Method Article
Molecular Probe Optimization to Determine Cell Mortality in a Photosynthetic Organism (Microcystis aeruginosa) Using Flow Cytometry
W tym Artykule
Podsumowanie
Microbial populations contain substantial cell heterogeneity, which can dictate overall behavior. Molecular probe analysis through flow cytometry can determine physiological states of cells, however its application varies between species. This study provides a protocol to accurately determine cell mortality within a cyanobacterium population, without underestimating or recording false positive results.
Streszczenie
Microbial subpopulations in field and laboratory studies have been shown to display high heterogeneity in morphological and physiological parameters. Determining the real time state of a microbial cell goes beyond live or dead categories, as microbes can exist in a dormant state, whereby cell division and metabolic activities are reduced. Given the need for detection and quantification of microbes, flow cytometry (FCM) with molecular probes provides a rapid and accurate method to help determine overall population viability. By using SYTOX Green and SYTOX Orange in the model cyanobacteria Microcystis aeruginosa to detect membrane integrity, we develop a transferable method for rapid indication of single cell mortality. The molecular probes used within this journal will be referred to as green or orange nucleic acid probes respectively (although there are other products with similar excitation and emission wavelengths that have a comparable modes of action, we specifically refer to the fore mentioned probes). Protocols using molecular probes vary between species, differing principally in concentration and incubation times. Following this protocol set out on M.aeruginosa the green nucleic acid probe was optimized at concentrations of 0.5 µM after 30 min of incubation and the orange nucleic acid probe at 1 µM after 10 min. In both probes concentrations less than the stated optimal led to an under reporting of cells with membrane damage. Conversely, 5 µM concentrations and higher in both probes exhibited a type of non-specific staining, whereby 'live' cells produced a target fluorescence, leading to an over representation of 'non-viable' cell numbers. The positive controls (heat-killed) provided testable dead biomass, although the appropriateness of control generation remains subject to debate. By demonstrating a logical sequence of steps for optimizing the green and orange nucleic acid probes we demonstrate how to create a protocol that can be used to analyse cyanobacterial physiological state effectively.
Wprowadzenie
The cell is a complex system, which constantly responds to the environment by modifying physiological parameters and altering its function. The population dynamics of isogenic microbial populations both in nature and the laboratory are affected by the development of subpopulations, occurring even under relatively constant environmental conditions1-3. The variability of natural microbial communities arises due to the highly variable nature of environmental conditions. These sometimes stochastic processes subsequently produce subpopulations that are very different to the population average. Recent evidence has revealed that these physiological subpopulations respond differently to environmental conditions and can produce signal compounds or inhibitors that dramatically affect and influence the overall population3,4.
Establishing a method to define heterogeneity within a population is key to understanding the ecology of microbes in various environments and is essential when building knowledge of nuisance cyanobacteria, such as the toxic Microcystis, which impacts heavily on human water security. Species such as Anabaena display morphological heterogeneity in response to environmental fluctuations, developing specialised cells like heterocysts and akinetes2. In contrast, Microcystis cells do not display obvious morphological heterogeneity during a stress response. The discrimination between viable and non-viable cells is the most important aspect of physiological differentiation and allows a better understanding of microbial population dynamics. However, the conceptual problem of bacterial viability itself remains difficult and poorly characterised1,5,6.
Flow cytometry (FCM) is a reliable and rapid method of analysing individual cells. To increase the understanding of single cell physiology through FCM, molecular probes have been used to distinguish a number of metabolic and biochemical processes7. This has led to increased knowledge of species on a cellular and population level and in turn helped water resource management8,9. However, organisms differ in terms of molecular probe uptake and efflux due to the pores and pumps in cellular walls and membranes, which have led to a number of molecular probe design and protocol implementation6,10,11. Molecular probes available for commercial and research purposes are often supplied with a generic protocol which may be applicable to a very different cell type. One must be very cautious in transferring protocols developed for one cell type to another6, it is therefore an essential task to optimize molecular probes effectively before use.
The green and orange nucleic acid probes bind to both double and single stranded nucleic acids with minimal base selectivity and are used to assess the plasma membrane integrity of cells. The green nucleic acid probe has a markedly improved cell labelling fluorescence signal compared to other molecular probes, such as propidium iodide-based compounds12, which can also act as an indicator of cell viability. The term 'cell viability' here assumes that DNA degradation occurs after the loss of plasma membrane integrity. The nucleic acid probes are unsymmetrical cyanine dyes with three positive charges and cannot enter cells with intact membranes under characterised concentrations, in both eukaryotic11,13 and prokaryotic14,15 organisms. The binding of a nucleic acid probe to nucleic acids can result in up to a >500 fold increase of fluorescence emissions from endogenous signals in cells that have their membrane integrity compromised. Although molecular probes such as the green nucleic acid probe can be a good indicator of single cell physiology, there is a need to optimize each probe with the intended target organism, as incubation times have varied from 7 min - 30 min and concentration ranges from 0.1 µM - 0.5 µM in Microcystis experiments alone15-19.
Here we present a protocol to optimize the cytometric assays of green and the relatively new orange nucleic acid probes (which to date not been tested on the cyanobacterial species M.aeruginosa). The following developed methodology can then be transferred to other species and used as a platform for optimizing protocols in other molecular probes, thereby increasing the understanding of microbes and their ecological behavior.
Protokół
1. Preparation of the Molecular Probe and Flow Cytometer
- Dilute stock solutions of the nucleic acid probes, which are supplied as a 5 mM solution in dimethylsulfoxide (DMSO) to aliquots of required concentrations in ultrapure filtered H2O.
- Store the nucleic acid probes in dark conditions between -5 oC and - 25 oC until use.
- Turn on the flow cytometer and load software package (see table of Materials/Equipment for FCM specifications).
- Place an empty haemolysis tube (12 x 75 mm) on the sample injection probe (SIP), click unclog and then back flush to start the FCM cleaning process.
NOTE: Some sample stages for the SIP can accommodate several types of tubes including microcentrifuge tubes. The molecular probe diluent and sheath fluid used in the FCM apparatus is from an analytical grade “type 1” 0.22 µm membrane filtered source. - Place a fresh haemolysis tube with 2 ml of ultrapure filtered H2O on the SIP, set a time limit for 10 - 15 min and a fluidic speed to fast (or a flow rate of >66 µl/min).
- Select a new data cell, put on relevant thresholds for fluorescence and light scatter channels to reduce background noise and click 'run'.
- If the total events per second are not below the manufacturers' recommendation, then run a 2 ml sample of decontamination solution for 2 min on fast and then repeat steps 1.4 & 1.5.
NOTE: Please check manufacturers’ recommendations for FCM start-up cleaning protocols, as they can vary between models. Testing for specific thresholds also needs to be in line with the FCM model as some equipment allows the user to apply voltages gains to the photomultiplier tubes (PMTs) enhancing or decreasing the electrical signal recorded from the optical detectors. The FCM model used in this experiment has fixed voltage PMTs and used a threshold of 80,000 forward light scatter (FSC-H) to exclude particles smaller than 2.0 µm and electronic noise.
2. Preparation of Cultures and Initial Cell Counts
- Autoclave 98 ml of ultrapure filtered H2O and 2 ml of algal media (x50 concentration) in a 250 ml beaker for 20 min at 120 oC.
- From an initial monoculture of M.aeruginosa (PCC 7806) in a high steady state density place 2 ml of the sample into a tube under the SIP. Disaggregate any colony formation by vortexing or sonication20 and confirm evenly dispersed cells through light microscopy.
NOTE: Excessive exposure to ultrasonic waves can lead to cell lysis and should be used with caution. Since sonication can collapse gas vesicles (as found in species like M.aeruginosa) outputs such as side light scatter (SSC-H) intensity can become more sensitive. - Within the FCM software, select a histogram plot to record data from forward light scatter (FSC-H) and configure the plot specifications by clicking "log" to view in a log scale on the axis.
- In a separate output, select another histogram (log axis) to record natural fluorescence such as the accessory photosynthetic pigment phycocyanin (FL4-H, 675 ± 12.5 nm), found in M.aeruginosa.
- Use a light source that can excite phycocyanin and a detector which can filter the emissions from the resulting fluorescence.
NOTE: Photosynthetic pigment such as chlorophyll can be excited with the commonly used 488 nm blue laser, whereas phycocyanin is excited over 600 nm and will only be detected with a red light source21. Check with the flow cytometers manufacturer for excitation light sources and the spectra of emission detectors, here both a 488 nm and a 640 nm laser were used along with a 675 ± 12.5 nm optical filter. - For recording of the highest resolution select settings closest to the core size of the target organism (10 µm) and a relatively slow flow rate (14 µl/min).
NOTE: For the best resolution samples should be run according to manufactures recommendation of events per second. - Before acquiring data use a threshold to gate out light scatter and / or fluorescence signals that are caused by electronic background noise or cell sample debris.
- Select a new data cell, create a density plot with FSC-H and SSC-H parameters on a log scale and click run.
- If sample density leads to an excessive event rate, dilution steps can be taken to increase accuracy and precision.
- On the density plot apply software gates from the previous histogram to exclude low level scatter signals, produced by background noise or debris (FSC <320,000). Conversely gate the higher relative fluorescence phycocyanin signals from cells and only include these events (FL4, 56,000 - 1,950,000).
- Use the number of cells recorded in the gated areas and divide it by the total volume of sample that has passed through the FCM, to work out how many cells per ml.
NOTE: The FCM model used here has a microprocessor controlled peristaltic pump system allowing sample volume to be determined. Other FCM equipment may require a calibrated bead suspension or a calculation of H2O weight/volume differences to verify total sample volume. - Add the required volume of cells to the freshly prepared media in order to start a batch growth cycle (250,000 cells/ml) of M.aeruginosa.
NOTE: Species will differ in growth rates depending on their in-situ environment parameters and nutrient availability, so a batch cycle should be pre-recorded to determine life cycle phases.
3. Optimization of Molecular Probe Cell Uptake
- Harvest half of the M.aeruginosa culture prepared in step 2 from an exponential phase and use as a 'live' control.
NOTE: Samples diluted from a high density culture straight to an exponential phase may affect optimization results through dead cell turnover, compared to that of cultures inoculated from an initial lag / induction phase. - Prepare the other half as a 'dead' control by using methods such as 70% ethanol, heating samples at 60 oC for 1 hr, paraformaldehyde or 4% formaldehyde for 30 min6,11,22. Check variations in the samples microenvironment (e.g., pH).
NOTE: The positive, heat-killed, ‘dead’ control in M.aeruginosa is distinguished from a ‘live’ sample through its decrease in phycocyanin signals. Inducing mortality by other methods may not cause the same output and will vary in species. - Set up mixed samples using different ratios of 'live' and 'dead' samples (e.g., 0%, 25%, 50%, 100%).
- Disaggregate colony formation by vortexing or sonication and check pH.
- Select a 488 nm laser alongside detectors which can record fluorescence from the green (FL1, 530 ± 15 nm) and orange (FL2, 585 ± 20 nm) nucleic acid probes and the 640 nm laser to record phycocyanin signals through its respective detector.
NOTE: When bound to DNA, the green nucleic acid probe has an approximate fluorescence excitation wavelength of 504 nm and emission maxima of 523 nm, whilst the orange nucleic acid probe has an excitation wavelength of 547 nm and emissions maxima of 570 nm. A 488 nm argon ion solid state laser can be employed to excite both molecular probes, however, a green laser (up to 547 nm) will produce a higher orange fluorescence. - As a starting point, introduce the molecular probe with manufacturers recommended concentration to the 50% 'live' and 50% 'dead' culture and incubate in the dark.
- Select a new data cell, place the sample under the SIP with thresholds and triggers that will reduce background noise (FSC-H 80,000).
- Create a density plot with FSC-H and SSC-H parameters, and three histograms. One histogram using the respective molecular probe optical detector channel (FL1 or 2), one to detect phycocyanin emissions (FL4-H) and the other FSC-H, all on a log scale.
- Incubate the samples of M.aeruginosa with the nucleic acid probes in darkness for up to 60 min, recording in separate data cells, at a number of time points (1, 5, 10, 15, 30 and 60 min).
NOTE: When adjusting parameters such as pH check with manufactures for potential reactions from certain chemicals (for the tested nucleic acid probes a buffer cannot contain phosphates or high levels of monovalent or divalent cations, as the binding with DNA will be reduced). - Apply a software gate to only include the FSC-H histogram (320,000 - 1,500,000) for the target organisms cell size, into the respective fluorescence probe channel histogram.
NOTE: The concentrations used in this protocol were 0.05, 0.1, 0.5, 1, 5, 10, 50 and 100 µM, which can be altered by either increasing or decreasing the volume of M.aeruginosa sample or initial stock solution of nucleic probes. - In the fluorescence probe channel, apply another inclusive software gate to the highest peak in the histogram (green FL1-H, 240,000 - 1,650,000, orange FL2-H, 30,000 - 165,000) and subsequently gate that positive probe fluorescence into the density plot.
- Run steps 3.1 - 3.11 with 100% "live", 100% 'dead' and all mixed culture samples, adjusting the molecular probe concentrations (e.g x 0.1 to x 10) and / or temperature and pH levels if necessary.
- Compare the number of positive molecular probe fluorescence signals to the original cell density of 'dead' cells (taken from half total FSC-H or a 100% 'dead' culture) to find the total percentage of cells stained with the nucleic acid probes.
- Do this for each time period within each concentration to find the optimal protocol for the highest percentage of cell nucleic probe uptake without producing non-specific staining (use the means in a One-Way ANOVA or Kruskal-Wallis one-way analysis of variance, if the data is non-parametric).
4. Molecular Probe Fluorescence Discrimination
- For testing of fluorescence interference / overlap from intrinsic or non-specific cell staining select the 50% 'live' and 50% 'dead' mixed culture data and take off all gates.
- Apply a software gate to only include the FSC-H histogram (320,000 - 1,500,000) for the target organisms cell size, into the phycocyanin channel histogram.
- Gate the highest phycocyanin peak and label as 'live', with the addition of the lowest peak labelled as 'dead'.
- Perform a further gate step into the respective molecular probe fluorescence histogram channel, using one at a time, the 'live' and then 'dead' phycocyanin (FL4) signals, recording both mean wavelengths.
NOTE: The mode for inducing mortality in this experiment was done by heat treatment, which under this protocol clearly decreases phycocyanin signals. Other methods of producing populations of ‘dead’ cells may yield different outputs in the acquired runs. - Ratio the mean wavelengths of the positive molecular probe fluorescence ('dead') and the intrinsic / non-specific signal ('live') to determine protocol sensitivity.
NOTE: To improve fluorescence discrimination of ‘live’ and ‘dead’ populations, increase the carbon source to active stain uptake in nutrient depleted cells or ethylenediaminetetraacetic acid (EDTA) to improve cell wall permeability and resulting fluorescence signals6. Limited compensation in some FCM models for spectral overlap can be performed by user-manipulated pairwise correction during sample acquisition (PMT voltage changes) or from post-analytical specialised software. - Select the optimized protocol where the highest amount of 'dead' cells has been stained without the occurrence non-specific staining. If a number of test have similar results accept the protocol with the lowest concentration and incubation time, along with good fluorescence signal discrimination. Follow manufactures instructions for the cytometer shutdown procedure.
Wyniki
Forward light scatter (FSC) and side light scatter (SSC) outputs from an M.aeruginosa batch culture in exponential phase provides information on cell size (diameter) and internal granularity respectively (Figure 1A). FSC can discriminate cells that are too large and / or small to be M.aeruginosa. This discrimination or gating can be done by refining data between certain points of a FSC output (Figure 1C). Phycocyanin, a major constitution of M.aeruginosa photosynthetic apparatus produces a strong signal when interrogated by a red light source (ex: 640; em: 675 ± 12.5 nm), which can be used to further gate populations, similar to that done in FSC gating but with fluorescence (Figure 1D). From FSC and fluorescence signals, data can be gated from the original output (Figure 1A) to specific data on M.aeruginosa for final cell counts (Figure 1B).
Autofluorescence per cell in cyanobacteria populations differs as a function of physiological state. Here cells were harvested from an exponential phase culture showing relatively high far red fluorescence for a 'live' control population and the 'dead' control was exposed to 60 oC heat for 1 hr. When 'live' higher pigmented and 'dead' lower pigmented controls are mixed the decreasing shift in autofluorescence is clear (Figures 2A&B). The 'dead' cell fluorescence and FSC parameters can be used to discriminate the total cells that have taken up the molecular probe. In membrane compromised or 'dead' cells the nucleic acid probes will produce an additional signal either on; FL1 channel (530 ± 15 nm) for the green nucleic acid probe or FL2 channel (585 ± 20 nm) for the orange nucleic acid probe (Figures 3A&B), compared to the native 'live' fluorescence signal. Confirmation of both nucleic acid probes in cells with damage membranes can be seen through epifluorescence microscopy (Figures 4A&D).
The total percentage of cells that had been stained by the green nucleic acid probe was obtained from mixed samples, which were pre-gated to include only M.aeruginosa sized cells from a FSC-H histogram (320,000 - 1,500,000). In a 50:50 'live:dead' sample, green fluorescence cells gated from the highest peak in a FL1 (530 ± 15 nm) histogram was compared to original density in the 'dead' cell population (either by halving the total FSC-H or running a separate cell count on only the 100% 'dead' cell samples). Above 100% would indicate that non-specific staining occurs. The mean percentage of cells that had taken up the green nucleic acid probe ranged from; 15 ± 0.3 % in 0.05 µM after 1 min to 177.1 ± 5.1 % in 100 µM during 10 min of incubation (Table 1). Concentrations over 1 µM (not including) showed non-specific staining (i.e., 'live' cells that started to take up the molecular probe). A Two-Way ANOVA between the concentrations that did not exhibit non-specific staining (0.05 - 1 µM) and showed an overall significant difference in the interaction between the concentration of green nucleic acid probe and incubation time in M.aeruginosa,F (12, 40) = 6.48, p <0.001. There was a main effect across the concentrations of green nucleic acid probe used F (3, 40) = 836.92, p <0.001 and incubation times F (4,40) = 347.98, p <0.001. A Post-Hoc Tukey test indicated a significant difference between all concentrations except 0.5 and 1 µM (p <0.001) and a significant difference between all incubation times of 1 - 60 min (p <0.001). However, a Post Hoc Tukey Test from a One Way ANOVA revealed the mean uptake of green nucleic acid probe does not differ between 30 and 60 min for 0.05 µM (p >0.05) and 1 µM (p >0.05) (Figure 5A). The mean ratio of 'live' and 'dead' relative fluorescence signals in FL1 for discrimination of populations (measured through FL4) ranged in the green nucleic acid probe from the highest in 0.1 µM after 60 min of 714 ± 14.8 (RFU) to the lowest of 0.8 ± 0.0 (RFU) in 100 µM after 30 min (Table 2). Comparing the ratios of relative fluorescence units (RFU) for intensity discrimination in 'live' and 'dead' FL1 signals a Two Way ANOVA reports an overall significant difference in the interaction between concentrations and incubation times F (28,80) = 23.9, p <0.001 with the green nucleic acid probe . There was also a significant difference in the main effect across all concentrations F (7,80) = 475.41, p <0.001 and incubation times F (4,80) = 78.28, p <0.001. The discrimination ratios of fluorescence signals between the 'live' and heat killed 'dead' cells increased with time between concentrations of 0.05 µM and 1 µM but decreased between 5 µM and 100 µM (Figure 5B).
The percentage of cells which were stained with the orange nucleic acid probe saw a mean low percentage of 4 ± 0.3 %, in a concentration of 0.05 µM after 1 min, rising to 166 ± 3.5 % in 100 µM after 10 min of incubation (Table 3). Again concentrations over 1 µM also presented non-specific staining of live cells. A Two-Way ANOVA between the concentrations which showed no non-specific staining (0.05 - 1 µM) reported an overall significant difference in the interaction between all concentrations of the orange nucleic acid probe and incubation times in M.aeruginosa, F (12, 40) = 133.55, p <0.001. There was a statistically significant main effect across concentrations F (3, 40) = 6919.67, p <0.001 and incubation times F (3, 40) = 1161.45, p <0.001. However a Post Hoc Tukey test through a One Way ANOVA on just 1 µM indicated that incubation times between 10, 30 and 60 min had no statistical difference in the uptake of orange nucleic acid probe (all p >0.05) (Figure 6A). Fluorescence signal discrimination between 'live' and 'dead' stained populations in FL2 ranged in orange nucleic acid probe from the lowest of 0.3 ± 0.0 (RFU) after 60 min in 50 µM and 30 min in 100 µM to the highest of 158.3 ± 0.4 (RFU) after 60 min at a concentration of 0.1 µM (Table 4). A Two Way ANOVA reported an overall statistical significance in the interactions between concentrations and incubation time F (28, 80) = 28.12, p <0.001. The main effects of concentration F (7, 80) = 607.9, p <0.001 and incubation times F (4, 80) = 31.24, p <0.001 were also found to be all significantly different. Fluorescence discrimination between the signals from 'live' and heat killed 'dead' cells in FL2 increased with time between concentrations of 0.05 µM and 0.5 µM but decreased between 1 µM and 100 µM (Figure 6B). Therefore the optimal concentration for the green nucleic acid probe is 0.5 µM with an incubation time of 30 min and for the orange nucleic acid probe the optimal concentration is 1 µM incubated for 10 min.

Figure 1. FCM Outputs of M.aeruginosa Through FSC, SSC & Far Red Fluorescence (675 ± 12.5 nm). (A) FSC and SSC of M.aeruginosa PCC 7806. (B) The same output as Figure 1A with gated FSC and far red fluorescence (ex: 640; em: 675 ± 12.5 nm). (C) FSC representing the sizes of the cell. The red lined arrow denotes the area that is gated to reduce background noise or other size particles. (D) High phycocyanin signals produced from M.aeruginosa in an exponential phase batch culture with red arrowed gated areas. Please click here to view a larger version of this figure.
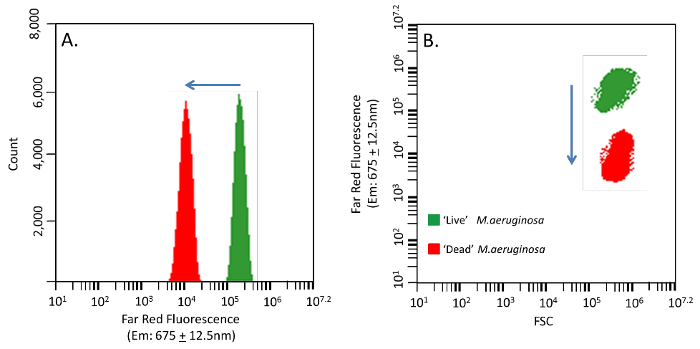
Figure 2. Phycocyanin Signal Shift in M.aeruginosa When Heat Treated. Change of M.aeruginosa autofluorescence in 'live' and 'dead' cells used to validate nucleic acid probe uptake. (A) Far red signals shifting from a higher pigmented 'live' population (green) to a lower pigmented 'dead' population (red). (B) FSC and far red fluorescence combined outputs with a shift of nearly two orders of magnitude. Please click here to view a larger version of this figure.

Figure 3. Orange Fluorescence Shift in M.aeruginosa From 'Live' Unstained to 'Dead' Stained Cells. M.aeruginosa 'live' and 'dead' mixed controls incubated with orange nucleic acid probe at a concentration of 1.0 µm for 30 min. (A) FL2 (585 ± 20 nm) channel reports an increased shift from a 'live' unstained population (green) to a 'dead' stained population (orange) through two orders of magnitude.(B) A 'dead' stained population (orange) which has decreased far red signals but increased FL2 signals from a 'live' population (green). Please click here to view a larger version of this figure.

Figure 4. Epifluorescence Microscopy of M.aeruginosa with Molecular Probes. Microscope images from a M.aeruginosa 50:50 live:dead population incubated with nucleic acid probes, all images x1,000 magnification. (A) An epifluorescence image of green nucleic acid probe taken up by 'dead' cells. 'Live' cells appear red due to the autofluorescence of chlorophyll. (B) Light microscope view of Figure 4A showing 'live' green pigmented cells and lesser pigmented 'dead' cells, with membrane damage. (C) Live:dead 50:50 mixed M.aeruginosa population. (D) Orange nucleic acid probe incubation from Figure 4C, with 'dead' cells revealing an orange colour through epifluorescence microscopy. Please click here to view a larger version of this figure.
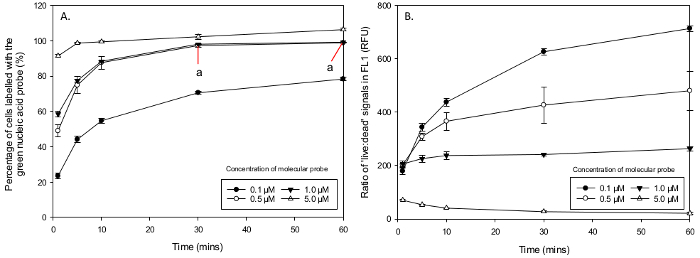
Figure 5. Percentage of Cells Labelled with Green Nucleic Acid Probe and the Ratio of 'Live' to 'Dead' FL1 Signals. Mean percentage of M.aeruginosa cells stained with the green nucleic acid probe in different concentration and incubation times. (A) 0.1 µM did not stain enough cells, 5 µM show non-specific staining and concentrations of 0.5 µM and 1 µM stained nearly 100% of the population, showing optimal incubation times after 30 min (a). (B) 'Live:dead' ratios of FL1 signals from an exponential growth phase and heat treated samples. Concentrations of 0.5 µM and 1 µM show good discrimination of FL1 signals increasing with incubation time (RFU ratios two orders of magnitude). Concentrations of 10 µM and over (not represented on graph) show poor fluorescence signal discrimination and overlapping of 'live' and 'dead' populations. Please click here to view a larger version of this figure.
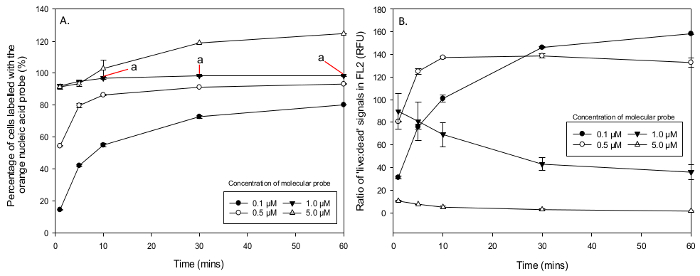
Figure 6. Percentage of Cells Labelled with Orange Nucleic Acid Probe and the Ratio of 'Live' to 'Dead' Signals. Results from concentration and incubation time changes in populations of M.aeruginosa. (A) Mean percentage of cells that had recorded an orange fluorescence signal in FL2 with concentrations of; 0.1 µM and 0.5 µM not staining enough cells, 5 µM staining live cells (non-specific staining), along with the optimal concentration of 1 µM after 10 min (a). (B) The orange nucleic acid probe 'live:dead' ratios in FL2 show good discrimination and no over lapping of fluorescence signals in concentrations of 1 µM or less and poor overlapping FL2 signals (ratios lower than one order of magnitude) with concentrations over 1 µM. Please click here to view a larger version of this figure.
| Conc. | Incubation time (min) | |||||||||
| (µM) | 1 | 5 | 10 | 30 | 60 | |||||
| 0.05 | 15.0 | 0.3 | 22.1 | 0.9 | 28.1 | 1.9 | 41.0 | 1.9 | 50.2 | 2.1 |
| 0.1 | 23.5 | 1.5 | 44.1 | 1.8 | 54.7 | 1.6 | 70.5 | 0.8 | 78.3 | 1.0 |
| 0.5 | 49.0 | 3.4 | 74.8 | 4.8 | 87.6 | 3.6 | 97.3 | 0.8 | 98.8 | 0.1 |
| 1 | 58.5 | 1.6 | 77.4 | 0.7 | 88.4 | 0.4 | 98.0 | 0.1 | 99.2 | 0.1 |
| 5 | 91.4 | 0.9 | 98.7 | 0.5 | 99.3 | 0.7 | 102.2 | 1.3 | 106.3 | 0.8 |
| 10 | 96.1 | 0.5 | 97.7 | 0.1 | 97.9 | 0.1 | 102.0 | 0.5 | 113.4 | 5.1 |
| 50 | 94.6 | 0.6 | 99.5 | 2.3 | 112.7 | 3.8 | 148.7 | 2.4 | 153.9 | 13.0 |
| 100 | 165.8 | 3.1 | 174.4 | 5.7 | 177.1 | 5.1 | 161.5 | 2.5 | 159.7 | 6.6 |
Table 1. Percentage Uptake of Green Nucleic Acid Probe in Cells. Table showing data from the optimization experiment using the green nucleic acid probe and a 50% 'live' and 50% 'dead' (heat treated) population sample. The mean percentages of cells that have recorded green nucleic acid probe fluorescence (FL1) have been calculated against the total number of 'dead' cells obtained from a FSC-H count. Values above 100% show non-specific staining (S.E. underlined).
| Conc. | Incubation time (min) | |||||||||
| (µM) | 1 | 5 | 10 | 30 | 60 | |||||
| 0.05 | 92.9 | 5.4 | 170.0 | 10.9 | 237.2 | 14.9 | 385.5 | 15.0 | 481.9 | 17.7 |
| 0.1 | 178.3 | 18.1 | 343.0 | 19.5 | 437.0 | 20.6 | 619.1 | 12.1 | 714.1 | 14.8 |
| 0.5 | 202.5 | 5.9 | 308.3 | 13.1 | 365.8 | 33.5 | 426.8 | 67.2 | 480.4 | 73.2 |
| 1 | 205.8 | 12.1 | 225.9 | 13.7 | 237.3 | 15.6 | 241.5 | 5.8 | 263.1 | 8.1 |
| 5 | 69.9 | 2.6 | 53.0 | 0.6 | 40.2 | 1.7 | 27.1 | 3.7 | 21.1 | 3.4 |
| 10 | 44.3 | 2.9 | 28.7 | 5.6 | 23.6 | 4.9 | 11.6 | 1.2 | 7.6 | 1.4 |
| 50 | 6.9 | 0.1 | 3.9 | 0.1 | 2.9 | 0.1 | 1.2 | 0.1 | 1.1 | 0.1 |
| 100 | 1.7 | 0.1 | 1.3 | 0.1 | 1.1 | 0.1 | 0.8 | 0.1 | 0.8 | 0.1 |
Table 2. Ratio of Green Fluorescence Signals in 'Live' and 'Dead' Stained M.aeruginosa Cells. FL1 signal discrimination between cells stained with the green nucleic acid probe and non-specific intrinsic recordings. The sample contained a 50% 'live' and 50% 'dead' (heat treated) M.aeruginosa of the same population measured over all concentration and incubation times (S.E. underlined).
| Conc. | Incubation time (min) | |||||||||
| (µM) | 1 | 5 | 10 | 30 | 60 | |||||
| 0.05 | 4.0 | 0.3 | 14.9 | 0.2 | 23.5 | 0.8 | 39.1 | 2.4 | 37.6 | 1.5 |
| 0.1 | 14.6 | 0.4 | 42.2 | 0.5 | 55.0 | 1.1 | 72.8 | 0.9 | 80.2 | 0.1 |
| 0.5 | 54.5 | 0.4 | 79.8 | 1.2 | 86.4 | 0.4 | 91.2 | 0.1 | 93.2 | 0.1 |
| 1 | 92.0 | 0.8 | 94.8 | 0.1 | 96.9 | 0.6 | 98.4 | 0.6 | 98.4 | 0.3 |
| 5 | 91.4 | 1.5 | 93.5 | 1.8 | 102.9 | 5.4 | 118.9 | 0.1 | 124.8 | 0.1 |
| 10 | 95.9 | 1.0 | 102.4 | 1.8 | 132.9 | 6.0 | 148.9 | 4.8 | 132.8 | 5.1 |
| 50 | 107.5 | 3.7 | 130.6 | 16.6 | 145.2 | 0.2 | 135.4 | 16.2 | 114.6 | 6.6 |
| 100 | 97.1 | 0.1 | 130.7 | 7.8 | 166.1 | 3.5 | 144.7 | 6.8 | 115.1 | 6.2 |
Table 3. Percentage Uptake of Orange Nucleic Acid Probe in Cells. Table with optimization data using the orange nucleic acid probe and a 50% 'live' and 50% 'dead' (heat treated) population sample. The mean percentage of cells that have recorded orange nucleic acid probe fluorescence (FL2) have been calculated against the total number of 'dead' cells from a FSC-H count (S.E. underlined).
| Conc. | Incubation time (min) | |||||||||
| (µM) | 1 | 5 | 10 | 30 | 60 | |||||
| 0.05 | 20.4 | 0.9 | 41.3 | 1.3 | 56.1 | 2.4 | 80.4 | 3.6 | 83.3 | 1.8 |
| 0.1 | 31.2 | 1.4 | 76.1 | 2.4 | 100.9 | 3.1 | 146.2 | 0.6 | 158.3 | 0.4 |
| 0.5 | 80.6 | 0.4 | 124.9 | 2.4 | 137.3 | 1.1 | 138.7 | 2.1 | 132.8 | 3.9 |
| 1 | 89.7 | 16.0 | 80.9 | 16.9 | 69.1 | 10.7 | 43.0 | 5.7 | 35.9 | 6.5 |
| 5 | 10.4 | 0.6 | 7.5 | 0.1 | 5.0 | 1.0 | 2.7 | 0.5 | 1.5 | 0.1 |
| 10 | 7.0 | 0.1 | 3.6 | 0.3 | 2.2 | 0.4 | 1.6 | 0.1 | 1.2 | 0.1 |
| 50 | 2.2 | 0.4 | 1.6 | 0.3 | 1.3 | 0.0 | 0.6 | 0.1 | 0.3 | 0.0 |
| 100 | 1.7 | 0.1 | 1.1 | 0.1 | 0.7 | 0.1 | 0.3 | 0.0 | 0.5 | 0.2 |
Table 4. Ratio of Orange Nucleic Acid in 'Live' and 'Dead' Stained M.aeruginosa Cells. FL2 signal discrimination between cells stained from the orange nucleic acid probe (FL2) and non-specific intrinsic recordings. The sample contained a 50% 'live' and 50% 'dead' (heat treated) M.aeruginosa of the same population measured over all concentration and incubation times (S.E. underlined).
Dyskusje
The increased numbers of publications using molecular probes indicates that reliable and informative data can be obtained5,6,8-15,19,22,23. As of yet there is no perfect stain for cell viability that can be effective across all species with the same concentration and incubation time6,10. Even the same type of probe with altered fluorescence emissions shows a need to establish the correct concentration and incubation time (Tables 1 & 3). As seen when using this protocol, the optimal concentration and incubation time for orange nucleic acid probe is 10 min at 1 µM, whereas the green nucleic acid probe would only need half the concentration but takes three times as long (Figures 5 & 6). Both of these cell-impermeant cyanine monomers with concentrations above 1.0 µM started to produce an overlap in emissions spectra with non-membrane injured cells. This overlap of signals provides evidence that non-specific staining occurs where 'live' cells are taking up the molecular probe. The resulting over fluorescence from non-specific staining generated false positives, underlining the need for protocol development to be carefully established for each molecular probe and target organism. The most critical steps in optimizing a molecular probe would be: 1) understanding how the molecular probe interacts with the FCM instrument; 2) adopting suitable live and dead controls; 3) developing knowledge of gating parameters from FCM outputs and 4) modifying the protocol through understanding of in-situ environments.
The light source, optical filters, ability to change PMT voltage and detectors within flow cytometers vary from manufacturer to manufacturer, so an awareness of what excitation wavelength from the laser and where the emission spectra lies in relation to the fluorescence channels are crucial. With acquisitions of data, thresholds can be imposed increasing resolution that would otherwise be lost to perhaps background noise, debris or dead cell aggregates. Molecular probes may offer insight into the viability of species without cultivation, through single parameters such as, membrane potential, DNA content and enzyme activity. However, within environmental samples there can be many organisms of a similar cell size and fluorescence. In addition within uniagal cultures, populations of cells will inevitably have a degree of physiological states3, which may affect molecular probe uptake. One of the main advantages of FCM is its ability to distinguish and characterise physiological states at the individual cell level in real time, which is essential when studying microorganisms viability in-situ, as they are often subjected to rapid and dynamic conditions. Despite its frequent use, the concept of cell viability still remains difficult to define. Usually applied to cellular proliferation, viability is very much scored on a growth based approach. This underpinning assumption may fall short, as conditions may not suit individual cells to reproduce but they may still be tolerable. Cells in a nonproliferating physiological state which have entered a quiescence or a G(0) cell cycle phase27 may not interact with a molecular probe giving false negatives in DNA cycle or metabolic based probes.
Using laboratory grown or freshly isolated cells to optimize molecular probes will aid the refining of data used in ecological studies. Cells used in optimization from batch cultures are commonly taken at exponential or early stationary phases and assumed to have the highest viability. However, extra caution must be taken when using high cell densities, those in a late stationary phase or microbes producing extracellular matrices. In this protocol the control for the dead population of cells were exposed to 60 oC heat for 1 hr, with confirmation of membrane damage and pigment degradation seen through microscopy (Figure 4) and FCM fluorescence outputs (Figures 2 & 3). Simulating the causes of natural cell death is extremely difficult as; ingestion by grazers, viral lysis, programmed cell death or the possibility of asymmetric division24 are not always feasible or be observed under laboratory conditions. Experimental modes of morality are often questioned as they may not be equivalent to stresses induced from the environment (e.g., heat killing) and there may be a heterogeneous response, by which certain individuals are killed and other cells show no observable cell degradation or death3,25. To avoid such a paradox the operational definition by which the experiments measured must be clearly established so as not to lead to confusion6,23, easing normalisation between studies.
Various alternate methods have been employed to analysis cell viability in cultures in conjunction with molecular probes. Epifluorescence microscopy is a traditional technique used, although photobleaching or the introduction of artefact signals from nearby cells may give an under or over estimate of a populations physiological state if not recorded quickly enough. This makes optimization through imaging very difficult, with potentially low accuracy. Genomic assessment of cellular viability using both DNA and RNA amplification methods such as; polymerase chain reaction (PCR), reverse transcriptase (RT-PCR) and nucleic acid sequence-based amplification (NASBA) have also been used. However, genomic assessment serves only as an indirect method of viability validation as the persistence of nucleic acid heavily depends on environmental conditions, with the correlation of actual cell viability potentially varying immensely28. By using a combination of light scatter and / or fluorescence through natural photosynthetic pigments (Figure 1) gating of populations can: increase resolution of data, identify rare population responses and reduce false positive results. FCM along molecular probes stands out from the previous mentioned techniques in testing single cell physiological for its speed, accuracy and recording of multiple parameters.
FCM is a powerful analytical tool in aquatic microbiology and with the addition of molecular probes has a wide potential in a number of areas including, single cell and community analysis to industrial sectors (e.g., monitoring drinking water supplies). Future advances could see FCM incorporate fluorescence in situ hybridization (FISH), which can detect and localise specific genetic sequences in individual cells within dense heterogeneous samples. The development of FCM and molecular probe portability for in-situ recordings could provide vital data on water sources to implement early strategies for resource management. Following the optimization protocol developed here, FCM and molecular probes can potentially play a critical role in providing valuable data about microbial physiology in aquatic environments.
Ujawnienia
The authors declare that they have no competing financial interests.
Podziękowania
The authors would like to acknowledge PhD student Dave Hartnell and Bournemouth University for support and funding for the research and facilities.
Materiały
| Name | Company | Catalog Number | Comments |
| Cyanobacteria Media | Sigma-Aldrich | C3061-500ML | BG-11 Freshwater concentrated solution (x50 dilution) |
| Decontamination Fluid | BD Biosciences | 653155 | Run for 2 min when outputs are more than 12 events per second on fast or a flow rate of 66 µl/min. Followed by 2 min of sheath H2O. |
| Flow Cytometer | BD Biosciences | by request | BD Accuri C6 |
| SYTOX Green | Life Technologies | S7020 | Nucleic acid stain – 5 mM solution in DMSO |
| SYTOX Orange | Life Technologies | S11368 | Nucleic acid stain – 5 mM solution in DMSO |
Odniesienia
- Kell, D. B., Kaprelyants, A. S., Weichart, D. H., Harwood, C. R., Barer, M. R. Viability and Activity in Readily Culturable Bacteria: A Review and Discussion of the Practical Issues. Anton. Leeuw. Int. J. G. 73, 169-187 (1998).
- Adams, D. G., Duggan, P. S. Tansley Review No. 107. Heterocyst and Akinete Differentiation in cyanobacteria. New Phytol. 144 (1), 3-33 (1999).
- Lidstrom, M. E., Konopka, M. C. The Role of Physiological Heterogeneity in Microbial Population Behavior. Nat. Chem. Biol. 6 (10), 705-712 (2010).
- Dagnino, D., de Abreu Meireles, D., de Aquino Almeida, J. C. Growth of Nutrient-Replete Microcystis. PCC 7806 Cultures is Inhibited by an Extracellular Signal Produced by Chlorotic Cultures. Environ. Microbiol. 8 (1), 30-36 (2006).
- Davey, H. M., Kell, D. B., Weichart, D. H., Kaprelyants, A. S. Estimation of Microbial Viability Using Flow Cytometry. Curr. Protoc. Cytom. Chapter. Chapter 11, (2004).
- Davey, H. M. Life, Death, and In-Between: Meanings and Methods in Microbiology. Appl. Environ. Microb. 77 (16), 5571-5576 (2011).
- Shapiro, H. M. Chapter 7, Parameters and Probes. Practical Flow Cytometry. , 273-410 (2003).
- Hammes, F., Berney, M., Wang, Y., Vital, M., Köster, O., Egli, T. Flow-Cytometric Total Bacterial Cell Counts as a Descriptive Microbiological Parameter for Drinking Water Treatment Processes. Water Res. 42 (1-2), 269-277 (2008).
- Wang, Y., Hammes, F., De Roy, K., Verstraete, W., Boon, N. Past, Present and Future Applications of Flow Cytometry in Aquatic Microbiology. Trends Biotechnol. 28 (8), 416-424 (2010).
- Shapiro, H. M., Nebe-von-Caron, G. Multiparameter Flow Cytometry of Bacteria. Methods. Mol. Biol. 263, 33-44 (2004).
- Peperzak, L., Brussaard, C. P. D. Flow Cytometric Applicability of Fluorescent Vitality Probes on Phytoplankton. J. Phycol. 47 (3), 692-702 (2011).
- Roth, B. L., Poot, M., Yue, S. T., Millard, P. J. Bacterial Viability and Antibiotic Susceptibility Testing with SYTOX Green Nucleic Acid Stain. Appl. Environ. Microbiol. 63 (6), (1997).
- Franklin, D., Airs, R., Fernandes, M. Identification of Senescence and Death in Emiliania huxleyi. and Thalassiosira pseudonana. Cell Staining, Chlorophyll Alterations, and Dimethylsulfoniopropionate (DMSP) Metabolism. Limnol. Oceanogr. 57 (1), 305-317 (2012).
- Lebaron, P., Catala, P., Parthuisot, N. Effectiveness of SYTOX Green Stain for Bacterial Viability Assessment. Appl. Environ. Microbiol. 64 (7), 2697-2700 (1998).
- Mikula, P., Zezulka, S., Jancula, D., Marsalek, B. Metabolic Activity and Membrane Integrity Changes in Microcystis aeruginosa.- New Findings on Hydrogen Peroxide Toxicity in Cyanobacteria. Eur. J. Phycol. 47 (July), 195-206 (2012).
- Regel, R. H., Brookes, J. D., Ganf, G. G., Griffiths, R. W. The Influence of Experimentally Generated Turbulence on the Mash01 Unicellular Microcystis aeruginosa Strain. Hydrobiologia. 517 (1-3), 107-120 (2004).
- Kameyama, K., Sugiura, N., Inamori, Y., Maekawa, T. Characteristics of Microcystin production in the Cell Cycle of Microcystis viridis. Environ. toxicol. 19 (1), 20-25 (2004).
- Gustafsson, S., Hultberg, M., Figueroa, R. I., Rengefors, K. On the Control of HAB Species using Low Biosurfactant Concentrations. Harmful Algae. 8 (6), 857-863 (2009).
- Bouchard, J. N., Purdie, D. A. Effect of Elevated Temperature, Darkness, and Hydrogen Peroxide Treatment on Oxidative Stress and Cell Death in the Bloom-Forming Toxic Cyanobacterium Microcystis. J. Phycol. 47 (6), 1316-1325 (2011).
- Reynolds, C. S., Jaworski, G. H. M. Enumeration of Natural Microcystis Populations. Br. Phycol. J. 13 (3), 269-277 (1978).
- Marie, D., Simon, N., Vaulot, D., Andersen, R. A. Phytoplankton Cell Counting by Flow Cytometry. Algal culturing techniques. , 253-268 (2005).
- Assunçao, P., Antuees, N. T., Rosales, R. S., de la Fe, C., Poveda, C., Poveda, J. B., Davey, H. M. Flow cytometric method for the assessment of the minimal inhibitory concentrations of antibacterial agents to Mycoplasma agalactiae. Cytom Part A. 69, 1071-1076 (2006).
- Davey, H. M., Kell, D. B. Flow Cytometry and Cell Sorting of Heterogeneous Microbial Populations: The Importance of Single-Cell Analyses. Microbiol. rev. 60 (4), 641-696 (1996).
- Franklin, D. J. Explaining the Causes of Cell Death in Cyanobacteria: What Role for Asymmetric Division?. J. Plankton Res. 36 (1), 11-17 (2013).
- Kaprelyants, A. S., Mukamolova, G. V., Davey, H. M., Kell, D. B. Quantitative Analysis of the Physiological Heterogeneity within Starved Cultures of Micrococcus luteus. by Flow Cytometry and Cell Sorting. Appl. Environ. Microbiol. 62 (4), 1311-1316 (1996).
- Waggoner, A., Melamed, M. R., Lindmo, T., Mendelsohn, M. L. Fluorescent probes for cytometry. Flow cytometry and sorting. , 209-225 (1990).
- Gray, J. V., Petsko, G. A., Johnston, G. C., Ringe, D., Singer, R. A., Werner-washburne, M. "Sleeping Beauty": Quiescence in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 68 (2), 187-206 (2004).
- Keer, J. T., Birch, L. Molecular Methods for the Assessment of Bacterial Viability. J. Microbiol. Methods. 53 (2), 175-183 (2003).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaPrzeglądaj więcej artyków
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone