Method Article
Expression, Detergent Solubilization, and Purification of a Membrane Transporter, the MexB Multidrug Resistance Protein
W tym Artykule
Podsumowanie
In this protocol we demonstrate the expression, solubilization, and purification of a recombinantly expressed membrane protein, MexB, as a soluble protein detergent complex. MexB is a multidrug resistance membrane transporter from the opportunistic bacterial pathogen Pseudomonas aeruginosa.
Streszczenie
Multidrug resistance (MDR), the ability of a cancer cell or pathogen to be resistant to a wide range of structurally and functionally unrelated anti-cancer drugs or antibiotics, is a current serious problem in public health. This multidrug resistance is largely due to energy-dependent drug efflux pumps. The pumps expel anti-cancer drugs or antibiotics into the external medium, lowering their intracellular concentration below a toxic threshold. We are studying multidrug resistance in Pseudomonas aeruginosa, an opportunistic bacterial pathogen that causes infections in patients with many types of injuries or illness, for example, burns or cystic fibrosis, and also in immuno-compromised cancer, dialysis, and transplantation patients. The major MDR efflux pumps in P. aeruginosa are tripartite complexes comprised of an inner membrane proton-drug antiporter (RND), an outer membrane channel (OMF), and a periplasmic linker protein (MFP) 1-8. The RND and OMF proteins are transmembrane proteins. Transmembrane proteins make up more than 30% of all proteins and are 65% of current drug targets. The hydrophobic transmembrane domains make the proteins insoluble in aqueous buffer. Before a transmembrane protein can be purified, it is necessary to find buffer conditions containing a mild detergent that enable the protein to be solubilized as a protein detergent complex (PDC) 9-11. In this example, we use an RND protein, the P. aeruginosa MexB transmembrane transporter, to demonstrate how to express a recombinant form of a transmembrane protein, solubilize it using detergents, and then purify the protein detergent complexes. This general method can be applied to the expression, purification, and solubilization of many other recombinantly expressed membrane proteins. The protein detergent complexes can later be used for biochemical or biophysical characterization including X-ray crystal structure determination or crosslinking studies.
Protokół
1. Day 1:
MexB from Pseudomonas aeruginosa is encoded by pFB101. The MexB gene was amplified from P. aeruginosa genomic DNA and inserted in the NdeI and XhoI restriction sites of the pET30b+ vector. The construct contains a C-terminal hexahistidine tag.
- The plasmid is used to transform E. coli strain C43(DE3) 12, and the transformants are plated on LB agar containing 30 ug/mL kanamycin.
2. Day 2: Overnight Cultures:
- In the evening, 4 X 3 mL LB cultures containing 30 ug/mL kanamycin are inoculated from the fresh transformant colonies. Alternatively, the cultures can be inoculated from a frozen perm.
These small cultures are grown on a roller at 37°C overnight.
3. Day 3: Growing 6 Liter Cultures:
- In the morning, use the overnight cultures to inoculate 150 mL LB containing 30 ug/mL kanamycin. Grow the culture at 37° C on a shaker.
- In the afternoon, use the small culture to inoculate 6 x 1L 2XYT media containing 30 ug/mL kanamycin in Fernbach flasks. (Use 25 mL per culture for a 1:40 dilution). Grow the cultures at 37°C until they reach an OD600 of 0.4-0.6, about 1.5 hours
- When the cultures reach the proper density, induce protein expression by adding 0.5 mL 1M IPTG. Put all the flasks back in the shaker and continue to grow them at 30°C overnight.
4. Day 4: Harvesting Cells and Purifying the Protein:
- Add protease inhibitors, DNAse, and lysozyme to the buffer solutions as follows: To 50 mL of cell resuspension buffer, add 10 mg DNaseI (0.1 mg/mL final concentration), 1 Complete EDTA-free protease inhibitor tablet, and a pinch of lysozyme. To 60 mL of membrane resuspension buffer, add 1 protease inhibitor tablet. To another 50 mL of membrane resuspension buffer, add 1 protease inhibitor tablet. Keep all three solutions on ice.
- Centrifuge the cultures 30 min 5,000 rpm in large-scale centrifuge to harvest the cells.
- Resuspend the cells in 100 mL cell resuspension buffer (50 mM NaP, pH 7.0, 300 mM NaCl, 2 mM MgCl2, 1 Complete EDTA-free protease inhibitor tablet, 0.1 mg/mL DNAse I, pinch of lysozyme)
- Pass the cell solution twice through a French pressure cell at 12,000 psi (762 gauge pressure). Collect the cell lysate in a bottle kept cold on ice.
- Transfer the cell lysate to SS34 centrifuge tubes and centrifuge to remove cell debris for 30 min at 10,000 rpm at 4°C in an SS-34 rotor.
- Carefully remove the supernatant into Ti647.5 ultracentrifuge tubes. Centrifuge 50 min at 40,000 rpm at 4°C. Discard the supernatant.
- Resuspend the pellet, which contains the cell membranes, in approx. 25 mL of membrane resuspension buffer (50 mM NaP, pH 7.0, 300 mM NaCl, 5% glycerol, 1 Complete EDTA-free protease inhibitor tablet).
- Transfer the membrane suspension to a clean centrifuge tube and centrifuge at 40,000 rpm in a Ti647.5 rotor for 50 min at 4 °C.
- Discard the supernatant and resuspend the washed membrane pellet in 25 mL membrane resuspension buffer (50 mM NaP, pH 7.0, 300 mM NaCl, 5% glycerol, 1 Complete EDTA-free protease inhibitor tablet).
5. TM Protein Solublization:
- To the resuspended membranes (about 25 mL), add 6 mL 10% DDM (final detergent concentration = 2% DDM) Rock the mixture at 4 °C for 2 hours.
- Centrifuge the mixture at 40,000 rpm for 40 min at 4°C in the Ti647.5 rotor to separate the soluble protein detergent complexes from the insoluble proteins. Save the supernatant, which contains the MexB protein detergent complexes.
6. IMAC:
- Mix the supernatant obtained from the high speed spin with the talon metal affinity beads equilibrated in resuspension buffer. Incubate for 1hr on a roller at 4°C.
- Pour the slurry into a gravity flow column body and discard the flow through.
- Wash the column with 20 mL (10 column volumes) of IMAC Binding and Wash Buffer (50 mM NaP, pH 7, 300 mM NaCl, 5% glycerol, 0.2% DDM)
- Elute the protein with IMAC elution buffer (50 mM NaP, pH 7.0, 300 mM NaCl, 5% glycerol, 250 mM imidazole, 0.2% DDM).
- Take 15 μL samples of the elution fractions, mix each with 15 μL 2X SDS sample buffer for analysis by polyacrylamide gel electrophoresis. Spin 30sec in a microcentrifuge. Analyze the samples on a 10% polyacrylamide SDS gel to estimate the amount and purity of MexB in each fraction.
- Pool the fractions containing the MexB protein detergent complexes and concentrate them in a spin concentrator at 4° C. Be careful that the protein does not precipitate out at this step.
7. Gel Filtration Column:
- Pre-equilibrate a Superose 12 HL 30/10 column with 24 mL running buffer (50 mM NaP, pH 7.0, 300 mM NaCl, 5% glycerol, 0.2% beta-octylglucoside), and wait for a flat baseline.
- Rinse the Akta system loading loop with running buffer.
- Filter the protein solution using a syringe filter before applying it to the column.
- Load up to 240 μL of the protein solution onto the column, with a protein concentration of up to 5mg/ mL.
- Run 1.5 column volumes (36 mL) of buffer, collect 0.25 mL fractions. The MexB protein detergent complexes should elute as a peak at around 10 - 15 mL of elution volume.
- Take 5 μL samples of the peak fractions. Mix each sample 1:1 with 2X SDS sample buffer. Analyze the samples on a 10% polyacrylamide gel to estimate the amount and purity of MexB in each fraction
- Pool the fractions containing pure MexB.
8. Representative Results:
Figure 1 includes a polyacrylamide gel with pooled column fractions from the IMAC column and individual fractions from the gel filtration column. After the gel filtration column the protein appears pure by Coomassie stained polyacrylamide gel. Figure 2 includes a trace from the gel filtration column showing the main peak of the protein detergent complex eluting from the column. The average yield of MexB protein is approximately 2 mg per 6 liters of 2XYT culture.
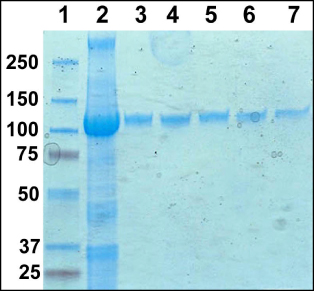
Figure 1. SDS-PAGE gel of purification of MexB PDCs. Lane 1, Molecular weight markers. 2, Pooled IMAC fractions. 3-7, Gel filtration column fractions.
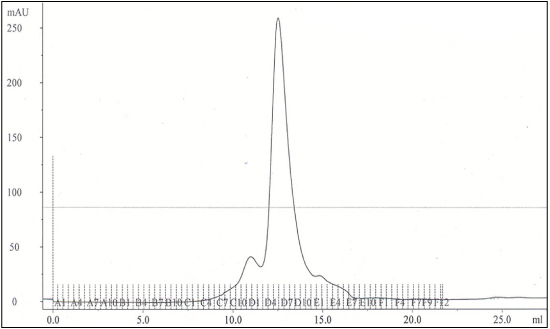
Figure 2. Example of Gel Filtration Results for MexB protein detergent complexes (PDC).
Dyskusje
In addition to multidrug resistance, many vital cellular activities, including ion transport, cell-cell communication, vesicle transport, maintenance of cellular structure, and host-pathogen interactions, involve proteins that are embedded in the cell membrane. Transmembrane proteins make up over 30% of known proteins and are the targets for the majority of pharmaceuticals in use today. The improper folding or activity of transmembrane proteins lead to important genetic diseases, including cystic fibrosis and diabetes. In spite of the vast importance of transmembrane proteins, there is far less known about their structures and molecular mechanisms than for soluble proteins. The presence of hydrophobic sequences can make it difficult to express and isolate large amounts of these proteins and makes them refractory to many biochemical and structural methods.
This protocol demonstrated the expression, detergent solubilization and purification of an MDR membrane protein as a soluble protein detergent complex. These methods can be used with some modification for many recombinantly expressed transmembrane proteins. The resulting purified protein detergent complexes are soluble and can be used for crystallization trials for X-ray crystallographic structure determination and for other biophysical or biochemical characterization, including reconstitution into liposomes or crosslinking studies.
During the purification procedures, it is important to be careful that protein detergent complexes do not precipitate out during the spin concentration steps. Different methods might also be used to help concentrate the PDC before or after the gel filtration step, such as repeating the IMAC step with a very small column and small elution volume.
Ujawnienia
No conflicts of interest declared.
Podziękowania
This project was supported by grants to CJJ from the National Science Foundation and the Society for Biomolecular Sciences.
Materiały
| Name | Company | Catalog Number | Comments |
| SDS sample buffer | Bio-Rad | 161-0737 | |
| C43(DE3) E. coli strain | Lucigen | 60345-1 | |
| kanamycin sulfate | Sigma-Aldrich | K4378 | |
| 2XYT media | Fisher Scientific | BP2466-2 | |
| LB media | Fisher Scientific | AC61189-5000 | |
| IPTG | Sigma-Aldrich | I6758 | |
| DNaseI | Fisher Scientific | BP3226-1 | |
| Lysozyme | Sigma-Aldrich | L7651 | |
| Complete EDTA-free protease inhibitor tablets | Roche Group | 11 873 580 001 | |
| NaP monobasic | Sigma-Aldrich | S6566 | |
| NaP dibasic | Sigma-Aldrich | S5136 | |
| NaCl | Sigma-Aldrich | S6191 | |
| MgCl2 | Sigma-Aldrich | M1028 | |
| Glycerol | Fisher Scientific | BP229-1 | |
| n-dodecyl-β-D-maltopyranoside | Anatrace | D310 | |
| 15ml tubes | Corning | 430052 | |
| See-Saw Rocker | Fisher Scientific | SSL 4 | |
| Talon metal affinity resin | Clontech Laboratories | 635503 | |
| imidazole | Sigma-Aldrich | I5513 | |
| 10% polyacrylamide SDS PAGE gels | Bio-Rad | 161-1454 | |
| Tris/glycine/SDS PAGE running buffer | Bio-Rad | 161-0732 | |
| Kaleidascope prestained molecular weight markers | Bio-Rad | 161-0324 | |
| Superose 12 30/10 column | GE Healthcare | 12 10/300 GL | |
| Amicon centrifugal concentrator | EMD Millipore | UFC801024 | |
| Syringe filter | Fisher Scientific | SLFG R04 NL | |
| Fernbach flasks | Fisher Scientific | 09-552-39 | |
| Shaker to hold Fernbach flasks | Fisher Scientific | ||
| Akta system | GE Healthcare | ||
| J6 Large scale centrifuge with JLA-8.1000 rotor | Beckman Coulter Inc. | ||
| 1 l centrifuge bottles | Beckman Coulter Inc. | 969329 | |
| RC-5 centrifuge | Thermo Fisher Scientific, Inc. | ||
| SS34 fixed-angle rotor and tubes | Thermo Fisher Scientific, Inc. | ||
| Sorvall floor model Ultracentrifuge | Thermo Fisher Scientific, Inc. | ||
| T647.5 rotor and tubes with caps | Thermo Fisher Scientific, Inc. | 08322 | |
| French Pressure Cell | Thermo Fisher Scientific, Inc. | FA-032 |
Odniesienia
- Eda, S., Maseda, H., Nakae, T. An elegant means of self-protection in gram-negative bacteria by recognizing and extruding xenobiotics from the periplasmic space. J. Biol. Chem. 278, 2085-2088 (2003).
- Li, X. Z., Ma, D., Livermore, D. M., Nikaido, H. Role of efflux pump(s) in intrinsic resistance of Pseudomonas aeruginosa: active efflux as a contributing factor to beta-lactam resistance. Antimicrob. Agents Chemother. 38, 1742-1752 (1994).
- Li, X. Z., Nikaido, H., Poole, K. Role of MexA-MexB-OprM in antibiotic efflux in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 39, 1948-1953 (1995).
- Masuda, N., Sakagawa, E., Ohya, S., Gotoh, N., Tsujimoto, H., Nishino, T. Substrate specificities of MexAB-OprM, MexCD-OprJ, and MexXY-oprM efflux pumps in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 44, 3322-3327 (2000).
- Okusu, H., Ma, D., Nikaido, H. AcrAB efflux pump plays a major role in the antibiotic resistance phenotype of Escherichia coli multiple-antibiotic-resistance (Mar) mutants. J. Bacteriol. 178, 306-308 (1996).
- Srikumar, R., Kon, T., Gotoh, N., Poole, K. Expression of Pseudomonas aeruginosa multidrug efflux pumps MexA-MexB-OprM and MexC-MexD-OprJ in a multidrug-sensitive Escherichia coli strain. Antimicrob. Agents Chemother. 42, 65-71 (1998).
- Tikhonova, E. B., Zgurskaya, H. I. AcrA, AcrB, and TolC of Escherichia coli Form a Stable Intermembrane Multidrug Efflux. Complex. J. Biol. Chem. 279, 32116-3224 (2004).
- Yoneyama, H., Ocakatan, A., Tsuda, M., Nakae, T. The role of mex-gene products in antibiotic extrusion in Pseudomonas aeruginosa. Biochem. Biophys. Res. Commun. 233, 611-618 (1997).
- Berger, B. W., Gendron, C. M., Robinson, C. R., Kaler, E. W., Lenhoff, A. M. The role of protein and surfactant interactions in membrane-protein crystallization. Acta. Crystallogr. D Biol. Crystallogr. 61, 724-730 (2005).
- Jones, M. Surfactants in membrane solubilisation. Int. J. Pharm. 177, 137-159 (1999).
- Maire, M. l. e., Champeil, P., Moller, J. V. Interaction of membrane proteins and lipids with solubilizing detergents. Biochim. Biophys. Acta. 1508, 86-111 (2000).
- Miroux, B., Walker, J. E. Over-production of proteins in Escherichia coli: mutant hosts that allow synthesis of some membrane proteins and globular proteins at high levels. J. Mol. Biol. 260, 289-298 (1996).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaPrzeglądaj więcej artyków
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone