Zaloguj się
Method Article
Three-dimensional Patterning of Engineered Biofilms with a Do-it-yourself Bioprinter
* Wspomniani autorzy wnieśli do projektu równy wkład.
W tym Artykule
Podsumowanie
This article describes a method of transforming a low-cost commercial 3D printer into a bacterial 3D printer that can facilitate printing of patterned biofilms. All necessary aspects of preparing the bioprinter and bio-ink are described, as well as verification methods to assess the formation of biofilms.
Streszczenie
Biofilms are aggregates of bacteria embedded in a self-produced spatially-patterned extracellular matrix. Bacteria within a biofilm develop enhanced antibiotic resistance, which poses potential health dangers, but can also be beneficial for environmental applications such as purification of drinking water. The further development of anti-bacterial therapeutics and biofilm-inspired applications will require the development of reproducible, engineerable methods for biofilm creation. Recently, a novel method of biofilm preparation using a modified three-dimensional (3D) printer with a bacterial ink has been developed. This article describes the steps necessary to build this efficient, low-cost 3D bioprinter that offers multiple applications in bacterially-induced materials processing. The protocol begins with an adapted commercial 3D printer in which the extruder has been replaced with a bio-ink dispenser connected to a syringe pump system enabling a controllable, continuous flow of bio-ink. To develop a bio-ink suitable for biofilm printing, engineered Escherichia coli bacteria were suspended in a solution of alginate, so that they solidify in contact with a surface containing calcium. The inclusion of an inducer chemical within the printing substrate drives expression of biofilm proteins within the printed bio-ink. This method enables 3D printing of various spatial patterns composed of discrete layers of printed biofilms. Such spatially-controlled biofilms can serve as model systems and can find applications in multiple fields that have a wide-ranging impact on society, including antibiotic resistance prevention or drinking water purification, among others.
Wprowadzenie
There is currently an increasing need to develop environmentally-friendly, sustainable solutions for the production of spatially-patterned materials, due to the expanding number of markets for such materials1. This article presents a simple, economical method for the production of such materials and therefore offers a large spectrum of future applications. The method presented here allows three-dimensional (3D) printing of spatially-patterned structures using a bio-ink containing living bacteria. Bacteria remain viable within the printed structures for over one week, enabling the bacteria to perform natural or engineered metabolic activities. Printed bacteria can thereby produce and deposit desired components within the printed structure, for example creating a functional cross-linked biofilm2.
Traditional methods for the production of advanced materials involve high energy expenditures (e.g., high temperatures and/or pressures) and can produce large quantities of chemical waste, often toxic substances that require cost-extensive utilization3,4. In contrast, multiple bacterial species are able to produce materials that can be readily applicable in various industries. These materials include polymers such as polyhydroxyalkanoates (PHA)5 or poly(glycolide-co-lactide) (PGLA)6, bacterial cellulose7, bacterial concrete materials8, biomimetic composites9, amyloid-based adhesives10, or bio-based electrical switches11, among others. Moreover, bacterial production of valuable materials typically takes place at near-ambient temperatures and pressures and in aqueous environments, without requiring or producing toxic compounds. While producing materials with bacteria has been demonstrated in the literature and some industrial applications have already emerged12,13, a reliable method for spatial patterning of such materials remains a challenge.
This article demonstrates a straight-forward method of converting a low-cost commercial 3D printer into a 3D bacterial printer. The protocol shows how to prepare a bio-ink containing and sustaining the living bacteria, as well as how to prepare substrates onto which the 3D printing can be performed. This method is appropriate to use with a variety of natural and engineered bacterial strains able to produce materials. These bacteria can be spatially distributed within a 3D printed structure and still continue their metabolic activity, which will result in a spatial distribution of the desired materials produced by the bacteria.
This printing method enables additive manufacturing of biofilms, aggregates of bacteria surrounded by a self-produced extracellular matrix. Biofilms are heterogeneous 3D networks in which proteins, polymers, bacterial cells, oxygen, and nutrients are all spatially structured14. While in the form of a biofilm, bacteria exhibit an increased antibiotic resistance and structural robustness, making them difficult to eradicate from surfaces including medical catheters and implants. The key to biofilm properties, and also the largest challenge to biofilm research, seems to be the heterogeneity of biofilms15,16,17. Production of spatially-controlled model biofilms is of special interest as it would allow for either reproducing or tuning the spatial patterns of biofilm components, aiding the understanding of the stable deposition of biofilms on virtually any surface in nature.
This article presents a method for the production of biofilms using 3D-printed hydrogels containing engineered E. coli bacteria that produce biofilm proteins in the presence of an inducer, as well as methods of verification of biofilm formation2. The major extracellular matrix components of these biofilms are curli amyloid fibers18 that contain self-assembled CsgA proteins. When engineered E. coli bacteria are induced to express CsgA proteins, they form a stable model biofilm that protects the cells against being washed off of the printing surface. Such a 3D printed biofilm can be spatially controlled and can serve as a useful research tool for the investigation of multiscale biofilm structure-function mechanics or materiomics19. These bespoke biofilms will aid the understanding of the principles of biofilm formation and their mechanical properties, enabling further research into the mechanisms of antibiotic resistance among other applications.
Protokół
1. Conversion of a commercial 3D printer into a 3D bioprinter
- Remove the extruder and the heater of a commercial 3D printer (Table of Materials) from the printer frame, and unplug the wiring controlling these elements from the main circuit board (Figure 1A). Since the sensor that controls the operational temperature of the printer needs to be functional to communicate with the printer software, remove from the printing software the algorithm that delays printing until operational temperature is reached.
- Connect a pipette tip (200 µL tip) via silicon tubing (inner diameter of 1 mm) to a 5 mL syringe loaded into a syringe pump. Mount the pipette tip onto the 3D printer extruder head as a replacement for the original extruder (Figure 1B).
- If more than one type of bio-ink will be used, mount additional tubing system(s) and pipette tip(s) to the printer.
2. Substrate preparation for 3D printing
- Add 4 mL of 5 M CaCl2 solution to 400 mL of 1% w/v agar dissolved in Luria-Bertani broth (LB) medium, supplemented with appropriate antibiotics and inducers (here 34 µg/mL chloramphenicol and 0.5% rhamnose).
- Dispense 20 mL of the LB-agar solution into each 150 mm x 15 mm Petri dish. Dry 30 min at room temperature with the lid half-open.
NOTE: The protocol can be paused here by storing these printing substrates at 4 °C for up to several days.
3. Bio-ink preparation
- Prepare a sodium alginate solution (3% w/v), and heat to the boiling point three times to sterilize the solution. Store at 4 °C until used.
- Grow E. coli MG1655 PRO ΔcsgA ompR234 (E. coli ΔcsgA) bacteria carrying plasmids pSB1C3-green fluorescent protein (GFP) (constitutive GFP expression)2 or pSB1C3-GFP-CsgA (constitutive GFP expression, rhamnose-inducible CsgA expression) overnight at 37 °C with shaking at 250 rpm in 50 mL of LB medium containing 34 µg/mL chloramphenicol and 0.5% rhamnose.
- Centrifuge the cell culture for 5 min at 3,220 x g to pellet the bacteria. Remove the supernatant.
- Re-suspend the bacteria pellet in 10 mL of LB medium and add 10 mL of sodium alginate (3% w/v).
4. 3D printing process
- Install and open the 3D printing software (Table of Materials) on a computer. Connect the 3D printer to the computer. Move the printhead to its home position by clicking the home button for the X, Y, and Z axes.
- For each print, place a prepared printing substrate onto a particular location on the printing bed.
- Calibrate the height of the printhead in the Z axis.
- Raise the printhead to a height of 22 mm under manual control, so that it will not collide with the edge of the petri dish when moving to the desired position. Position the printhead overtop of the plate, and move it down until the pipette tip contacts the printing surface. Assign this Z-axis position as Z1 (the height of the printing surface).
- Raise the printhead, and move it outside of the plate area by manual control in the X, Y, and Z axes. If the working distance between the printhead and the plate surface is defined as Z2, enter Z1 + Z2 into the printing program as the Z-value during printing.
- Program the printing shape by a self-developed point-by-point coordinate-determined method according to the desired trajectory.
- If the desired trajectory is a straight line, define only the start and end points. Including additional points on curved lines will result in smoother curves. Move the printhead manually to every point sequentially, and record the coordinates of these points in order. Enter all of these coordinates as well as the printhead moving speed for each printed segment into the G-code editor.
- Both before and after printing, lift the printhead to a distance higher than the plate edge (20 mm), and move directly out of the plate region. Save this program as a G-code file and load directly for use in subsequent prints, while re-measuring the Z axis height for each new printing substrate.
NOTE: See Table 1 for an example G-code for printing a square. - Load the pre-programmed G-code file. Open the G-code editor in the software, and program in the commands for printing the desired shape. At each command line, the position of the printhead may be changed in the X, Y, and/or Z axis. Input the Z value during all printing steps as Z1 + Z2 (height of printing surface + working distance).
NOTE: The moving speed is also adjustable; 9,000 mm/min is a suitable value for typical printing rates. - Load the liquid bio-ink into syringe(s), and mount them in the syringe pump(s) of the 3D bioprinter.
- Print the bio-ink onto the printing substrate by clicking the Print button.
- During printing, control the printhead movement entirely by the software. Manually start the syringe pump before the printhead comes into contact with the printing surface.
NOTE: The coordination of the syringe pump and the printer is empirically determined depending on the extrusion speed, the speed at which the printhead moves to the first print point, and the initial position of the printhead. If the initial printhead position is 20 mm, with a printhead speed of 9,000 mm/min and an extrusion speed of 0.1 mL/h, start the syringe pump immediately after the printing is started. If the extrusion speed is changed from 0.1 mL/h to 0.3 mL/h, then wait 2−3 s to start the syringe pump after the printing is started. - Stop the syringe pump as soon as the printhead arrives at the last point of printing. Halt the syringe pump before the printhead lifts up at the end of the printing process, otherwise excess bio-ink will drop onto the printing substrate and reduce the printing resolution.
- For the construction of 3D structures, control all movements of the printhead in the G-code editor. Type in the printing height of the first layer. Increase the Z-value in the G-code by 0.2 millimeters for the second layer to increase the printing height. Thereafter, increase the Z-value by 0.1 millimeters when moving to a higher layer. Do not move the plate during the printing process.
- To measure the width and height of the printed hydrogel, use a steel ruler placed underneath or alongside the sample.
5. Growing and testing the effectiveness of biofilm production by E. coli
- Incubate the printed samples at room temperature for 3−6 days to allow the production of biofilm components (curli fibers). Image the plates using a camera or fluorescent scanner.
- To dissolve the alginate matrix, add 20 mL of 0.5 M sodium citrate solution (pH = 7 adjusted with NaOH) to the printing substrates, and incubate for 2 h with 30 rpm shaking at room temperature. Discard the liquid and image the plates again to compare with the images of the plates before citrate treatment.
Wyniki
The first step for successful 3D printing of biofilms is converting a commercial 3D printer into a bioprinter. This conversion is achieved by removing the extruder and heater of the printer, designed for printing with a polymeric ink, and replacing these with components appropriate for printing bio-ink containing living bacteria (Figure 1A). The extruder is replaced by a pipette tip (or tips, if multiple bio-inks will be used in the printing process) attached to a tubing system connected to a syringe pump (Figure 1B). The successful conversion of the commercial printer into a bioprinter can be assessed based on the ability to transfer desired bio-ink(s) from the syringe pump through the tubing system and pipette tip(s) onto a printing surface without leaking or heating the bio-ink. If the tubing bulges due to the flow of bio-ink during printing, it may be replaced by tubing with thicker walls. It should be noted that this printing technique should be able to work with any type of commercial 3D printer for which tubing can be attached to the printhead.
The 3D bioprinter can create bacteria-encapsulating hydrogels in a variety of two-dimensional (2D) and 3D shapes (Figure 2). Calcium ions in the printing substrate induce solidification (chelation of calcium ions with alginate carboxyl groups) of the bio-ink upon printing, converting the liquid bio-ink into a solid hydrogel. The resolution of bioprinting will depend on the extrusion speed, the size of the pipette tip, the speed of the printhead, the volume and concentration of CaCl2 solution added to the agar solution, the flatness of the printing surface, and the viscosity of the bio-ink used. The concentration of CaCl2 solution has a great influence on hydrogel sharpness. Four different concentrations of CaCl2 (0.1 M, 0.2 M, 1 M, and 5 M) were sampled, and only 5 M CaCl2 solution resulted in hydrogel that did not become blurred after printing. Therefore, 5 M was chosen as the optimal concentration of CaCl2 solution.
In an earlier version of this protocol, the CaCl2 solution was applied onto the surface of the agar plate rather than mixed into the agar solution before pouring the agar plate. When using this version, the volume of the CaCl2 solution has a critical influence over printing quality and resolution. When using a 150 x 15 mm Petri dish, applying a volume of calcium chloride solution of more than 100 µL (or 30 µL for a 90-mm Petri dish) results in too much liquid remaining on the printing surface. This liquid may spread unevenly when the plate is moved, which can change the working distance and cause blockage of the pipette tip. Too much volume of CaCl2 can also cause printed hydrogels to float and slide across the solution, changing the shape and position of the printed hydrogel. If the volume of calcium chloride solution is too small, some regions of the plate may not receive CaCl2 solution and will have poor hydrogel solidification. In this improved protocol, adding the CaCl2 solution directly into the agar solution prior to pouring the agar plate resulted in substantially less moisture on the surface of the printing substrate compared to the surface-applied method, resulting in dramatically improved printing resolution.
The extrusion speed and printhead movement are interdependent and can be tuned in a coordinated manner to alter the printing resolution. For example, if the printer is operated with extrusion speed between 0.1 mL/h and 0.5 mL/h with a constant printhead movement speed of 300 mm/min, the diameter of the printed hydrogel increases with the increase of extrusion speed2,20. At extrusion speeds over 0.5 mL/h, the outer edges of the printed lines of hydrogel change from straight, parallel lines to wavy lines, and the line width also increases. The velocity of the printhead also has an influence on the printing resolution. With a constant extrusion speed of 0.3 mL/h, increasing the speed of the printhead from 300 mm/min to 500 mm/min results in the width of the printed hydrogel becoming narrower, decreasing from 1.8 mm to 0.9 mm. If the printhead moving speed is over 500 mm/min, the gel line will easily become discontinuous. For a 200 µL pipette tip and the bio-ink used in the current study, several combinations of the printing resolution are considered optimal (Table 2). At pumping speed 0.3 mL/h, printhead movement speed 500 mm/min, and working distance 0.2 mm, printed hydrogel is produced with a width of approximately 0.9 mm.
One crucial achievement of the bacterial 3D printing method is its ability to create engineered biofilms. To create an engineered and spatially-controlled biofilm, the bacteria should not only survive the 3D printing process but should also produce biofilm components while remaining within the printed pattern. The engineered E. coli bacteria used in this protocol, E. coli ΔcsgA bacteria carrying the plasmid pSB1C3-GFP-CsgA, enable controllable expression of curli proteins. The use of a csgA-knockout strain ensures that CsgA protein is only expressed when it is induced from a plasmid with rhamnose. The bacteria export the induced CsgA protein subunits, which then self-assemble21 onto CsgB proteins on the bacterial outer membrane22 to form curli fibers. These amyloid-like fibers are the major proteinaceous components of biofilm extracellular matrix: a connected network of proteins and polymers in which the bacteria are embedded. The printed alginate matrix of the 3D-printing bio-ink lends physical support and structure to the bacteria during the curli production process. The use of constitutive GFP expression allows for visualization and quantification of printed cells via fluorescence imaging.
In order to assess whether the formation of biofilm was successful, the alginate matrix was dissolved using a sodium citrate solution, and the shape of the printed bio-ink was assessed after the citrate treatment (Figure 3). In the case of bio-ink without the inducible curli production plasmid, the printed pattern was completely dissolved after the sodium citrate treatment, signifying that no biofilm curli network had formed (Figure 3A,B). In the case of bacteria containing the inducible curli production plasmid, the gel was not dissolved after sodium citrate treatment (Figure 3C,D). This result indicates that the printed bacteria were able to form a curli network extensive enough to stabilize the printed pattern of bacteria2.
To construct multi-layered structures, additional layers were printed (Figure 4) by adjusting the print height and print trajectory in the G-code editor. Increasing the number of printed layers in a sample caused the width and the height of the printed structures to increase incrementally (Figure 5)2,20, but even 5-layer printed structures could be created with a resolution of millimeters to sub-millimeters. When E. coli engineered to inducibly produce curli proteins were printed into multi-layered structures, sodium citrate treatment did not dissolve the samples, whereas multi-layer structures containing non-curli-producing E. coli were dissolved in sodium citrate solution (Figure 6). This experiment demonstrates that engineered biofilms can be created in multi-layered, three-dimensional printed structures, as well as in single-layer printed structures.

Figure 1: Photos showing the conversion of a commercial 3D printer into a 3D bioprinter. (A) The components of the 3D bioprinter after conversion from a commercial 3D printer. (B) The bio-ink extruder formed by a tubing system attached to a pipette tip. Additional printing tips can be added in the second printhead hole or by adding additional holes to the printhead, for use in printing multiple types of bio-ink. Please click here to view a larger version of this figure.
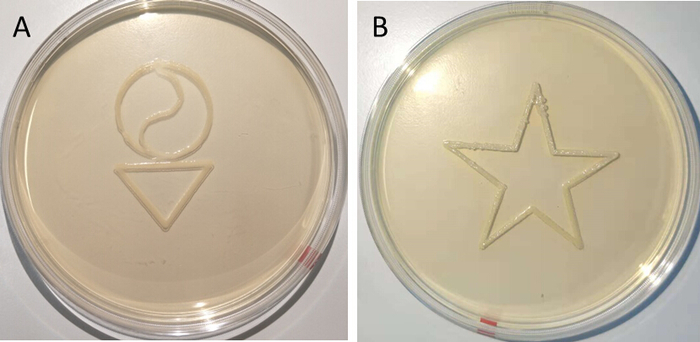
Figure 2: Examples of 3D bioprinted patterns containing E. coli pSB1C3-GFP-CsgA. These images were taken two days after printing. This printing resolution was obtained with pumping speed 0.3 mL/h, printhead movement speed 300 mm/min, and working distance 0.2 mm. The G-codes for printing these shapes may be found in the Supplemental Files. Please click here to view a larger version of this figure.

Figure 3: A method of verifying whether biofilm components have been produced by E. coli bacteria within a printed pattern. When printed E. coli contained a plasmid that did not encode for curli induction, the printed pattern was completely dissolved by sodium citrate treatment (A and B). When E. coli containing a plasmid encoding inducible curli proteins was used, the printed biofilm was resistant to sodium citrate treatment (C and D). The programming process and explanations of the G-code for printing this square pattern are provided in Table 1. Please click here to view a larger version of this figure.

Figure 4: Top view (A) and side view (B) of multi-layered printed structures containing E. coli pSB1C3-GFP-CsgA. This sample was printed with pumping speed 0.3 mL/h, printhead movement speed 200 mm/min, and working distance 0.2 mm. Please click here to view a larger version of this figure.

Figure 5: The line width and height of printed hydrogels containing different numbers of printed layers. The measurements were performed on samples printed with pumping speed 0.3 mL/h, printhead movement speed 500 mm/min, and working distance 0.2 mm. Please click here to view a larger version of this figure.

Figure 6: A method of verifying whether biofilm components have been produced by E. coli bacteria within multi-layer printed structures. Engineered E. coli was printed into 1-, 3-, or 5-layer hydrogels and incubated for 6 days. When the printed E. coli contained a plasmid that did not encode for curli induction, the printed pattern was completely dissolved by sodium citrate treatment (A and B). When the printed E. coli contained a plasmid encoding inducible curli proteins, the printed biofilm was resistant to sodium citrate treatment (C and D). Please click here to view a larger version of this figure.
| G-code commands | Tasks |
| G1 Z20 F9000 | Lift the Z-axis to a height of 20 mm with a 9,000 mm/min moving speed. |
| G1 X95 Y65 F9000 | Move to the starting point of the first line with a 9,000 mm/min moving speed. |
| G1 Z6 F9000 | Move downwards in the Z-direction to a proper (here Z = 6 mm) printing distance. |
| G1 X95 Y105 F300 | End point of the first line and starting point of the second line. |
| G1 X135 Y105 | End point of the second line and starting point of the third line. |
| G1 X135 Y65 | End point of the third line and starting point of the fourth line. |
| G1 X95 Y65 | End point of the fourth line and starting point of the first line; a square is formed. |
| G1 Z20 F9000 | Lift the Z-axis to a height of 20 mm at 9,000 mm/min. |
| G1 X55 Y40 F9000 | Move to a coordinate (55, 40) outside of the Petri dish range. |
Table 1: Programming process and explanations of G-code for printing a square.
| Extrusion speed (mL/h) | Printhead moving speed (mm/min) | Gel width (mm) |
| 0.1 | 100 | 1.6 ± 0.1 |
| 0.1 | 200 | 1.1 ± 0.1 |
| 0.1 | 300 | 1.0 ± 0.1 |
| 0.3 | 300 | 1.8 ± 0.1 |
| 0.3 | 400 | 1.2 ± 0.1 |
| 0.3 | 500 | 0.9 ± 0.1 |
| 0.5 | 200 | 2.2 ± 0.2 |
| 0.5 | 1,200 | 1.2 ± 0.2 |
| 0.7 | 200 | 2.8 ± 0.1 |
| 0.7 | 1,200 | 1.3 ± 0.1 |
Table 2: The optimal printing parameters for hydrogels with high resolution. Five points were measured for each condition. The average value and standard deviation are shown in the table.
Dyskusje
The protocol presented here for 3D printing of engineered biofilms has two critical steps. First is the preparation of the agar printing surface, which is the most critical factor to producing a specific printing resolution. It is important to ensure that the printing surface is flat and that the pipette tip on the printhead is positioned at the correct height from the surface. If the surface is not flat, the working distance will change during the printing process. If the working distance is less than 0.1 mm, the CaCl2 solution could enter inside the pipette tip and cause hydrogel formation, causing the pipette tip to become clogged. If the working distance is more than 0.3 mm, the gel cannot be printed continuously. The optimal working distance in this study is 0.2 mm. Good approaches for preparing flat agar printing surfaces are to use larger-diameter Petri dishes (150-mm-diameter Petri dish rather than a 90-mm-diameter plate), place the plates on a flat table, pour the agar solution with fast and even speed, and avoid moving the agar plate during its solidification.
The second critical step is the selection of desired printing parameters including pumping speed, viscosity of the bio-ink used, and printhead speed, which determine the resulting printing resolution. To select these parameters in an efficient manner, the user can sample several extreme values for printhead speed with a constant extrusion rate, noting the width of the printed hydrogel for each set of conditions. Then, repeat this experiment with 4 other extrusion rates. Next, take the five combinations that produced the best printing resolution for the application, and vary both printing parameters (pumping and printhead speeds) in smaller and smaller steps until the desired resolution is obtained.
The thickness of the printed lines has an impact on the ability of the printed engineered bacteria to form stable biofilms. Under optimal printing conditions (pumping speed 0.3 mL/h, printhead speed 300 mm/min, and working distance 0.2 mm), printed lines of bio-ink will produce stable biofilms after 3-6 days of incubation at room temperature. If the lines become thicker, such as by increasing the pumping speed, the middle regions of each line may not be induced sufficiently to produce citrate-stable biofilms.
When printing a multi-layer bio-ink hydrogel, each printed layer is solidified upon contacting the calcium ions that have diffused into the previous printed layer. Since the Ca2+ concentration in the printing substrate is high, Ca2+ ions can rapidly diffuse up through the lower layers. Therefore, the upper layers can be printed immediately after the previous layers have been printed, simply by adjusting the print height in the G-code editor. Additionally, the printing distance of the upper layer should be restricted to only 0.1−0.2 mm higher than the printing distance of the previous layer. If the added printing distance is less than 0.1 mm, the tip will drag across the first layer and reduce the resolution of the printed hydrogel. If the added printing distance is larger than 0.2 mm, the bio-ink will form drops of liquid during extrusion, causing the printed hydrogel to become discontinuous.
The current bioprinting approach enables the production of reproducible, spatially-controlled engineered biofilms, suitable for use in the study of biofilm mechanical properties or biological resistance of biofilm bacteria to various factors including antibiotics, surfactants, etc. This capability ensures a direct usability of the proposed method. The development of higher-precision do-it-yourself (DIY) bioprinters will likely be possible by maintaining the printing working distance but lowering the pumping speed and the moving speed of the printhead, or by sampling different extruder geometries and bio-ink chemistries. With future improvements to the printing resolution, additional applications can be enabled such as tissue engineering or drug delivery. The 3D bioprinting approach described here should also be able to be expanded to printing additional types of bacteria species that are biocompatible with our alginate-based bio-ink. The current protocol provides sufficient sterility by repeatedly boiling the bio-ink during preparation, using sterile syringes and printing tips, and utilizing antibiotics in both the bio-ink and printing plate. Future experiments using wild-type bacteria may require additional sterilization measures such as replacing or disinfecting the tubing system between prints.
To the authors' best knowledge, the presented method (originally developed in Lehner et al.20) is the first published example of an additive manufacturing style for 3D printing of bacteria. In the first part of this protocol, this general method is described in detail for the 3D printing of bacteria, which is applied to the production of engineered biofilms2. Multiple future applications of 3D-printed biofilms are possible using this method. In nature, multiple bacterial systems have evolved that create various types of biofilms, of which in this article a single system was explored. Multiple other systems can be easily examined by creating 3D-printed biofilms with other bacterial systems, such as Bacillus subtilis or Acetobacter xylinum. Alternative methods23,24 have also been developed for spatial patterning of bacteria at high resolution using optical signals. These approaches require more expensive, complicated equipment to achieve them in comparison to this printer, and are only suitable for patterning of genetically engineered bacteria.
The ability to spatially pattern 3D-printed biofilms with this method can allow for the creation of engineered biofilms that reproduce the spatial heterogeneity of natural biofilms25. Because of the highly detailed arrangement of protein and polymeric fibers within a biofilm, bacteria in a biofilm state achieve a much higher resistance to chemical and physical stimuli, such as an increased resistance to antibiotics as compared to the same bacteria in a planktonic state. Moreover, bacteria within a biofilm show an increased resistance to fluid flow, making the maintenance and sterility of implantable medical devices much more difficult26. Printed engineered biofilms that attempt to reproduce the specific spatial distributions of biofilm components are powerful tools for studying the mechanisms by which bacteria within a biofilm achieve resistance phenotypes.
Ujawnienia
The authors have nothing to disclose.
Podziękowania
This work was supported by an AOARD grant (No. FA2386-18-1-4059), the Netherlands Organization for Scientific Research (NWO/OCW) as part of the Frontiers of Nanoscience program, and the Advanced Materials NWO-NSFC program (No. 729.001.016). The authors acknowledge laboratory assistance of Ramon van der Valk and Roland Kieffer.
Materiały
| Name | Company | Catalog Number | Comments |
| 3D printer | CoLiDo | 3D-P Kit | |
| 3D printing software | CoLiDo | Print-Rite ColiDo Repetier-Host v2.0.1 | |
| Agar | Sigma-Aldrich | 05040 | |
| CaCl2 dihydrate | Sigma-Aldrich | C7902 | |
| Centrifuge | Eppendorf | 5810 R | |
| Chloramphenicol | Sigma-Aldrich | 3886.1 | |
| LB broth powder | Sigma-Aldrich | L3022 | |
| Orbital shaker | VWR | 89032-092 | Model 3500 |
| Petri dish | VWR | 25384-326 | 150 x 15 mm |
| Rhamnose | Sigma-Aldrich | 83650 | |
| Silicon tubing | VWR | DENE 3100103/25 | |
| Syringe pump | ProSense B.V. | NE-300 | |
| Sodium alginate | Sigma-Aldrich | W201502 | |
| Sodium citrate monobasic | Sigma-Aldrich | 71498 | |
| Sodium hydrooxide | VWR | 28244.295 |
Odniesienia
- Tibbitt, M. W., Rodell, C. B., Burdick, J. A., Anseth, K. S. Progress in material design for biomedical applications. Proceedings of the National Academy of Sciences of the United States of America. 112 (47), 14444-14451 (2015).
- Schmieden, D. T., et al. Printing of Patterned, Engineered E. coli Biofilms with a Low-Cost 3D Printer. ACS Synthetic Biology. 7 (5), 1328-1337 (2018).
- Mao, L. B., et al. Synthetic nacre by predesigned matrix-directed mineralization. Science. 354 (6308), 107-110 (2016).
- Gao, H. L., et al. Mass production of bulk artificial nacre with excellent mechanical properties. Nature Communications. 8 (1), 287 (2017).
- Poirier, Y., Nawrath, C., Somerville, C. Production of Polyhydroxyalkanoates, a Family of Biodegradable Plastics and Elastomers, in Bacteria and Plants. Nature Biotechnology. 13, 142-150 (1995).
- Choi, S. Y., et al. One-step fermentative production of poly(lactate-co-glycolate) from carbohydrates in Escherichia coli. Nature Biotechnology. 34 (4), 435-440 (2016).
- Mohammadi, P., Toivonen, M. S., Ikkala, O., Wagermaier, W., Linder, M. B. Aligning cellulose nanofibril dispersions for tougher fibers. Scientific Reports. 7 (1), 11860 (2017).
- Jonkers, H. M. Bacteria-based self-healing concrete. Heron. 56 (1/2), (2011).
- Schmieden, D. T., Meyer, A. S., Aubin-Tam, M. E. Using bacteria to make improved, nacre-inspired materials. MRS Advances. 1 (8), 559-564 (2016).
- Zhong, C., et al. Strong underwater adhesives made by self-assembling multi-protein nanofibres. Nature Nanotechnology. 9 (10), 858-866 (2014).
- Chen, A. Y., et al. Synthesis and patterning of tunable multiscale materials with engineered cells. Nature Materials. 13 (5), 515-523 (2014).
- Gatenholm, P., Klemm, D. Bacterial Nanocellulose as a Renewable Material for Biomedical Applications. MRS Bulletin. 35, 208-213 (2010).
- Rodriguez-Carmona, E., Villaverde, A. Nanostructured bacterial materials for innovative medicines. Trends in Microbiology. 18 (9), 423-430 (2010).
- Hung, C., et al. Escherichia coli biofilms have an organized and complex extracellular matrix structure. MBio. 4 (5), (2013).
- Donlan, R. M., Costerton, J. W. Biofilms: Survival Mechanisms of Clinically Relevant Microorganisms. Clinical Microbiology Reviews. 15 (2), 167-193 (2002).
- Wu, H., Moser, C., Wang, H. Z., Hoiby, N., Song, Z. J. Strategies for combating bacterial biofilm infections. International Journal of Oral Science. 7 (1), 1-7 (2015).
- Stewart, P. S., Franklin, M. J. Physiological heterogeneity in biofilms. Nature Reviews Microbiology. 6 (3), 199-210 (2008).
- Kikuchi, T., Mizunoe, Y., Takade, A., Naito, S., Yoshida, S. Curli Fibers Are Required for Development of Biofilm Architecture in Escherichia coli K-12 and Enhance Bacterial Adherence to Human Uroepithelial Cells. Microbiology and Immunology. 49 (9), 875-884 (2005).
- Cranford, S., Buehler, M. J. Materiomics: biological protein materials, from nano to macro. Nanotechnology, Science and Applications. 3, 127-148 (2010).
- Lehner, B. A. E., Schmieden, D. T., Meyer, A. S. A Straightforward Approach for 3D Bacterial Printing. ACS Synthetic Biology. 6 (7), 1124-1130 (2017).
- Wang, X., Smith, D. R., Jones, J. W., Chapman, M. R. In vitro polymerization of a functional Escherichia coli amyloid protein. Journal of Biological Chemistry. 282 (6), 3713-3719 (2007).
- Hammar, M., Bian, Z., Normark, S. Nucleator-dependent intercellular assembly of adhesive curli organelles in Escherichia coli. Proceedings of the National Academy of Sciences of the United States of America. 93 (13), 6562-6566 (1996).
- Huang, Y. J., Xia, A. G., Yang, G., Jin, F. Bioprinting Living Biofilms through Optogenetic Manipulation. ACS Synthetic Biology. 7 (5), 1195-1200 (2018).
- Jin, X. F., Riedel-Kruse, I. H. Biofilm Lithography enables high-resolution cell patterning via optogenetic adhesin expression. Proceedings of the National Academy of Sciences of the United States of America. 115 (14), 3698-3703 (2018).
- Stewart, P. S., Franklin, M. J. Physiological heterogeneity in biofilms. Nature Reviews Microbiology. 6 (3), 199-210 (2008).
- Percival, S. L., Suleman, L., Vuotto, C., Donelli, G. Healthcare-associated infections, medical devices and biofilms: risk, tolerance and control. Journal of Medical Microbiology. 64, 323-334 (2015).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
ISSN 2578-2614
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone
Używamy plików cookie, aby ulepszyć Twoje wrażenia z korzystania z naszej witryny.
Kontynuując korzystanie z naszej witryny lub klikając „Kontynuuj”, wyrażasz zgodę na akceptację naszych plików cookie.