Method Article
Live Imaging of Glial Cell Migration in the Drosophila Eye Imaginal Disc
W tym Artykule
Podsumowanie
Here we describe a protocol to examine the migration of glial cells into the developing Drosophila eye using live microscopic analysis paired with GFP tagged glial cells.
Streszczenie
Protokół
Part 1: Pre-experimental set-up.
- One week in advance, mate flies to generate larva that express GFP under the control of a glial-specific promoter. For our experiment we visualized GFP tagged with a nuclear localization sequence expressed in glial cells using the reversed polarity (repo) promoter (3, 4).
- Prepare cover slips at least one day in advance by soaking 18 mm round cover slips for 10 minutes in 1% poly-L-lysine solution and air dry overnight.
- On the day before the experiment, clean a Chamlide magnetic culture chamber by soaking components overnight in 70% ethanol.
- On the day of the experiment, prepare culture medium by adding fetal bovine serum, penicillin-streptomycin solution, and insulin to 10 ml of Schneider's insect medium using aseptic technique. The working concentrations are: 1X fetal bovine serum; 100U/ml penicillin; 0.1mg/ml streptomycin; and 0.2 mg/ml insulin in Schneider’s insect medium (modified from (5)).
- Allow the culture chamber to air dry in a cell culture hood. Wash off residual ethanol from the culture chamber by rinsing with prepared culture medium.
Part 2: Dissection of the Drosophila eye-brain complex.
- Select a third-instar wandering larva from the side of a fly vial and place in a drop of chilled culture medium on a petri dish on ice for several minutes. Chilling the larva is recommended to slow peristaltic body contractions for ease of dissection.
- Place the chilled larva in a drop of culture medium on a Sylgard coated dish under a dissection microscope.
- Under the dissection microscope, use a pair of Dumont fine forceps to firmly grasp the larva approximately one third of the way from the posterior end. Wait until the larva extrudes its mouth hooks from the anterior end. Grasp the mouth hooks with a second pair of forceps upon full extension. To dissect the larva, slowly pull the two pairs of forceps in opposite directions. The eye-brain complex, along with salivary glands, fat bodies, and imaginal tissue, will pull away from the larval body. Don’t pull the mouth hooks too quickly otherwise they will tear off of the eye-brain complex.
- Trim away salivary glands, fat bodies, and imaginal discs using ultra-fine clipper scissors. The two hemispheres of the brain and eye-discs will be attached to the ventral nerve cord and mouth hooks.
- Place an 18 mm cover slip onto a glass slide. Leave one edge of the cover slip hanging off the edge of the slide in order to later pick up the cover slip. Add a drop of culture medium onto the 18 mm cover slip. Transfer the eye-brain complex into the culture medium by grasping the mouth hooks with forceps. Don’t grasp the eye-brain complex directly with the forceps or the tissue will be damaged.
- Using fine dissection scissors, trim off the mouth hooks from the eye-brain complex and discard. Removal of the mouth hooks is important for live imaging as the mouth hooks will continue to contract in the culture medium causing the tissue to move during microscopy.
- To visualize glial migration within the eye imaginal disc, carefully cut the optic stalk, the thin tissue that connects the brain and eye disc and push away or discard the brain (see figure 1A). To visualize glia within the optic stalk, leave the brain, optic stalk, and eye imaginal disc intact (see figure 1E).
- Pick up the coverslip using forceps and draw a circle underneath around the tissue of interest with a permanent marker. This circle will aid in locating the tissue for microscopy in Part 4.
Part 3: Mounting the eye imaginal disc in a magnetic culture chamber.
- Using forceps, grasp the hanging edge of the coverslip. Transfer the coverslip to the bottom plate of the Chamlide magnetic chamber without disturbing the tissue in culture.
- Place the silicone O-ring onto the main body of the Chamlide chamber. Install the main body on top of the bottom plate.
- Slowly add culture medium to the chamber. Don’t add the culture medium too quickly or the tissue of interest will be disturbed. Don’t completely fill the chamber to allow for gas exchange under the cover.
- Gently place the invisible cover on top of the Chamlide chamber.
Part 4: Visualization of migrating glial cells in the eye disc.
- Place the culture chamber on the stage of a confocal or fluorescence microscope. Locate the sample using the circle on the coverslip as a reference on low magnification.
- Focus on the sample using a 40x lens. Allow the tissue to settle onto the coverslip. Capture images of the eye disc or optic stalk every 10-15min over 3-4 hours. The tissue may twitch and move out of focus. Manually focusing the tissue before image capture will alleviate this problem.
Part 5: Representative results:
Performed correctly, our protocol allowed us to collect a series of images of GFP-tagged glial cells that migrated from the optic stalk into the eye imaginal disc (Figure 1 B-D). While live imaging for a 60-minute period was sufficient to observe changes in the positions of glial nuclei within a wild type eye imaginal disc, glial nuclei in a mutant for a gene necessary for glial cell migration completely failed to exit the optic stalk (arrows Figure 1 F-H).
We have cultured eye-brain complexes for periods as long as 240 minutes before observing deterioration of the cultured tissue. Following the 240-minute time-point we begin to observe GFP positive cells in the culture medium surrounding cultured eye-brain complexes (arrow Figure 2 C). In addition GFP will accumulate in diffuse spots throughout the tissues suggesting a breakdown in tissue integrity.
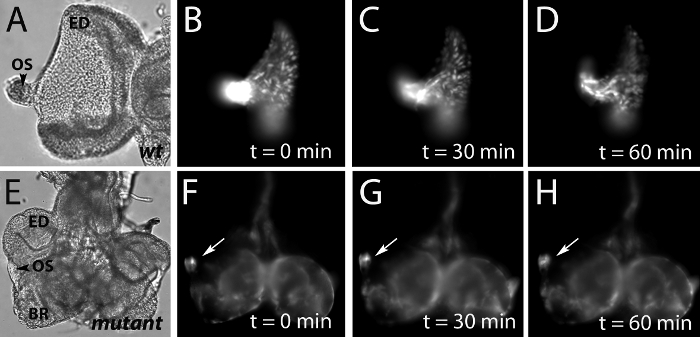
Figure 1: Live imaging of GFP-tagged glial nuclei in the developing wild type and mutant visual systems.
A, E) Images taken using differential interference contrast (DIC) microscopy of cultured wild type and mutant eye-imaginal discs (ED). In the wild type, glial cells are born in and migrate from the optic stalk (OS) into the eye-disc. The brain has been removed to facilitate flattening and imaging of the eye imaginal disc. B-D) Fluorescence microscopy of the wild type cultured eye imaginal disc in (A) at 0, 30, and 60 minute time points reveals GFP-tagged glial nuclei migrate within the eye disc.
F-H) Fluorescence microscopy of the mutant eye-brain complex in (E) at 0, 30, and 60 minute time points demonstrates a stalling of GFP-tagged glial nuclei within the optic stalk (arrow). The brain (BR) has been left attached to the optic stalk to facilitate imaging of glia within the stalk.

Figure 2: Live imaging of GFP-tagged glial membranes in a wild type eye imaginal disc. We have successfully cultured eye-brain complexes for 240-minute periods. Tissue cultured longer than 240 minutes begins to break down. The culture medium surrounding the eye imaginal disc (ED), optic stalk (OS), and brain lobe (BR) at 0 (A) and 150 minutes (B), compared to the 300 minute time-point (C), is free of GFP-positive cells, indicated by an arrow.
Dyskusje
In this protocol we describe observation of glial cell migration into the eye imaginal disc using live microscopy. In our wild type example (figure 1 A-D), we used a nuclear GFP marker to observe glial cell movement in the eye disc over the course of one hour. In a mutant for a candidate gene required for glial cell migration currently under study in our laboratory, we observed a stalling of glial cell nuclei within the optic stalk during a one-hour period (figure 1 E-H). Our strategy can be adapted to visualize further detail of cellular behavior regulated by our gene of interest. For example, a membrane targeted GFP molecule, such as mCD8-GFP, can be expressed in glia to visualize cellular processes. Use of a membrane bound GFP marker will allow us to determine if glial cells in our mutants are capable of extending cellular processes such as filopodia into the eye disc. Similarily actin tagged with GFP or tubulin tagged with GFP could be expressed in the developing glia to visualize changes in the cytoskeleton during glial cell migration. Additionally, the examination of single GFP-labeled mutant glial cells can be visualized using live microscopy (6). This technique will allow us to more accurately describe the requirement for our gene of interest in regulating the migration of glial cells.
Ujawnienia
The authors have nothing to disclose.
Podziękowania
Patrick Cafferty is supported by a postdoctoral fellowship from the Multiple Sclerosis Society of Canada.
Materiały
| Name | Company | Catalog Number | Comments | |
| Poly-L-lysine | Reagent | Sigma-Aldrich | P8920 | |
| Schneider’s Insect Media | Reagent | Sigma-Aldrich | S0146 | |
| Penicillin-Streptomycin | Reagent | Sigma-Aldrich | P4458 | |
| Insulin solution from bovine pancreas | Reagent | Sigma-Aldrich | I0516 | |
| Chamlide Magnetic chamber | Tool | Live cell Instrument | CM-R-10 | 35 mm dish type chamber for 18 mm coverslip |
| Ultra fine clipper scissors | Tool | Fine Science Tools | 15200-00 | |
| Dumont #5 forceps | Tool | Fine Science Tools | 11251-20 | |
| Fluorescent microscope | Microscope | Carl Zeiss, Inc. | Any fluorescent imaging system that has the necessary filters and excitation for GFP can be used. |
Odniesienia
- Aigouy, B., Lepelletier, L., Giangrande, A. Glial chain migration requires pioneer cells. J. Neurosci. 28, 11635-11641 (2008).
- Silies, M., Yuva, Y., Engelen, D., Aho, A., Stork, T., Klambt, C. Glial cell migration in the eye disc. J. Neurosci. 27, 13130-13139 (2007).
- Brand, A. H., Perrimon, N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 118, 401-415 (1993).
- Sepp, K. J., Auld, V. J. Conversion of lacZ enhancer trap lines to GAL4 lines using targeted transposition in Drosophila melanogaster. Genetics. 151, 1093-1101 (1999).
- Gibson, M. C., Patel, A. B., Nagpal, R., Perrimon, N. The emergence of geometric order in proliferating metazoan epitheia. Nature. 442, 1038-1041 (2006).
- Lee, T., Luo, L. Mosaic analysis with a repressible cell marker (MARCM) for Drosophila neural development. Trends Neurosci. 24, 251-254 (2001).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaPrzeglądaj więcej artyków
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone