Method Article
Cryosectioning and Immunostaining of Mouse Retina
* These authors contributed equally
In This Article
Summary
A protocol for preparing mouse retinal cryosections and performing immunostaining on photoreceptors is described. This article enables researchers to consistently produce mouse retinal frozen sections with well-preserved morphology and high-quality immunostaining results.
Abstract
Tissue sectioning and immunohistochemistry are essential techniques in histological and pathological studies of retinal diseases using animal models. These methods enable detailed examinations of tissue morphologies and the localization of specific proteins within the tissue, which provide valuable insights into disease processes and mechanisms. Mice are the most widely used model for this purpose. However, because mouse eyeballs are small and mouse retinas are extremely delicate tissues, obtaining high-quality retinal sections and immunostaining images from mouse eyeballs is typically challenging. This study describes an improved protocol for cryosectioning mouse retinas and performing immunohistochemistry. An essential point of this protocol involves coating the eyeball with a layer of super glue, which prevents deformation of the eyeballs during the processes of cornea removal, lens extraction, and embedding. This step ensures the integrity of retinal morphologies is well preserved. This protocol highlights critical technical considerations and optimization strategies for consistently producing high-quality retinal sections and achieving excellent immunostaining results.
Introduction
Cryosectioning and immunohistochemistry (IHC) are indispensable techniques in biomedical research, particularly for studying complex biological structures such as the retina1. These advanced methodologies are integral to understanding the intricate cellular composition and molecular organization of the retina. They provide researchers with the ability to investigate retinal functionality and pathology at a detailed level, offering insights that are critical for advancing knowledge in this field.
Cryosectioning plays a vital role in maintaining the morphological integrity of retinal tissue. It ensures that the delicate structure of the retina remains intact, allowing sections to be used in subsequent immunofluorescent studies with high accuracy and reliability. Compared with other methods, such as paraffin embedding, cryosectioning has significant advantages as it better preserves both tissue morphology and antigenicity, making it particularly suitable for immunohistochemical staining2. The frozen section technique is widely adopted for studying a range of complex tissues and even fine cellular structures3, enabling precise analyses of their architecture.
IHC is a powerful and versatile laboratory technique that allows for the visualization of the localization of specific proteins within tissues. This technique has become a cornerstone in both clinical and research settings, where it is extensively utilized for diagnostics, disease monitoring, and biological investigations. The success of an IHC experiment depends heavily on meticulous sample preparation, careful handling of the tissue, and precise control of immunostaining conditions. Small variations in protocol can greatly impact the quality of results, underscoring the importance of standardization and optimization1.
When combined, cryosectioning and IHC offer unparalleled advantages for researchers seeking to explore the spatial distribution, expression levels, and cellular interactions of various proteins within the retina. These methodologies allow for detailed investigations into the molecular mechanisms underlying retinal development, function, and disease. Such insights are particularly valuable in studying retinal disorders, including age-related macular degeneration, diabetic retinopathy, and retinitis pigmentosa. By elucidating the pathophysiology of these conditions, cryosectioning and IHC contribute to identifying potential biomarkers and developing novel therapeutic strategies.
Despite its utility, working with mouse retinas presents unique challenges. Mice are widely used as animal models in ophthalmic research due to their genetic similarity to humans and their well-characterized retinal structure. However, obtaining high-quality cryosections is inherently difficult because of the small size and delicate nature of mouse retinal tissue. This study provides a detailed methodology for cryosectioning and performing IHC on mouse retinas, highlighting critical technical considerations and offering optimization strategies to address these challenges. By refining these techniques, researchers can achieve consistent and high-quality results, advancing the study of retinal biology and pathology.
Protocol
The procedure adhered to the guidelines established by the Association for Research in Vision and Ophthalmology for the Use of Animals in Research. Approval was obtained from the Institutional Animal Care and Use Committee (IACUC) of Sichuan Provincial People's Hospital. Male C57Bl/6J mice, aged two to three months and weighing 25-30 g, were used for this protocol. A comprehensive list of the reagents and equipment utilized in this study is provided in the Table of Materials.
1. Reagent preparation
- 1x PBS buffer
- Weigh 8 g of NaCl, 1.42 g of Na2HPO4, 0.42 g of KH2PO4, and 0.2 g of KCl using a scale. Transfer these into a beaker and add an appropriate amount of double-distilled water (ddH2O) to dissolve the components. Pour the solution into a 1 L volumetric flask and adjust the volume to 1 L with ddH2O.
- 4% Paraformaldehyde (PFA) solution
- Add 0.4 g of PFA powder and 15 µL of 1 M NaOH to a 15 mL conical tube containing 8 mL of 1x PBS buffer. Place the tube in a water bath set to 60 °C to dissolve the PFA powder completely. Adjust the final volume to 10 mL with 1x PBS.
- IHC blocking solution
- Combine 1 mL of normal donkey serum, 20 µL of 20% NaN3, 200 µL of 20% Triton X-100, and 18.78 mL of 1x PBS buffer. Mix thoroughly.
- 30% sucrose solution
- Dissolve 15 g of sucrose in 40 mL of 1x PBS buffer. Adjust the volume to 50 mL with 1x PBS buffer and mix until fully dissolved.
- IHC secondary antibody solution
- Dilute Alexa Fluor 488-conjugated goat anti-rabbit antibody at a 1:300 ratio and DAPI at a 1:2,000 ratio using the prepared blocking buffer.
2. Mouse eyeball cryosectioning
- Eyeball dissecting
- Anesthetize a 3-month-old C57Bl/6 mouse (approximately 25 g) via intraperitoneal injection of 2% Tribromoethanol at a dosage of 15 µL/g body weight. Sacrifice the mouse using cervical dislocation (following institutionally approved protocols).
- Mark the superior side of the eyeball with a blue marker on the sclera (Figure 1A). Remove the eyeballs using scissors.
- If any blood is present on the eyeball surface, gently wipe it off using a lint-free wipe. Under a dissecting microscope, carefully remove the extraocular muscles attached to the eyeballs.
- Fixation
- Transfer the dissected eyeballs to a 2-mL round-bottom microcentrifuge tube containing 1 mL of 4% PFA. Fix the eyeballs for 10 min. Subsequently, transfer the eyeballs to an inverted 3- or 6-cm Petri dish.
- Under a dissecting microscope, make a small incision (approximately 1-2 mm) on the cornea using fine forceps and ophthalmic scissors. Place the eyeballs back into the 4% PFA solution for an additional 2 h of fixation on ice (Figure 1B).
- Cryoprotection
- Remove the fixative and wash the eyeballs three times with 1x PBS buffer solution. Transfer the eyeballs to a 2-mL round-bottom microcentrifuge tube containing 1 mL of 30% sucrose solution for cryoprotection at 4 °C. Allow the eyeballs to settle at the bottom of the tube or leave them overnight.
- Coating the eyeball
- Transfer an eyeball onto an inverted Petri dish with the cornea side facing upward. Blot the excess solution from the cornea using a lint-free wipe. Dip a 2-3 cm segment of tennis string into the glue (composed of cyanoacrylates) contained in a 0.2-mL microcentrifuge tube, then remove it quickly.
- Under a dissecting microscope, attach one end of the tennis string with residual super glue to the center of the moist cornea. Allow the glue to solidify for 10-20 s. Grasp the other end of the tennis string and dip the eyeball into a 200-µL microcentrifuge tube filled with super glue, ensuring the sclera is completely immersed for approximately 1 s.
- Quickly remove the eyeball and immerse it in PBS (Figure 1C). The glue on the sclera surface will solidify immediately.
- Removal of the cornea
- Blot the excess PBS on the surface of the solidified glue using a lint-free wipe. Under a dissecting microscope, remove the cornea with ophthalmic scissors while holding the attached tennis string.
- Embedding
- Using forceps, carefully extract the lens from the eyecup. Absorb any excess sucrose solution trapped within the eyecup with a strip of lint-free wipe. Transfer the eyecup to an embedding mold filled with the optimal cutting temperature (OCT) compound.
- Fill the eyecup completely with OCT. Position the eyecup so that it faces the sidewall of the embedding mold, ensuring the sagittal plane is parallel to the bottom of the mold. Use the blue mark on the sclera as a reference. Transfer the embedding mold containing the eyecup to a -80 °C freezer (Figure 1D). The eyecup will be frozen within five min.
- Cryosectioning
- Transfer the frozen eyecup to a cryostat set to -20 °C and allow it to equilibrate within the chamber for 30 min. Section the eyecup at a thickness of 12 µm. Mount the sections onto positively charged glass slides for subsequent use.
3. Immunohistochemical staining
- Baking
- Place the frozen sections onto slides and bake them in a 37 °C oven for 30-60 min to ensure proper adhesion of the tissue to the slide.
- Washing
- Using a PAP pen, draw a circle around the sections on the slide. Immerse the slide into a Coplin jar containing 1x PBS buffer. Place the Coplin jar on a slow shaker and wash the sections for 2 × 10 min to remove the OCT compound.
- Blocking and permeabilization
- Place the slide horizontally in a moisture chamber. Add the blocking solution to the circled sections and allow for blocking and permeabilization at room temperature for 30 min.
- Primary antibody incubation
- Aspirate the blocking solution carefully from the slide. Add the primary antibody, diluted in the blocking solution, to the sections. Incubate the sections in the moisture chamber at 4 °C overnight to ensure optimal binding of the antibody to the target antigen.
- Secondary antibody incubation
- Aspirate the primary antibody solution and wash the slide twice with 1x PBS for 2 × 10 min. Add the appropriate secondary antibody, mixed with DAPI, and diluted in the blocking solution.
- Incubate the sections in a dark moisture chamber at room temperature for 1 h to allow for secondary antibody binding and counterstaining with DAPI.
- Mounting
- Aspirate the secondary antibody solution and rinse the slide with 1x PBS for 2 × 10 min. Use a Kimwipe to blot away any remaining PBS on the slide.
- Apply an appropriate amount of antifade mounting medium to the sections and cover them with a coverslip. Store the mounted samples at 4 °C in the dark to preserve fluorescence and prevent photobleaching.
4. Imaging
- Acquire fluorescence signals from the immunostained retinal sections using a laser scanning confocal microscope. Adjust the settings for optimal resolution and sensitivity to capture clear and distinct fluorescence signals.
- Ensure appropriate filters are used to detect the specific wavelengths of the fluorophores employed in the experiment.
Results
Following the protocol outlined above, eyes from 1-month-old wild-type C57Bl/6J mice were fixed in 4% PFA. The fixed samples were then embedded in OCT and cryosectioned. The sections were immunostained with an anti-PDE6B antibody and counterstained with DAPI to label the nuclei. PDE6B is a phototransduction protein specifically expressed in rod photoreceptor cells4. Compared to traditional protocols, this protocol significantly improves both the morphology of mouse retinas and the quality of immunofluorescent images, providing great consistency. The representative results demonstrate successful immunostaining of retinal cryosections using anti-PDE6B (Figure 2). Photoreceptor outer segments were clearly labeled with anti-PDE6B. All photoreceptor outer segments appeared morphologically intact, and individual segments were discernible. No retinal detachment was observed in the section. Additionally, the inner segments and outer nuclear layer remained intact.
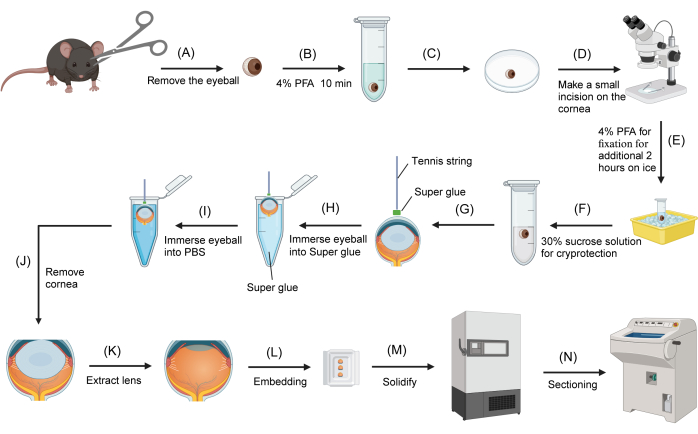
Figure 1: Mouse eye cryosectioning procedure. (A) The eyeballs are removed from sacrificed mice using scissors. (B) The eyeballs are transferred to a 2-mL round-bottom microcentrifuge tube containing 4% PFA and fixed for 10 min. (C) An eyeball is transferred to the surface of a 3-cm inverted Petri dish. (D) Under a dissecting microscope, a small incision (approximately 1 mm) is made on the cornea. (E) The eyeball is returned to the 4% PFA fixative for additional fixation on ice for 2 h. (F) The fixative is replaced with 30% sucrose for cryoprotection. (G) The tennis string is dipped into super glue, and the end is attached to the central surface of the cornea. (H) After the tennis string is firmly attached to the cornea, the other end of the string is held, and the eyeball is immersed in the glue. (I) The eyeball is immediately removed from the super glue and immersed into PBS. (J) The eyeball is removed from PBS, and the cornea is cut away with scissors, holding the free end of the tennis string. (K) The lens is extracted with forceps. (L) The eyecup is transferred to an embedding mold filled with OCT. (M) The embedding mold is transferred to a -80 °C freezer. (N) The frozen OCT block containing the eyecup is sectioned using a cryostat. Please click here to view a larger version of this figure.
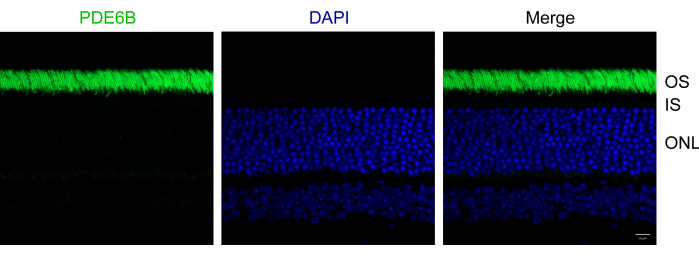
Figure 2: Immunofluorescent images of mouse retinal sections. Retinal sections were immunostained with anti-PDE6B (green) and counterstained with DAPI (blue). OS, outer segment; IS, inner segment; ONL, outer nuclear layer. Scale bar: 10 µm. Please click here to view a larger version of this figure.
Discussion
A number of factors influence the quality of tissue sections, including the composition of the fixation solution, fixation and cryoprotection time, and embedding methods5. When enucleating the eyeball from the mouse, it is essential to remove the extraocular muscles and other connective tissue attached to the eyeball. If not properly removed, these tissues can cause deformation of the eyeball during extraction from the eye socket, potentially leading to retinal detachment. During the fixation process, an incision should be made on the cornea to release intraocular pressure (IOP). Without this incision, the eyeball may shrink, resulting in deformation and retinal detachment. The increased permeability of the eyeball during fixation allows IOP to force the efflux of aqueous humor from the intraocular space. By making an incision in the cornea, the internal pressure of the eyeball is relieved, which helps prevent shrinkage and allows the fixative to more effectively penetrate the intraocular space for optimal fixation.
The retina consists of 10 layers of structurally distinct and delicate tissues. To preserve morphological integrity, it is crucial to fix the tissue properly. PFA solution is commonly used as a fixative6, as it can be freshly prepared. Using a pre-made formalin solution should be avoided, as it is prone to oxidation7, which can lead to poor fixation and compromised retinal morphology. Furthermore, fixation should not exceed 2 h, as extended fixation can cause excessive autofluorescence, particularly in the retinal pigment epithelium (RPE) cells, which may interfere with result interpretation. This issue is often observed in paraffin retinal sections, as these are typically prepared from eyeballs that have been fixed for extended periods, sometimes overnight or even longer.
We previously demonstrated that coating the eyeball with a layer of super glue significantly improves the quality of mouse retinal cryosections8. Initially, a pipette tip was used to spread super glue on the surface of the sclera; however, this method occasionally caused the eyeball to adhere to the supporting surface, such as the petri dish. To address this issue, the coating technique was modified by dipping the eyeball into the glue after attaching a segment of tennis string to the cornea9, as described in the protocol above. The attached tennis string segment serves as a handle for easy handling and facilitates the subsequent cutting and removal of the cornea.
The distribution of photoreceptors is not homogeneous across the mouse retina. Specifically, S-cone photoreceptors are predominantly localized on the inferior side of C57Bl/6J retinas, while ML-cones are more evenly distributed throughout the retina10. Therefore, marking the eyeball before enucleation is essential to ensure the desired orientation (dorsal-ventral or nasal-temporal) during processing.
However, there are limitations to this protocol. The mouse is not perfused before sacrifice. When the target for labeling is retinal vasculature, and an anti-mouse secondary antibody is used, non-specific labeling of vascular tissues by the secondary antibody may occur. In such cases, we recommend perfusing the mouse before sacrificing to prevent this issue.
In summary, a protocol has been described that enables researchers to consistently produce high-quality mouse retinal cryosections for immunostaining. This protocol is relatively easy to follow, making it particularly useful for researchers with limited experience.
Disclosures
The authors have no conflicts to disclose.
Acknowledgements
This research project was supported by the National Natural Science Foundation of China (82371059 (H.Z.), 82102470 (J.W.)), Sichuan Science and Technology Program (2023JDZH0002 (H.Z.)).
Materials
| Name | Company | Catalog Number | Comments |
| -80 °C freezer | Haier | DW-86L626 | |
| Adhesion microscope slides | CITOTEST | 80312-3161 | |
| Alexa488-Goat anti-Rabbit | Proteintech | SA00006-2 | |
| C57BL/6J mouse | The Jackson Laboratory | 664 | |
| Cryosection microtome | Leica | N/A | |
| Cryostat | LEICA | N/A | |
| DAPI | Cell Signaling Technology | 4083S | |
| Dissecting microscope | ZEISS | 3943030830 | |
| Donkey serum | Solarbio | S9100 | |
| Embedding molds | Thermo Fisher Scientific | 1841 | |
| Fine dissection scissors | RWD | S13001-10 | |
| Fine forceps | RWD | F11020-11 | |
| Fluoromount aqueous mounting medium | Sigma-Aldrich | F4680 | |
| Incubator | Shanghai Yuejin | N/A | |
| KCl | Sigma-Aldrich | 1049330500 | |
| KH2PO4 | Sigma-Aldrich | 1048771000 | |
| Kimwipes | Thermo Fisher Scientific | FIS-06666 | |
| Laser confocal microscope | ZEISS | N/A | |
| Microscope cover Glass | CITOTEST | 80340-3610 | |
| Na2HPO4 | Sigma-Aldrich | 1065860500 | |
| NaCl | Sigma-Aldrich | S9888 | |
| NaOH | Sigma-Aldrich | 1091371003 | |
| O.C.T compound | Sakura | 4583 | |
| Pap pen | Sigma-Aldrich | Z672548 | |
| PFA | Sigma-Aldrich | 441244 | |
| Rabbit anti-PDE6B | Proteintech | 22063-1-AP | |
| Shaker | SCILOGEX | 8042210200 | |
| Spring scissors | RWD | S11036-08 | |
| Sucrose | BioFroxx | 1245GR500 | |
| Super glue | Deli | 7147S | |
| Tennis string (1.24 mm) | Gosen | TS761 | |
| Tribromoethanol | Macklin | T831042 | |
| Triton X-100 | Solarbio | IT9100 |
References
- Tokuyasu, K. T. Immunochemistry on ultrathin frozen sections. Histochem J. 12 (4), 381-403 (1980).
- Ramos-Vara, J. A., Miller, M. A., et al. When tissue antigens and antibodies get along: Revisiting the technical aspects of immunohistochemistry--the red, brown, and blue technique. Vet Pathol. 51 (1), 42-87 (2014).
- Usukura, E., et al. A cryosectioning technique for the observation of intracellular structures and immunocytochemistry of tissues in atomic force microscopy (AFM). Sci Rep. 7 (1), 6462 (2017).
- Park, P. S. Supramolecular organization of rhodopsin in rod photoreceptor cell membranes. Pflugers Arch. 473 (9), 1361-1376 (2021).
- Boonstra, H., Oosterhuis, J. W., Oosterhuis, A. M., Fleuren, G. J. Cervical tissue shrinkage by formaldehyde fixation, paraffin wax embedding, section cutting and mounting. Virchows Arch A Pathol Anat Histopathol. 402 (2), 195-201 (1983).
- Stradleigh, T. W., Ishida, A. T. Fixation strategies for retinal immunohistochemistry. Prog Retin Eye Res. 48, 181-202 (2015).
- Thavarajah, R., Mudimbaimannar, V. K., Elizabeth, J., Rao, U. K., Ranganathan, K. Chemical and physical basics of routine formaldehyde fixation. J Oral Maxillofac Pathol. 16 (3), 400-405 (2012).
- Li, L., et al. An improved method for preparation of mouse retinal cryosections. Eur J Histochem. 64 (3), 3154 (2020).
- Yang, J., et al. A quick protocol for the preparation of mouse retinal cryosections for immunohistochemistry. Open Biol. 11 (7), 210076 (2021).
- Szel, A., et al. Unique topographic separation of two spectral classes of cones in the mouse retina. J Comp Neurol. 325 (3), 327-342 (1992).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved