Method Article
Preclinical Model of Prenatal Delta-9-Tetrahydrocannabinol Exposure to Assess Its Impact on Neurodevelopmental Outcomes
In This Article
Summary
As legislative restrictions change, resulting in increased accessibility of cannabinoid products, it is critically important to develop models to study the impact of these exposures, particularly during pregnancy. A preclinical model of moderate prenatal cannabinoid exposure through voluntary ingestion has been developed, enabling more in-depth investigations.
Abstract
Prenatal cannabinoid exposure (PCE) is becoming increasingly frequent as more states across the United States legalize recreational marijuana (cannabinoids). The consumption of cannabinoid products during pregnancy has been associated with various abnormal outcomes, although historical studies were conducted during a time when the potency of these products was approximately 300% lower than that of current products. Given the rising use of cannabinoids, it is essential to understand the potential impacts PCE may have on fetal neurodevelopment and subsequent infant and child development. Previous studies have demonstrated that PCE negatively affects learning and memory, behavioral skills, sleep, and attention in offspring. The aim of this study is to recapitulate PCE through voluntary ingestion of delta-9-tetrahydrocannabinol (THC), the psychoactive component of cannabinoid products, during pregnancy in a preclinical model. This article outlines the procedure for achieving moderate PCE throughout gestation. In this model, the control group consumes plain mini-chocolate/peanut butter cookies, while the PCE group consumes THC mixed into peanut butter paired with mini-chocolate cookies. This approach enables further investigation into the impact of PCE on developmental outcomes.
Introduction
As rates of prenatal cannabinoid product use increase1, likely influenced by the growing legalization of cannabinoid products across the United States, studies are needed to better understand their impact on fetal development. Delta-9-tetrahydrocannabinol (THC), the psychoactive component of cannabinoid products, readily crosses the placenta and interacts with the endogenous endocannabinoid system2,3. The endogenous endocannabinoid system plays a critical role in neural development and may be a pathway through which developmental alterations occur. Cannabinoid use during pregnancy has been linked to reduced birth weight4 and an increased risk of premature birth5,6. Additional developmental abnormalities, such as deficits in social behavior and executive functions throughout life, have also been reported7,8,9.
Over time, changes in cannabinoid potency and patterns of use in the general population have highlighted the need for detailed studies on the potential effects of prenatal exposure on fetal development. The use of cannabinoid products during pregnancy is increasing, as these products are often perceived to alleviate symptoms such as depression, stress, pain, sleep disturbances, and nausea10,11. Dickson et al.12 conducted a study investigating recommendations from 400 cannabis dispensaries regarding first-trimester use in Colorado and found that approximately half of the dispensaries (50.5%) recommended edibles to pregnant individuals. It is often perceived that edibles avoid harm and health risks associated with smoking13,14, which results in many pregnant individuals consuming edibles.
Edibles deliver cannabinoids through the gastrointestinal system, where THC is absorbed into the bloodstream and transported via the portal vein to the liver for first-pass metabolism. In the liver, enzymes hydroxylate THC to produce 11-hydroxytetrahydrocannabinol (11-OH-THC), a highly potent psychoactive metabolite that easily crosses the blood-brain barrier15. Although it takes longer for the initial psychoactive effect of edibles (30-90 min), the resulting 'high' lasts longer, with a peak at 2-4 h after ingestion16,17. The pharmacokinetic half-life of THC is 1.3-7.3 h in rats18,19.
Given the increasing utilization during pregnancy and the paucity of data on its impact on fetal neurodevelopment, a preclinical model of voluntary THC ingestion during pregnancy was developed, aligning more closely with the reported typical human use (oral consumption to alleviate nausea) to further describe the impact on neurodevelopmental outcomes.
Protocol
All procedures described here were approved by the University of New Mexico Health Sciences Center (UNM HSC) Institutional Animal Care and Use Committee (IACUC). All experiments were conducted in compliance with the ARRIVE guidelines. Long-Evans (Blue Spruce, HsdBlu: LE) rats were obtained from a commercial source. The males used were proven adult breeder rats, 12 weeks old upon arrival and 15-16 weeks old at the start of the breeding protocol. The females weighed 125-150 g and were approximately 6-7 weeks old upon arrival. At the time of breeding, the females were approximately 9-10 weeks old. It is critical to ensure that all prospective dams have no prior history of pregnancy. Allow at least one week for acclimation to the facility and their new housing environment. The first part of this protocol addresses pre-pregnancy cannabinoid consumption, while the second part focuses on pregnancy cannabinoid exposure. Details of the animals, reagents, and equipment used are provided in the Table of Materials. The experimental design is provided in Figure 1.
1. Pre-pregnancy cannabinoid exposure
- Housing and maintenance
- House animals individually in standard rat static micro isolator cages with bedding at 22 °C on a reverse 12-h light/dark schedule (lights on from 21:00 to 09:00 daily).
- Provide access to irradiated food (see Table of Materials) and tap water ad libitum throughout the study.
- Material and chemical preparation
- Obtain all required materials and chemicals (as provided in the Table of Materials).
- Keep research-grade THC in vials containing 10 mg/mL or 20 mg/mL THC dissolved in ethanol.
NOTE: Use appropriate personal protective equipment (PPE), as THC is a skin irritant and an aspiration hazard. Secure THC in a steel cabinet, as it is classified as a Schedule I controlled substance by the U.S. Department of Health and Human Services and the U.S. Food and Drug Administration.
- Baseline measurements
- Obtain baseline body weights for each female rat.
- Pause the experiment here for 1-2 weeks if necessary.
- Preparation of THC-infused cookies
- Prepare cookies 30 min prior to administration at 14:00 daily to maintain consistent feeding times.
- Separate mini chocolate cookies and add approximately half a teaspoon of peanut butter to the cream.
- Mix the required dose of THC (1.5 mg/kg for the first 2 days, followed by 3 mg/kg for 7 days) with the peanut butter before assembling the cookie.
- Consumption evaluation
- Provide a plain peanut butter/mini chocolate cookie for 2-4 days until consistent consumption is achieved. Remove water from the cage and administer the treat to each rat using forceps at 14:00 daily.
- Observe until the treat is completely consumed. Remove remnants if necessary.
NOTE: Administer THC at a dose of 1.5 mg/kg for 2 days, followed by 3 mg/kg for 7 days. Leave treats in the cage for up to 2 h for rats that do not consume immediately. - Continue only with rats that show consistent treat consumption. Remove those with inconsistent ingestion from the experiment.
- Evaluate pre-pregnancy consumption for 2 weeks.
- Post-PCE group assignment
- Assign rats with consistent ingestion randomly to either the plain (control) or THC-infused (PCE) cookie group.
- Allow a minimum of 4 days without cookies prior to breeding to ensure THC clearance from their systems.
2. Pregnancy (prenatal) cannabinoid exposure
- Breeding and pregnancy confirmation
- Weigh the females to obtain a starting weight before placing them in a cage with a proven male breeder (male is THC-naïve).
- Ensure that the female rats do not consume a cookie during this time.
- Confirm pregnancy by the presence of a vaginal plug, which typically occurs 24 h after mating.
- Weigh the female rat and return it to the home cage. This is defined as Gestational Day (GD) 1.
- THC infusion during pregnancy
- Provide a plain or THC-infused (3 mg/kg) cookie at 14:00 daily starting at GD 1 for the duration of the pregnancy.
- Continue this regimen until the dam is noted to be nesting for delivery, at which point THC exposure is stopped.
- Monitoring maternal weight gain
- Weigh the rat dams twice a week to assess maternal weight gain.
NOTE: The new weights are also used to calculate how much THC to include in the cookie, adjusting the dose as necessary based on the dam's weight.
- Weigh the rat dams twice a week to assess maternal weight gain.
- Cessation of THC exposure and birth documentation
- Stop the THC exposure procedures when the dam is nesting around the expected time of delivery.
- Once offspring are born, record the number of live pups.
- Designate the day of birth as postnatal day (P) 0.
- Weaning and housing of offspring
- Wean the offspring at postnatal day (P) 23.
- Group house the weaned offspring with littermates based on sex.
- Euthanasia of dams
- Once the litter is weaned, euthanize the dams following institutionally approved protocols.
3. Post-procedural steps
- Offspring housing and monitoring
- Ensure all offspring are kept in appropriate housing conditions, ensuring proper temperature, light, and ventilation.
- Monitor pups regularly for any health or developmental issues, such as abnormal body weight or developmental delays.
- Baseline assessment
- If required, assess baseline measures such as body weight, reflexes, or other relevant developmental parameters before initiating experiments on the offspring.
- Experimental group assignment
- Assign pups to experimental groups randomly to minimize any biases in testing.
- Limit the number of pups used per experiment to 1-2 pups from each litter to reduce potential litter effects.
Results
To establish voluntary consumption of THC, numerous treats were trialed to determine which ones were palatable to the rats and would be consumed quickly and consistently.
Initially, sugar-free strawberry-flavored gelatin cubes (1 cubic mL size), both with and without THC, were trialed. The amount of strawberry syrup flavoring was increased, and THC was trialed at three different concentrations (2 mg/kg/dose, 3 mg/kg/dose, and 5 mg/kg/dose). The rats did not consistently consume the cubes and were noted to quickly discontinue consumption at the higher THC concentrations. It is known that rats can refuse food with a bitter or acidic taste, which raised concerns that this model might not be viable for voluntary consumption.
After consultation with the institutional veterinarian, various other treats were trialed, including crackers with either peanut butter or cheese, mini chocolate cookies with peanut butter, cheese crackers with peanut butter, and fish-shaped crackers with cheese, all obtained from Amazon. The female rats consistently consumed the mini chocolate cookies with peanut butter, often within 10-30 min of being offered the treat. THC was then added to the mini chocolate cookies with peanut butter, and the percentage of dams who consumed the daily treat was similar between the control and THC-exposed groups (67% vs. 62%, respectively; p = 0.14).
Dams were then exposed to THC as described in the protocol, with pre-pregnancy and prenatal cannabinoid exposure. Dams were randomized into the control group or the PCE group (THC exposure at 3 mg/kg/dose) before breeding. Litter size and maternal weight gain were similar between the groups. The mortality rate (percent death from birth to P2) and birth weight were approaching significance between controls and PCE (0% vs. 0.04%, p = 0.06 and 6.25 g vs. 5.96 g, p = 0.07, respectively). The mortality rate trended higher in the PCE group, and the offspring birth weight trended lower in the PCE group (Table 1 and Figure 2), which is consistent with the human literature20.
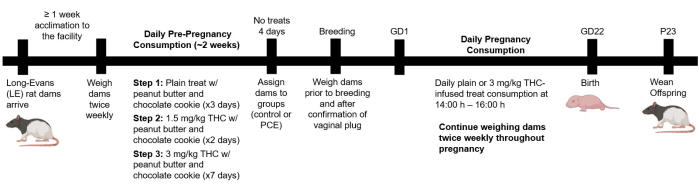
Figure 1: Experimental design. Dams voluntarily ingested THC-infused cookies daily before breeding to ensure consistent intake. Dams not consuming the cookies were excluded from the experiment. The remaining dams were randomized to consume either a plain or THC-infused (3 mg/kg) cookie during pregnancy. The birth occurred normally, and pups were weaned on postnatal day (P) 23. Please click here to view a larger version of this figure.

Figure 2: Graphical representation of dam and offspring characteristics for the PCE model. This figure provides a graphical representation of dam and offspring characteristics in the PCE model. Please click here to view a larger version of this figure.
| Sham (n = 6 dams) | PCE (n = 4 dams) | p Value* | |
| Maternal weight gain (g) | 107.00 ± 6.83 | 94.25 ± 10.03 | 0.48 |
| Litter size (number of live pups/litter) | 10.83 ± 1.31 | 12.75 ± 0.74 | 0.22 |
| Mortality rate (% death from birth to P2) | 0.00 ± 0.00 | 0.04 ± 0.02 | 0.06 |
| Offspring birth weight (g) | 6.25 ± 0.11 | 5.96 ± 0.10 | 0.07 |
| Offspring weight on postnatal day 21 (g) | 41.31 ± 1.40 | 37.61 ± 2.22 | 0.17 |
| *p value was calculated using a student's t-test | |||
Table 1: Dam and offspring characteristics for the PCE model. Maternal weight gain through gestational day (GD) 22 was measured as the increase in body weight in grams and was not significantly different between groups (p = 0.48). The table also includes the average litter size, the mortality rate (percentage of deaths from birth to P2), the birth weight of the offspring per litter, and the weight at P21. All data are shown as the mean value ± standard error of the mean (SEM); *p values were calculated using a Student's t-test.
Discussion
The PCE paradigm outlined here involves voluntary THC consumption by pregnant rat dams. This is the first study to explore THC administration to pregnant rat dams via voluntary ingestion in a preclinical model. Both groups of rat dams consumed the same rat chow diet, minimizing potential variability in nutrition and caloric intake between groups. Animal models offer the advantage of controlling the timing and dosage of PCE for study purposes.
Pre-pregnancy consumption is assessed to identify rats that consume THC at the desired levels for the subsequent phases of the consumption protocol. This approach ensures that all female rats have prior experience with THC ingestion before pregnancy, more accurately reflecting human behavior5, where individuals often use THC before pregnancy. In this model, PCE occurs throughout gestational development, roughly corresponding to the first two trimesters of human gestation. Evidence suggests that cannabinoid use is most common during the first trimester, decreases in the second, and further declines in the third1.
Endocannabinoid receptors are not expressed in the fetus until 5-6 weeks of gestation21. Exposure before this period may not directly influence fetal brain development, although indirect effects through endocannabinoid receptor expression in the placenta are still possible22. Consequently, researchers focused on neurodevelopmental outcomes will need to account for the timing of exposure in relation to the specific outcomes of interest.
A critical step in the protocol is the ability to measure THC levels frequently to ensure that consumption achieves the target serum levels. Ongoing experiments are measuring serum levels throughout pregnancy to ensure that a stable level can be maintained despite the metabolic changes that occur during pregnancy.
We considered other oral administration techniques, such as oral gavage. However, the goal of this model is to remain as clinically relevant as possible. Gavage administration can induce significant stress in rats, resulting in increased blood pressure, elevated plasma corticosterone levels, and other adverse effects23,24. Thus, we chose voluntary ingestion to minimize any unintended side effects.
The limitations of this study include ensuring consistent consumption and the need for caution when preparing the treats, as inaccuracies in THC dosage during preparation could affect the results. While individual rats may differ in metabolism, this is also a strength given the desire for clinical correlation.
Overall, a preclinical model of voluntary ingestion to produce moderate THC exposure is feasible. This model development will allow for further in-depth investigations into neurodevelopmental outcomes, as well as other areas of interest. Future studies will contribute to the growing literature concerning the impact of PCE on fetal development.
Disclosures
The authors have no conflicts of interest to disclose.
Acknowledgements
Thank you to the National Institute on Drug Abuse (NIDA) Drug Supply Program for the generous supply of THC. Supported by the University of New Mexico Department of Pediatrics Research Allocation Committee grant and the Division of Neonatology.
Materials
| Name | Company | Catalog Number | Comments |
| 5L0D PicoLab Laboratory Rodent Diet, irradiated | Purina LabDiet | 5L0D | |
| Cellulose/paper bedding BioFresh Comfort Bedding | BioFresh Vet | NA | https://biofreshvet.com/products/ |
| Digital Kitchen Scale | Etekcity | EK4150 | |
| Female rats, 125-150 g | Inotiv | 14005F | |
| Long-Evans rats (Blue Spruce, HsdBlu: LE) | Inotiv | 14016M | |
| N10 Rodent Plastic Cage Bottoms | Ancare | N10PLF | |
| N10 Rodent Wire Lids | Ancare | N10SS | |
| Narrow Pattern Forceps | Fine Science tools (FST) | 11002-14 | |
| OREO mini chocolate Sandwich cookies, Go Paks, 12-3.5 oz cups | Amazon | B0198TUO7W | |
| proven adult breeder males | Inotiv | 14016M | |
| Rat static polysulfone microisolator cages (R20): | |||
| Research Grade THC (only available with a DEA license, Schedule I drug) | NIDA | NA | https://nida.nih.gov/research/research-data-measures-resources/nida-drug-supply-program |
| SKIPPY Creamy Peanut Butter spread | Amazon | B0C75KZ28C | |
| THC Consumption Supplies: |
References
- Volkow, N. D., Han, B., Compton, W. M., McCance-Katz, E. F. Self-reported medical and nonmedical cannabis use among pregnant women in the United States. JAMA. 322 (2), 167-169 (2019).
- de Salas-Quiroga, A., et al. Prenatal exposure to cannabinoids evokes long-lasting functional alterations by targeting CB1 receptors on developing cortical neurons. Proc Natl Acad Sci USA. 112 (44), 13693-13698 (2015).
- Baglot, S. L., et al. Maternal-fetal transmission of delta-9-tetrahydrocannabinol (THC) and its metabolites following inhalation and injection exposure during pregnancy in rats. J Neurosci Res. 100 (3), 713-730 (2022).
- Ryan, S. A., Ammerman, S. D., O'Connor, M. E. Committee on Substance Use and Prevention; Section on Breastfeeding. Marijuana use during pregnancy and breastfeeding: implications for neonatal and childhood outcomes. Pediatrics. 142 (3), e20181889 (2018).
- Corsi, D. J., et al. Association between self-reported prenatal cannabis use and maternal, perinatal, and neonatal outcomes. JAMA. 322 (2), 145-152 (2019).
- Duko, B., Dachew, B. A., Pereira, G., Alati, R. The effect of prenatal cannabis exposure on offspring preterm birth: a cumulative meta-analysis. Addiction. 118 (4), 607-619 (2023).
- Scheyer, A. F., Melis, M., Trezza, V., Manzoni, O. J. J. Trends in Neurosciences. Trends Neurosci. 42 (12), 871-884 (2019).
- Nashed, M. G., Hardy, D. B., Laviolette, S. R. Prenatal Cannabinoid Exposure: Emerging Evidence of Physiological and Neuropsychiatric Abnormalities. Front Psychiatry. 11, 624275 (2021).
- Lin, A., et al. Prenatal cannabinoid exposure: why expecting individuals should take a pregnancy pause from using cannabinoid products. Front Pediatr. 11, 1278227 (2023).
- Chang, J. C., et al. Beliefs and attitudes regarding prenatal marijuana use: perspectives of pregnant women who report use. Drug Alcohol Depend. 196, 14-20 (2019).
- Young-Wolff, K. C., et al. Trends in cannabis polysubstance use during early pregnancy among patients in a large health care system in Northern California. JAMA Netw Open. 5 (6), e2215418 (2022).
- Dickson, B., et al. Recommendations from cannabis dispensaries about first-trimester cannabis use. Obstet Gynecol. 131 (6), 1031-1038 (2018).
- Murphy, F., et al. Baby boomers and cannabis delivery systems. J Drug Issues. 45 (3), 293-313 (2015).
- Barrus, D. G., et al. Tasty THC: Promises and challenges of cannabis edibles. Methods Rep, RTI Press. , 1-22 (2016).
- Mura, P., Kintz, P., Dumestre, V., Raul, S., Hauet, T. THC can be detected in brain while absent in blood. J Anal Toxicol. 29 (8), 842-843 (2005).
- Grotenhermen, F. Pharmacokinetics and pharmacodynamics of cannabinoids. Clin Pharmacol Ther. 42 (4), 327-360 (2003).
- Thompson, R., DeJong, K., Lo, J. Marijuana Use in Pregnancy: A Review. Obstet Gynecol Surv. 74 (7), 415-428 (2019).
- Ravula, A., et al. Pharmacokinetic and pharmacodynamic characterization of tetrahydrocannabinol-induced cannabinoid dependence after chronic passive cannabis smoke exposure in rats. Cannabis Cannabinoid Res. 4 (4), 240-254 (2019).
- Zgair, A., et al. Dietary fats and pharmaceutical lipid excipients increase systemic exposure to orally administered cannabis and cannabis-based medicines. Am J Transl Res. 8 (8), 3448-3459 (2016).
- Mulligan, M. K., Hamre, K. M. Influence of prenatal cannabinoid exposure on early development and beyond. Adv Drug Alcohol Res. 3, 10981 (2023).
- Paul, S. E., et al. Associations between prenatal cannabis exposure and childhood outcomes: Results from the ABCD study. JAMA Psychiatry. 78 (1), 64-76 (2021).
- Fügedi, G., et al. Increased placental expression of cannabinoid receptor 1 in preeclampsia: An observational study. BMC Pregnancy Childbirth. 14, 395 (2014).
- Walker, M. K., et al. A less stressful alternative to oral gavage for pharmacological and toxicological studies in mice. Toxicol Appl Pharmacol. 260 (1), 65-69 (2012).
- Brown, A. P., Dinger, N., Levine, B. S. Stress produced by gavage administration in the rat. Contemp Top Lab Anim Sci. 39 (1), 17-21 (2000).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved