic tape over the top of the sensors to further secure each sensor to the skin. Avoid applying excessive pressure, and as mentioned above, ensure that the wires from different sensors do not overlap.
2. Signal acquisition
- Select the pre-set template on the data acquisition software and press Open. The template will have the following pre-set parameters: A high pass filter (0.5-20 Hz) in the EMG signal to reduce low-frequency artifacts to facilitate real-time visualization.
- Set the sampling rate of the EMG signal of at least 1 kHz.
- Set the gain of the EMG signal to 1000.
- Set the template to acquire a synchronized recording of ECG and respiratory flow.
- Acquire sEMG and ECG data according to protocol, e.g., during a spontaneous breathing trial in a mechanical ventilation patient.
- After the protocol is complete, stop recording and save the data file.
NOTE: Figure 2 shows screenshots of the software showing applied filtering.
3. Preprocessing after data acquisition
- Open the software and confirm the parameters to be used for analysis of the EMG signal (a bi-directional high pass filter of 5 Hz, the Least Mean Square (LMS) Adaptive Filter to remove ECG contamination, root mean square transform with a moving window for 0.02 s) and press Continue.
- Select the file to be analyzed and press OK.
- Define the time interval to be analyzed (if the total duration of the file is to be analyzed, it will be from 0 s to maximum time), press Select the Range and Continue, and then press Conditioning.
- Press the Analyze button to apply the pre-selected parameters (see step 3.1). Visualize the analyzed EMG signal. Press Rescaled on 1 button to show the EMG signal normalized by its maximum value during the recorded period.
- Press the Continue to Calculate On Off button. Based on the derivative function of the EMG signal it will detect the onset timing of the EMG activity. Press the On and Off button.
- Select the EMG signal from the muscle that needs to be visualized. The visualization can be alternated between muscles to allow the visual inspection of all recorded EMG signals. Press the STOP Looking and Go To Saving button. Press Saving.
- Select the data to be saved. It is possible to reduce the signals before saving (e.g., from 1000Hz to 100Hz). Press Save Processed Data, select the computer folder in which the file is to be saved, and give it a name. Press Save again to confirm.
4. Postprocessing
- Open the saved file using software that provides the ability to compute calculations (e.g., Excel, R, Phyton, Matlab). Determine each breath either by the on and off time of the flow signal and calculate the EMG peak RMS and the EMG mean RMS for each breath.
- For EMG onset, calculate the Absolute difference (in milliseconds) between EMG onset and inspiratory flow onset (INSP, onset):
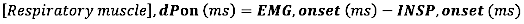
- For EMG offset, calculate the Absolute difference (in milliseconds) between EMG offset and the end of inspiratory flow (INSP,offset)
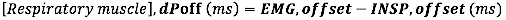
- For EMG onset relative to the duration of inspiratory time, calculate the relative difference (to the duration of Ti) between EMG onset and INSP,onset:
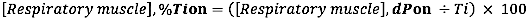
- For EMG offset relative to the duration of inspiratory time, calculate the relative difference (to the duration of Ti) between EMG offset and INSP,offset:
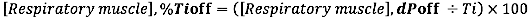
where dP is the time difference between the EMG,onset and inspiratory flow onset (INSP,onset) or between the EMG,offset and inspiratory flow offset (INSP,offset).)
Wyniki
Data is provided for a male participant (22 years old; weight: 100 kg; height: 185 cm; BMI: 29 kg/m2) with normal spirometry and inspiratory muscle strength (FEV1: 4.89 L/s [97% of predicted]; maximal inspiratory pressure: 151 cmH2O [136% of predicted]). He performed an incremental inspiratory threshold loading (ITL) up to task failure using a protocol previously described21,22,23. An overview of the data acquisition system is depicted in Figure 1. The participant sat comfortably in a chair with nose clips on, forearms resting on an adjustable desk, and the head supported neutrally on a head-chin rest. The participant breathed through a mouthpiece connected to a two-way non-rebreathing valve, which was connected to a heated pneumotach and an ITL device. This ITL device imposed a load during inhalation but none during exhalation. The ITL test began with a warm-up load (-12 cmH2O), followed by increments of loading the plunger by 50 g every 2 min until task failure. Task failure was defined as the point when the participant took his mouth off the mouthpiece or when he could no longer generate sufficient inspiratory pressure to lift the plunger on three consecutive breaths. For this participant, task failure was reached at -120 cmH2O.
Figure 3 shows raw and filtered diaphragm EMG signals in addition to the ECG and inspiratory flow signals during the ITL. Notably, the ECG artifacts depicted in diaphragm raw EMG (uppermost tracing) are not present (or less present) in the diaphragm filtered EMG (lowermost tracing). Moreover, the wandering baseline that can be noted in the diaphragm raw EMG does not appear after filtering was applied.
Figure 4 shows the onset timing of the respiratory muscle EMG at low and high loads. At low load, only the scalene and parasternal intercostal onset activity is detected before the onset of the inspiratory flow, whereas the diaphragm and sternocleidomastoid onset activity was detected after the onset of the inspiratory flow. However, while breathing to overcome higher loads during ITL, earlier activation (relative to flow) of the diaphragm, parasternal intercostal, scalene, and sternocleidomastoid is observed.
Figure 5 shows the duration time of the respiratory muscle EMG activity at low and high loads. The duration of the EMG activity of the diaphragm, parasternal intercostal, and scalene are similar at low and high loads. However, the duration of sternocleidomastoid activity was longer at the high load compared to the low load.
Figure 6 shows the EMG RMS of the diaphragm, parasternal intercostal, scalene, and sternocleidomastoid. At high loads the EMG RMS of all these muscles was higher compared to low loads, representing the greater muscle activity needed to overcome the increased loads.
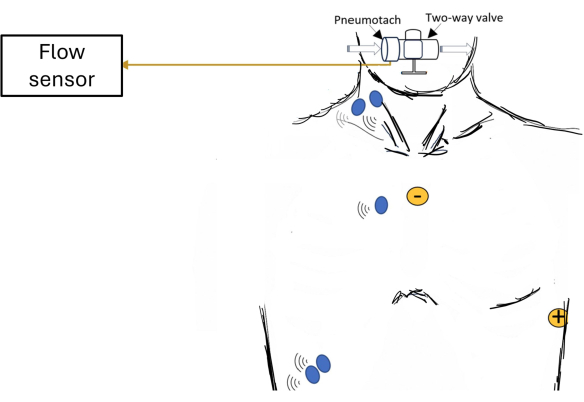
Figure 1: Schematic of participant set-up showing an overview of data acquisition. Examples of electrode placements are shown for surface electromyography (EMG; blue dots) of respiratory muscles and electrocardiogram (ECG; yellow dots). Please click here to view a larger version of this figure.

Figure 2: Example of working screens of the software showing applied filtering. (A) Initial screen showing recorded signals and filtering parameters. (B) Screen showing the RMS of the EMG after applications of filters (green tracing). Flow is shown in white. Horizontal lines demonstrated the onset of EMG activity (yellow), the onset of inspiratory flow (green line), the offset of EMG activity (dashed yellow line), and the end of inspiratory flow (red line). Abbreviations: SCM: sternocleidomastoid. RMS: root mean square. Please click here to view a larger version of this figure.
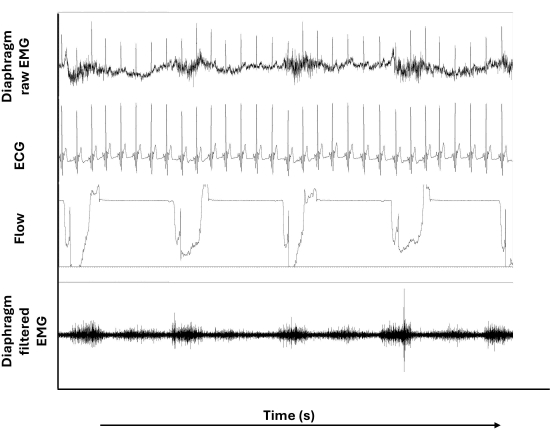
Figure 3: Raw and filtered diaphragm surface EMG. From top to bottom, panels show the raw EMG signal of the diaphragm, the electrocardiogram (ECG) signal, the inspiratory flow signal, and the filtered EMG signal of the diaphragm. Please click here to view a larger version of this figure.

Figure 4: Onset time of the respiratory muscle EMG signal during low (-12 cmH2O) vs. high loads (-120 cmH2O) during incremental inspiratory threshold loading to task failure. Data is from a male participant. Y-axes depict the time difference between the onset time of the surface EMG and inspiratory flow in seconds, where zero is the onset of the inspiratory flow. Negative values indicate that the EMG onset occurred before the onset of the inspiratory flow, whereas positive values indicate that the EMG onset occurred after the onset of the inspiratory flow. The panels show the onset time of the respiratory muscle EMG activity of the (A) diaphragm, (B) parasternal intercostal, (C) scalenes, and (D) sternocleidomastoid. Please click here to view a larger version of this figure.
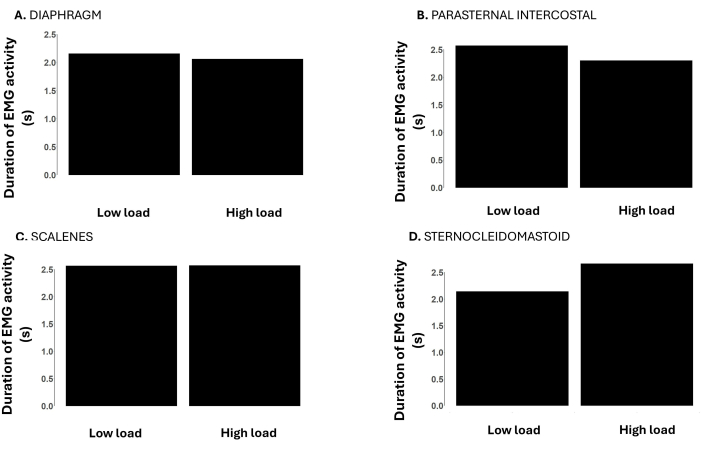
Figure 5: Duration time of the respiratory muscle EMG signal during low (-12 cmH2O) vs. high loads (-120 cmH2O) during an incremental inspiratory threshold loading up to task failure. Data is from a male participant. Y-axes depict the duration of the EMG activity (from EMG onset to offset) in seconds. The panels show the duration of the respiratory muscle EMG activity of the (A) diaphragm, (B) parasternal intercostal, (C) scalenes, and (D) sternocleidomastoid. Please click here to view a larger version of this figure.
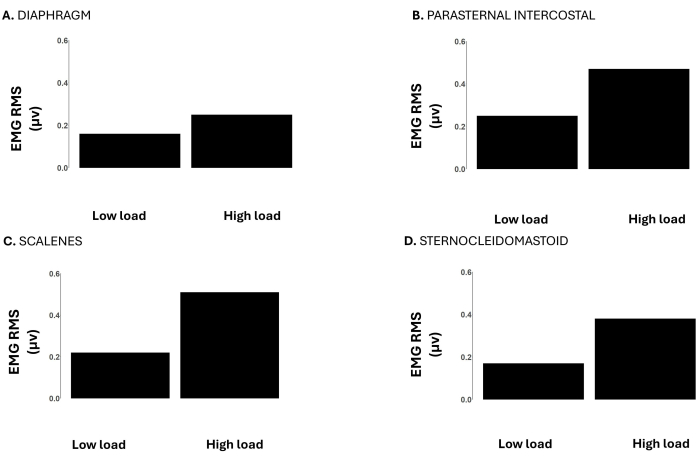
Figure 6: RMS of the respiratory muscle EMG signal during low (-12 cmH2O) vs. high loads (-120 cmH2O) during an incremental inspiratory threshold loading up to task failure. Data is from a male participant. Y-axes depict the EMG RMS in microvolts. The panels show the EMG RMS of the (A) diaphragm, (B) parasternal intercostal, (C) scalenes, and (D) sternocleidomastoid. Please click here to view a larger version of this figure.
Dyskusje
Removal of cardiac activity artifacts from the EMG signal is complex due to their overlapping bandwidth spectrums. The majority of the EMG frequency spectrum is between 20 and 250 Hz, while the ECG frequency spectrum is between 0 Hz and 100 Hz. For some analyses (i.e., timing), it is essential to derive the EMG signal without ECG contamination to achieve accuracy and interpretability of the EMG magnitude and timing. The least mean square (LMS) adaptive filter by utilizing frequencies, is an algorithm that recognizes a pattern. In this case, the algorithm removes the ECG frequency content from the combined ECG-EMG signal. It was determined that a filter length of 70 and step size of 0.01 are optimal coefficients that provide the least error and best overall results24. The ECG recorded synchronously with the EMG is used to tune the coefficients of the Finite Impulse Response (FIR) filter continuously. Thus, the removal is very precise and can accommodate a variable heart rhythm, which can occur throughout testing. The ECG filtering algorithm is pre-set, and the ECG channel is automatically recognized. Bi-directional filtering minimizes time shift on the detection of the onset time of the EMG signal. It is used to eliminate phase distortion, which can be common with standard (unidirectional) filtering methods.
The first derivative function of each muscle EMG RMS is calculated. A positive or negative derivative indicates an increasing or decreasing EMG RMS, respectively. The application of the derivative function to determine the increasing and decreasing phases of the EMG RMS enables the algorithm to perform accurately despite variations of "baselines" that do not return to zero. Because of the variability of the baseline among activation bursts, an algorithm utilizing the EMG RMS absolute values could not consistently identify the EMG onsets and offsets.
To detect the EMG onset, the beginning of each breath's inspiratory phase is determined within ±1 millisecond's accuracy from the flow signal (INSP,onset). Firstly, the maximum increase of the EMG RMS on a breath-by-breath basis is determined as a reference to detect the onset time of the EMG activity (EMG,onset). In order to account for the variable EMG baseline, EMG,onset is defined as the timepoint when it reaches 5% of its maximum (±1 ms) amplitude. Consideration of this 5% threshold avoids inadvertently identifying baseline EMG RMS variability as activations. Concurrent EMG filtering and the EMG,onset detection are applied to several muscles. Figure 2B shows the EMG,onset detection for the sternocleidomastoid in a representative breath.
The software allows modification of pre-set parameters. Different levels of high or low-pass filters can be used, and smoothing can be applied if required. The increase in the EMG signal to detect the EMG,onset is pre-set at 5%, but this threshold value can also be modified. When evaluating ventilatory loading, mouth pressure can be additionally measured as an index of load. Likewise, end-tidal CO2 can be monitored whereby efforts are made to maintain it close to the normal range by coaching the participant to adjust their level of ventilation or by altering inspired CO2.
The described protocol follows international recommendations for signal acquisition and processing and the developed algorithm for filtering has been validated25. Nonetheless, careful visual inspection of the EMG signal is required throughout each step to ensure that only good-quality signals are analyzed. Other approaches have been used in the literature to filter out ECG artifacts from the EMG signals, including high-pass filters with high cutoff frequencies (e.g., up to 200 Hz), gating, and wavelet denoising. High-pass filters with high cutoff frequencies will also delete much of the EMG signal, modifying its frequency spectrum and amplitude26. Gating detects strong ECG artifacts and deletes the contaminated EMG signal as well as EMG signals around it, causing loss of temporal information and affecting the detection of the EMG timing (e.g., onset and offset)27,28. Wavelet denoising is well balanced between complexity and performance; however, it can cut off large EMG activities burst29. A least mean square adaptive filter in the frequency domain was used here, which only removes frequencies of the signal associated with the patient's own ECG13,19. While it allows reliable measures of EMG time and amplitude, it requires continuous and simultaneous ECG recordings.
To date, this approach can only be applied in offline data analysis. Further development of the software and the establishment of real-time communication of available EMG systems with the software would provide real-time visualization and analysis of respiratory muscle EMG. This would offer the potential for utilizing respiratory muscle EMG to support real-time clinical decision-making.
Respiratory muscle EMG can provide information regarding muscle activity and respiratory drive. It is a relatively complex technique that encompasses several steps to assure good signal quality. This protocol describes steps to assure good skin preparation, signal acquisition, and processing and provides information relative to both the magnitude and timing of the activity of the respiratory muscles, which have both been associated with clinical outcomes. This protocol has received Research Ethics Authorization from several institutions internationally.
Ujawnienia
The authors declare they have no conflict of interest to disclose.
Podziękowania
AR is supported by a Canadian Institutes of Health Research (CIHR) Fellowship (#187900) and UM was funded by Mitacs (IT178-9 -FR101644).
Materiały
| Name | Company | Catalog Number | Comments |
| Adjustable table | Amazon | VIVO Electric Height Adjustable 102 cm x 61 cm Stand Up Desk | Enables fine adjustment for trunk and mouthpiece position |
| Air filters | Cardinal | https://cardinalfilters.com/ | |
| Analog output cable | A-Tech Instruments Ltd. | 25 pin D-sub Female to 16xBNC male; 16xRG-174 -16 x 3ft cable | To connect EMG (Noroxan) to data acquisition system (PowerLab) |
| Bioamp for ECG | ADInstruments | ML138 | |
| Desktop or Laptop | N/A | N/A | Capacity for data acquisition system including EMG |
| Double sticks for EMG probes | Noraxon | https://shop.noraxon.com/products/dual-emg-electrodes | |
| Electromyography | Noraxon | Noraxon Ultium Myomuscle with 8 smart leads. https://www.noraxon.com/our-products/ultium-emg/ | |
| EMG electrodes | Duotrode | N/A | |
| Gas analyzer | ADInstruments | ML206 | |
| Gloves | Medline | https://www.medline.com/jump/category/x/cat1790003 | |
| Metricide or protocol to disinfect valves & mouthpieces | Medline | https://www.medline.com/product/MetriCide-28-Disinfectant/Disinfectants/Z05-PF27961?question=metricide | |
| Oximeter pod | ADInstruments | ML320/F | https://www.adinstruments.com/products/oximeter-pods |
| Pneumotach | ADInstruments | MLT3813H-V | https://www.adinstruments.com/products/heated-pneumotach-800-l-heater-controller |
| Powerlab and Labchart Data Acquisition System | ADInstruments, Inc. | https://m-cdn.adinstruments.com/brochures/Research_PowerLab _Brochure_V2-1.pdf | Acquires mouth pressure, ECG, end-tidal CO2, flow (to derive respiratory rate, tidal volume, minute ventilation) and EMG. |
| Pressure transducer with single or dual channel demodulator | Validyne.com | Www.Validyne.Com/Product/Dp45_Low_Pressure_ Variable_Reluctance_Sensor/ | Range depends on population being tested i.e. patients or healthy (Www.Validyne.Com/Product/Cd280_Multi_Channel_Carrier_ Demodulator/; www.Validyne.Com/Product/Cd15_General_Purpose_Basic _Carrier_Demodulator/) |
| Silicone mouthpieces | Hans Rudolph | https://www.rudolphkc.com/ | Small bite size |
| Table model chin rest | Sacor Inc. | Model 600700 | https://sacor.ca/products/head-chin-rest-table-model-with-white-chin-rest-cup |
| Two-way t-piece nonrebreathing valve with sampling port | Hans Rudolph | 1410 Small | |
| Ultrasound | GE Healthcare | Vivid i BT12 Cardiac system with Respiration and 12L-RS Linear Array Transducer | Requires resolution to landmark respiratory muscles including appositional region of diaphragm |
Odniesienia
- Vaporidi, K., et al. Respiratory drive in critically ill patients. Pathophysiology and clinical implications. Am J Respir Crit Care Med. 201 (1), 20-32 (2020).
- Ciumas, C., Rheims, S., Ryvlin, P. fMRI studies evaluating central respiratory control in humans. Front Neural Circuits. 16, 982963 (2022).
- Domnik, N. J., Walsted, E. S., Langer, D. Clinical utility of measuring inspiratory neural drive during cardiopulmonary exercise testing (CPET). Front Med (Lausanne). 7, 483 (2020).
- Hudson, A. L., et al. Activation of human inspiratory muscles in an upside-down posture. Respir Physiol Neurobiol. 226, 152-159 (2016).
- Hodges, P. W., Gandevia, S. C. Pitfalls of intramuscular electromyographic recordings from the human costal diaphragm. Clin Neurophysiol. 111 (8), 1420-1424 (2000).
- Nguyen, D. a. T., et al. Differential activation of the human costal and crural diaphragm during voluntary and involuntary breaths. J Appl Physiol (1985). 128 (5), 1262-1270 (2020).
- Hudson, A. L., Gandevia, S. C., Butler, J. E. Common rostrocaudal gradient of output from human intercostal motoneurones during voluntary and automatic breathing. Respir Physiol Neurobiol. 175 (1), 20-28 (2011).
- Epiu, I., et al. Inspiratory muscle responses to sudden airway occlusion in chronic obstructive pulmonary disease. J Appl Physiol (1985). 131 (1), 36-44 (2021).
- Sinderby, C., et al. An automated and standardized neural index to quantify patient-ventilator interaction. Crit Care. 17 (5), R239 (2013).
- Estrada, L., Sarlabous, L., Lozano-Garcia, M., Jane, R., Torres, A. Neural offset time evaluation in surface respiratory signals during controlled respiration. 2019, 2344-2347 (2019).
- Luo, Y. M., Moxham, J. Measurement of neural respiratory drive in patients with COPD. Respir Physiol Neurobiol. 146 (2-3), 165-174 (2005).
- Jonkman, A. H., et al. Analysis and applications of respiratory surface EMG: Report of a round table meeting. Crit Care. 28 (1), 2 (2024).
- Rodrigues, A., et al. Semi-automated detection of the timing of respiratory muscle activity: Validation and first application. Front Physiol. 12, 794598 (2021).
- Parthasarathy, S., Jubran, A., Tobin, M. J. Cycling of inspiratory and expiratory muscle groups with the ventilator in airflow limitation. Am J Respir Crit Care Med. 158 (5 Pt 1), 1471-1478 (1998).
- De Troyer, A., Boriek, A. M. Mechanics of the respiratory muscles. Compr Physiol. 1 (3), 1273-1300 (2011).
- Laghi, F., et al. Diaphragmatic neuromechanical coupling and mechanisms of hypercapnia during inspiratory loading. Respir Physiol Neurobiol. 198, 32-41 (2014).
- Parthasarathy, S., Jubran, A., Laghi, F., Tobin, M. J. Sternomastoid, rib cage, and expiratory muscle activity during weaning failure. J Appl Physiol (1985). 103 (1), 140-147 (2007).
- Parthasarathy, S., Jubran, A., Tobin, M. J. Assessment of neural inspiratory time in ventilator-supported patients. Am J Respir Crit Care Med. 162 (2 Pt 1), 546-552 (2000).
- Dacha, S. R. A., Louvaris, Z., Janssens, L., Janssens, W., Gosselink, R., Langer, D. Effects of inspiratory muscle training (IMT) on dyspnea, respiratory muscle function and respiratory muscle activation in patients with COPD during endurance cycling. Eur Respir J. 54 (Suppl 63), PA2199 (2019).
- Bellissimo, C. A., Morris, I. S., Wong, J., Goligher, E. C. Measuring diaphragm thickness and function using point-of-care ultrasound. J Vis Exp. 201, e65431 (2023).
- Basoudan, N., et al. Scalene and sternocleidomastoid activation during normoxic and hypoxic incremental inspiratory loading. Physiol Rep. 8 (14), e14522 (2020).
- Basoudan, N., Shadgan, B., Guenette, J. A., Road, J., Reid, W. D. Effect of acute hypoxia on inspiratory muscle oxygenation during incremental inspiratory loading in healthy adults. Eur J Appl Physiol. 116 (4), 841-850 (2016).
- Melo, L. T., et al. Prefrontal cortex activation during incremental inspiratory loading in healthy participants. Respir Physiol Neurobiol. 296, 103827 (2022).
- Dacha, S., et al. Comparison between manual and (semi-)automated analyses of esophageal diaphragm electromyography during endurance cycling in patients with COPD. Front Physiol. 10, 885 (2019).
- Hermens, H. J., Freriks, B., Disselhorst-Klug, C., Rau, G. Development of recommendations for SEMG sensors and sensor placement procedures. J Electromyogr Kinesiol. 10 (5), 361-374 (2000).
- Petersen, E., Sauer, J., Graßhoff, J., Rostalski, P. Removing cardiac artifacts from single-channel respiratory electromyograms. IEEE Access. 8, 30905-30917 (2020).
- Hutten, G. J., van Thuijl, H. F., van Bellegem, A. C., van Eykern, L. A., van Aalderen, W. M. A literature review of the methodology of EMG recordings of the diaphragm. J Electromyogr Kinesiol. 20 (2), 185-190 (2010).
- van Leuteren, R. W., Hutten, G. J., de Waal, C. G., Dixon, P., van Kaam, A. H., de Jongh, F. H. Processing transcutaneous electromyography measurements of respiratory muscles, a review of analysis techniques. J Electromyogr Kinesiol. 48, 176-186 (2019).
- Jonkman, A. H., Juffermans, R., Doorduin, J., Heunks, L. M. A., Harlaar, J. Estimated ECG subtraction method for removing ECG artifacts in esophageal recordings of diaphragm EMG. Biomed Signal Process Control. 69, 102861 (2021).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaPrzeglądaj więcej artyków
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone