Method Article
Peripheral Subtractive Dissection of Glissonean Pedicles in Minimally Invasive Anatomic Liver Resection for Right Posterior Lobe Tumors
* These authors contributed equally
In This Article
Summary
Minimally invasive anatomic liver resection (MIALR) involving Glissonean pedicle ligation for the right posterior hepatic lobe may cause tumor rupture, hemorrhage, and ischemia-reperfusion injury (IRI). This study proposes a novel surgical approach, peripheral subtractive dissection of the Glissonean pedicle (PSDGP), aimed at mitigating these risks.
Abstract
Minimally invasive anatomic liver resection (MIALR) has recently garnered significant attention and has rapidly advanced in the field of hepatobiliary surgery. In particular, the dissection of the Glissonean pedicle, such as in Atsushi Sugioka's Gate Theory, represents a fundamental operative technique within MIALR. This technique is based on the anatomical structure of the Laennec capsule, thereby promoting and implementing MIALR in a scientifically rigorous manner. However, potential risks such as hemorrhage, ischemia-reperfusion injury (IRI), and tumor rupture may arise during MIALR in clinical practice, particularly when it is applied to tumors located in the right posterior hepatic lobe near the bifurcation of the Glissonean pedicles (excluding hilar cholangiocarcinoma). To address these challenges, this study introduces a unique surgical approach, termed peripheral subtractive dissection of the Glissonean pedicle (PSDGP), designed to mitigate these potential complications. During the PSDGP procedure in MIALR for liver tumors, the cystic plate approach is utilized to facilitate extrahepatic dissection. Initially, a non-absorbable suture is threaded from Gate VI to Gate IV under the guidance of non-traumatic forceps (or similar instruments). Subsequently, the non-traumatic forceps are passed through Gate V again to retrieve the non-absorbable suture from Gate IV. Finally, both Gate V and Gate VI are used to achieve the separation of the right posterior Glissonean pedicle. This method may improve surgical success rates and yield better oncological outcomes due to its strict adherence to the no-touch and en-bloc principles of tumor resection.
Introduction
Laparoscopic hepatic surgery has been continuously explored and developed for more than three decades, evolving from sporadic liver resections to precise minimally invasive anatomic liver resection (MIALR). It has become a prominent area within hepatobiliary surgery, gaining considerable attention in recent years1,2,3,4,5. This technique offers several advantages, including enhanced visualization and magnification of the surgical field, enabling precise surgical operations. Accurate understanding and refinement of the Glissonean pedicles approach are fundamental skills in MIALR, ensuring safety, efficiency, and accuracy3,4,5. Atsushi Sugioka's Gate Theory, based on the anatomical structure of the Laennec capsule, provides a well-established solution that has gained wide recognition6,7(Figure 1A,B). It also describes the surgical procedure for MIALR, which includes the initial isolation and ligation of the Glissonean pedicle corresponding to the hepatic lobe, followed by the resection of the lobe.
In clinical practice, conventional hepatectomy of the right posterior liver lobe is typically performed by dissecting and ligating the right posterior Glissonean pedicle in the Rouviere's groove or by directly accessing Gate V and Gate VI through the porta7,8. However, surgery limitations, diminished tactile sensitivity, reduced global visual control ability, and anatomical variations may cause intraoperative confusion in anatomical orientation for tumors located near the bifurcation of the Glissonean pedicle in the liver (excluding hilar cholangiocarcinoma). These challenges may lead to inadvertent damage to variant blood vessels of the posterior portal vein, subsequent bleeding, and rupture of the tumor capsule5,9,10,11. Furthermore, conventional hepatectomy of the right posterior liver lobe, or occlusion of the hepatoduodenal ligament or right Glissonean pedicle, is often necessary to minimize intraoperative bleeding2,3. However, this occlusion not only induces ischemia on the tumor-bearing side of the liver but also affects the normal side, exacerbating hepatic ischemia-reperfusion injury (IRI)1,12.
Takasaki et al. previously described the extrahepatic isolation of the posterior sectional pedicle using the subtraction method7,8, while Sugioka and Kato described subtraction techniques for the extrahepatic isolation of peripheral segmental pedicles13. These applications align with liver resection in the context of peripheral subtractive dissection of the Glissonean pedicle (PSDGP), with the primary objective of mitigating pedicle injury or tumor rupture during direct pedicle isolation. Therefore, this study proposes the use of PSDGP technology for specific tumor types located near the bifurcation of the Glissonean pedicle in the liver (excluding hilar cholangiocarcinoma). The primary objective is to mitigate bleeding risk during the separation of the right posterior hepatic pedicle and prevent rupture of the tumor capsule, while concurrently reducing IRI in the residual liver.
Protocol
1. Patient selection
- Select patients based on the following inclusion criteria: tumor in the right posterior lobe (both benign and malignant tumors).
- Exclude patients based on the following exclusion criteria: hilar cholangiocarcinoma and hepatic neoplasm without metastasis.
2. Surgical procedure
- Place patients in the lithotomy position after combined intravenous-inhalational anesthesia (following institutionally approved protocols). Insert five trocars into the upper abdomen. Maintain intra-abdominal pressure at 13 mmHg. Insert an umbilical tape to encircle the hepatoduodenal ligament and perform the intermittent Pringle maneuver in an intracorporeal manner.
- Insert a 10 mm trocar above the umbilicus for the observation port.
- Insert a 12 mm trocar below the xiphoid process for the assistant's port.
- Insert a 5 mm trocar at the upper one-third of the line connecting the xiphoid process to the umbilicus.
- Insert a 12 mm trocar at the intersection of the midclavicular line and the costal margin for the surgeon's port.
- Insert a 5 mm trocar at the intersection of the anterior axillary line and the costal margin for the second surgeon's port.
- Carefully expose the Laennec's capsule by dissecting along the cystic plate through the surface of the right Glissonean pedicle. Confirm the right Glissonean pedicle in the liver by identifying the continuation of the hepatoduodenal ligament. Continue the dissection until reaching the surfaces of both the right posterior and right anterior Glissonean pedicles (Figure 1C).
3. Pedicle management
- Retract the base of segment 4 using a laparoscopic long and curved grasper on the upper right side. Pull the hepatoduodenal ligament to the lower left side using the intracorporeal Pringle maneuver.
- Expose the visceral peritoneum at the base of segment 4 of the liver hilum (Figure 2A). Using non-traumatic forceps and an ultrasonic scalpel, carefully dissect the visceral peritoneum between Laennec's capsule covering segment 4 and the superior surface of the hilar plate.Dissect and ligate small branches from the right Glissonean pedicle to provide sufficient space for isolation. Ensure meticulous dissection of small branches to avoid biliary leakage (Figure 2B).
- Detach the left side of the hilar plate from the base of segment 4. Continue dissection to the right side of the umbilical plate (Figure 2C).
- Temporarily occlude the hepatoduodenal ligament during the descent of the hilar plate to minimize hemorrhage and prevent contamination of Laennec's capsules. This ensures optimal visibility and facilitates the subsequent procedural intervention.
- Prepare a 15 cm long size 0 non-absorbable suture (3.5 metric) or a similar suture, labeled as 'a' and 'b,' with the end 'b' secured in advance.
- Use non-traumatic forceps to guide end 'a' of the non-absorbable suture (3-0) through gate VI towards gate IV, facilitating access to the right Glissonean pedicle (Figure 3A).
- Use non-traumatic forceps to direct end 'a' of the non-absorbable suture from gate IV to gate V on the right side, resulting in the acquisition of the right anterior Glissonean pedicle (Figure 3B).
- Simultaneously pull both ends ('a' and 'b') of the non-absorbable suture to expose the right posterior Glissonean pedicle (Figure 3C).
- Apply tension to the 'a' and 'b' ends of the non-absorbable suture to occlude the right posterior Glissonean pedicle (Figure 4A). Use the Pringle maneuver to occlude the right Glissonean pedicle, reducing bleeding during liver resection and minimizing ischemia-reperfusion injury (IRI) (Figure 4B).
NOTE: This approach ensures an optimal outcome, ultimately achieving precise MIALR of the right posterior hepatic lobe (Figure 4C).
4. Post-operative patient care and monitoring
- Monitor the patient and administer low-flow oxygen on the 1st day after the operation.
- Initiate the Enhanced Recovery After Surgery (ERAS) protocol on the first post-operative day, allowing patients to commence liquid diets and engage in bedside activities.
- Administer hepatoprotective agents, albumin, anti-inflammatory drugs, analgesics, and low-dose spironolactone for diuretic therapy.
- Monitor liver function (Alanine aminotransferase [ALT], Aspartate aminotransferase [AST], total bilirubin [TBIL], direct bilirubin [DBIL]) and coagulation function (prothrombin time [PT], activated partial thromboplastin time [APTT], thrombin time [TT], fibrinogen [FIB]) on the 1st, 3rd, and 5th days after the operation.
Results
The surgery was completed in 176 min, with minimal intraoperative bleeding of 50 mL and no need for a blood transfusion. The right Glissonean pedicle was occluded twice, with the first occlusion lasting 15 min and the second lasting 20 min. The hepatoduodenal ligament was not occluded during the procedure, and an adequate blood supply to the left liver was maintained while preserving the right hepatic vein (Figure 4C). No short-term complications occurred, and the patient's post-operative recovery was successful. The total hospital stay was 11 days, with a post-operative stay of 9 days.
On the fifth post-operative day, an enhanced CT scan was performed to evaluate the surgical outcome. Compared with the pre-operative CT, the results indicated that the right hepatic vein (RHV) exhibited smooth blood flow, with no evidence of vascular stenosis or thrombosis (Figure 5A,B). The main portal vein (PV) and its left and right branches, including the continuation of the right branch as the right anterior branch, appeared normal, with no signs of vascular stenosis or thrombosis. Additionally, no hematoma or effusion was observed around the liver (Figure 5C,D).
The alanine aminotransferase (ALT) level on post-operative day 1 was 354 U/L but returned to normal by day 5. Total bilirubin levels remained stable throughout the perioperative period. Coagulation function indicators, monitored multiple times before and after surgery, remained within the normal range. Additionally, the alpha-fetoprotein (AFP) level decreased from 1,210 ng/mL before surgery to 36 ng/mL after surgery (Table 1).
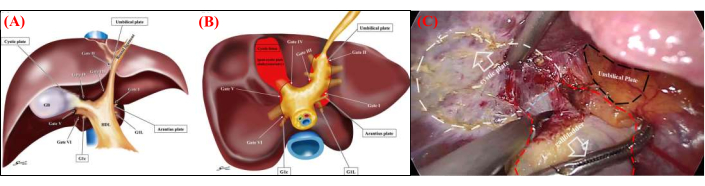
Figure 1: Anatomical structure of the liver's membrane. (A) Schematic representation of the four anatomical landmarks and six gates in the frontal view. Recognizing these landmarks and gates is essential for the standardization of extrahepatic Glisson pedicle isolation. (B) Schematic representation of the six gates and Laennec's capsule in the caudal view. The relationship between the six gates and Laennec's capsule (indicated by the red area) is shown. The gaps between Laennec's capsule and the Glisson pedicle (plate system) are accessible only through these six gates. Gate I: caudal end of the Arantius plate; Gate II: junction between the round ligament and the umbilical plate; Gate III: right edge of the Glisson pedicle root of the umbilical portion; Gate IV: left edge of the posterior extremity of the cystic plate or the anterior Glisson pedicle; Gate V: bifurcation of the right main Glisson pedicle; Gate VI: space between the posterior Glisson pedicle and G1c. (C) Atraumatic forceps are used to retract the gallbladder, enabling complete dissection of Laennec's membrane along the cystic plate to free the gallbladder up to the root of Calot's triangle. Please click here to view a larger version of this figure.

Figure 2: Exposure of the Glisson pedicle. (A) The Pringle maneuver is employed to occlude the initial porta hepatis, minimizing tissue damage. Atraumatic forceps are used to retract liver segment 4b and the hilar plate, while another atraumatic forceps gently separates Laennec's capsule. (B) The visceral peritoneum at the base of segment 4 of the liver hilum is exposed. (C) Complete dissection of the hilar plate allows full exposure of the right Glisson pedicle. Please click here to view a larger version of this figure.
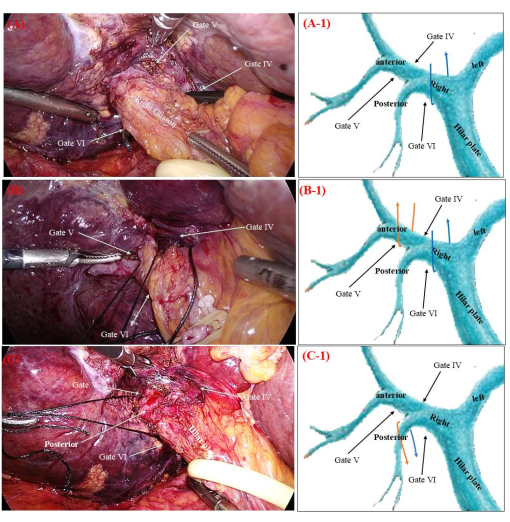
Figure 3: Peripheral subtractive dissection of Glissonean pedicles. (A) The gold finger (or non-damaging forceps) is used to guide the b end of the non-absorbable suture from Gate VI to Gate IV. (B) The gold finger is used to guide the b end of the non-absorbable suture from Gate IV to Gate V. (C) Manipulation of the a and b ends of the non-absorbable suture enables precise access to the right posterior Glisson pedicle. The orange and blue arrows in Figure A-1,B-1,C-1 indicate the trajectory of non-absorbable sutures. Please click here to view a larger version of this figure.

Figure 4: Precise anatomical resection of the liver. (A) Ligation of the right posterior Glisson pedicle results in evident ischemia in the right posterior liver lobe (white dashed line indicates the boundary). (B) Ligation of the right Glisson pedicle results in evident ischemia in the right hemiliver (yellow dashed line indicates the boundary). Given that the right posterior Glisson pedicle has already been ligated, occlusion of the right Glisson pedicle is equivalent to obstructing the right anterior Glisson pedicle. (C) Precise anatomical resection of the right posterior liver lobe is performed, preserving the right hepatic vein (RHV) during surgery (blue dashed line indicates RHV deformation). Please click here to view a larger version of this figure.

Figure 5: Comparative analysis of liver morphology pre-and post-surgery. (A) Pre-operative enhanced CT images reveal the anatomical structures of the left, middle, and right hepatic veins (dotted lines indicate the primary course of the hepatic veins). (B) Enhanced CT images obtained on post-operative day 5 confirm that the left, middle, and right hepatic veins remain intact. (C-D) Post-operative enhanced CT images demonstrate that the left and right branches of the portal vein, as well as the right hepatic vein, follow their normal courses without evidence of vascular stenosis or thrombosis. No hemorrhage or effusion is observed in the perihepatic region (solid lines indicate the primary course of the portal vein). Please click here to view a larger version of this figure.
| Items | Results | |
| Operation time (min) | 176 | |
| Intraoperative bleeding volume (mL) | 50 | |
| Blood transfusion volume (mL) | 0 | |
| Pringle maneuver for vascular occlusion time (min) | 35(15+20) | |
| Postoperative complication | None | |
| Duration of hospital stay (day) | 11 | |
| Postoperative hospital stay (day) | 9 | |
| Preoperative levels of ALT and amylase | 19 | |
| ALT amylase levels on POD1 (U/L) | 354 | |
| ALT amylase levels on POD3 (U/L) | 132 | |
| ALT amylase levels on POD5 (U/L) | 31 | |
| Preoperative levels of AST and amylase | 23 | |
| AST amylase levels on POD1 (U/L) | 381 | |
| AST amylase levels on POD3 (U/L) | 119 | |
| AST amylase levels on POD5 (U/L) | 22 | |
| Level of Tbil preoperatively (ng/mL) | 16.9 | |
| Level of Tbil on POD1 (ng/mL) | 25.8 | |
| Level of Tbil on POD3 (ng/mL) | 19.7 | |
| Level of Tbil on POD5 (ng/mL) | 18.5 | |
| Preoperative coagulation function(TT/APTT/PT/FIB) | normal | |
| Postperative coagulation function(TT/APTT/PT/FIB) | normal | |
| Level of AFP preoperatively (ng/mL) | 1210 | |
| Level of AFP postoperatively (ng/mL) | 36 | |
Table 1: Intraoperative and post-operative parameters.
Discussion
The extracorporeal dissection method of the Glissonean pedicles is safe, effective, and expedient, making it a fundamental technique for MIALR2,3,7,13. The Glissonean pedicles and their branches provide blood supply to the hepatic lobes and facilitate bile outflow. Safe and efficient dissection of the Glissonean pedicles during surgery is essential for the successful implementation of MIALR.
According to Atsushi Sugioka's "Gate Theory," which is based on Laennec's capsule anatomical theory, surgical procedures accurately determine the anatomical approach and levels of the Glissonean pedicles using the "gate" as a guide and Laennec's capsule as a boundary1,2,7,14. The primary operational space in the application of the "Gate Theory" lies in the gap between Laennec's capsule and the Glissonean sheath. The liver's portal system is formed by the thickened and fused Glissonean capsule in the first hepatic hilum region, composed of four distinct components: the hepatic plate, gallbladder plate, umbilical vein plate, and Arantius plate2,7.
Surgical techniques for hepatic and cystic plates have now reached a relatively mature stage and have gradually evolved into indispensable steps in MIALR surgery. The crucial step in MIALR surgery involves the ligation of the corresponding Glissonean pedicles based on the tumor's location, which requires opening the respective gate. For instance, left hemiliver resection requires passage through Gate 1 and Gate 3; left lateral section resection requires passage through Gate 1 and Gate 2; left medial section resection involves passage through Gate 2 and Gate 3; right hemiliver resection requires passage through Gate 4 and Gate 6; right anterior segment resection requires passage through Gate 4 and Gate 5; and right posterior segment resection requires passage through Gate 5.
However, potential risks of bleeding and tumor capsule rupture may occur during MIALR in clinical practice for tumors located in the right posterior hepatic lobe near the bifurcation of the Glissonean pedicle, excluding hilar-cholangiocarcinoma5,6,13. To mitigate these risks, the right posterior Glissonean pedicle is indirectly reached through the PSDGP method, circumventing direct passage through Gate V and Gate VI. The right posterior Glissonean pedicle obtained through indirect dissection offers several advantages over direct dissection.
First, effective control of the right Glissonean pedicle can be achieved by accessing Gate 4 to Gate 6, facilitating blood flow occlusion during right posterior liver lobe resection. Selective occlusion of the right Glissonean pedicle can be maintained for up to 30 min or longer without compromising blood flow in the left liver lobe, compared to the conventional method of direct hepatoduodenal ligament occlusion15,16,17. This approach reduces hepatic ischemia-reperfusion injury (IRI) and accelerates post-operative liver function recovery18. Second, accessing the right posterior Glissonean pedicle poses a significant surgical challenge due to its deep anatomical location and the presence of anatomical variations. Crossing directly from Gate V to Gate VI to access this pedicle may lead to unintended overlooking of anatomical variations, increasing the risk of damaging surrounding membranous structures and causing bleeding5,14,19. Finally, a significant risk of tumor rupture exists during the resection of benign tumors, such as vascular malformations, potentially leading to substantial hemorrhage when dissecting the Glissonean pedicles. If the tumor is malignant, its rupture during Glissonean pedicle dissection not only results in hemorrhage but also violates the principles of "no touch" and "en bloc" resection. Surgical resection becomes particularly challenging when the tumor, whether benign or malignant, is located in the right posterior lobe of the liver, especially if the tumor diameter is equal to or greater than 3 cm and is situated in segment 7 near major blood vessels and bile ducts. Therefore, the PSDGP technique can effectively occlude the Glissonean pedicle of the right anterior lobe to control bleeding and minimize IRI in the left hepatic parenchyma, ultimately achieving the goal of MIALR.
The pre-operative planning for hepatic lobectomy involves the use of CT, MRI, and auxiliary 3D visualization to develop a precise surgical plan4,8,20. The dissection of Glissonean pedicles during surgery follows the principles of Gate Theory, while intraoperative ultrasound and fluorescence imaging are used to accurately locate and guide liver resection. These techniques assist surgeons in performing MIALR according to the pre-operative plan.
This study proposes that the implementation of the PSDGP approach in Laennec's capsule during the surgical procedure enables precise MIALR while separating the right posterior Glissonean pedicle. In particular, in economically underdeveloped countries or regions with limited access to ultrasound equipment and indocyanine green fluorescence imaging devices in medical centers, this technique provides precise control of the right posterior Glissonean pedicle and ensures that no pedicles are missed. Subsequently, the liver parenchyma is incised along the ischemic line on the liver surface, providing a clear visualization of the underlying right hepatic vein. A meticulous resection of the right posterior lobe is then performed based on the accurate identification and complete dissection of the right hepatic vein.
The right Glissonean pedicle and the right anterior Glissonean pedicle were dissected along Laennec's capsule during the PSDGP procedure, while the right posterior Glissonean pedicles were obtained by subtractive dissection from the outside of the Glissonean pedicle sheath, effectively ligating both the pedicles and the surrounding connective tissue7,9,10. Surgeons should pay close attention to the caudate pedicles branching out from the right main pedicle during the subtractive dissection of the Glissonean pedicle for the posterior pedicle.
Accurate dissection of Gates IV and VI is of utmost importance for right pedicle isolation as the first step of PSDGP for the posterior pedicle. During this procedure, Gates IV and VI should be carefully dissected at the right, outside the branching point of the pedicles of the caudate process (G1c) and paracaval portion (G1r). If G1c or G1r is involved in the sling tape of the right pedicle isolation, these caudate pedicles remain included in the potential posterior pedicle during the subsequent subtractive dissection.
In the context of the PSDGP procedure, cholecystectomy can be performed concurrently to address gallbladder issues in patients with benign conditions such as gallstones. However, there are limitations associated with the PSDGP technique for patients with a normal gallbladder; therefore, explicit patient consent must be obtained before proceeding with the surgery. Additionally, according to Professor Cho A and colleagues in Japan21,22,23, the ligation of variant blood vessels surrounding the branching point of the Glissonean pedicle may supply part of segment 5, potentially resulting in localized liver ischemia at the resection margin. Consequently, this may lead to post-operative liver enlargement compared to preoperatively planned resection. Although the theoretical increase in the dimension of liver resection in the portal vein drainage area is not significant, further research is necessary to ascertain the clinical feasibility, safety, and limitations of the PSDGP procedure.
Clinical practice has revealed that PSDGP can be effectively used in resections involving the left lateral lobe, left medial lobe, and both lobes of the liver. However, further investigation is needed to determine its feasibility, safety, and limitations in clinical applications. This study is a retrospective analysis performed at a single center, involving a limited number of patients; half of them were diagnosed with benign tumors, and the other half with malignant tumors. Long-term follow-up results are currently unavailable. Therefore, further validation is required to assess the technical aspects, indications, contraindications, and long-term therapeutic effects of PSDGP in oncology. Prospective research objectives are currently underway.
Disclosures
The authors have no conflicts of interest or financial ties to disclose.
Acknowledgements
The study was financially supported by the Sichuan Medical Science and Technology Innovation Research Association (Project Code: YCH-KY-YCZD2024-075)
Materials
| Name | Company | Catalog Number | Comments |
| Electrocantery | Hangzhou Kangji Medical Instrument Co., Ltd | KJ-SJ0205 | Sterile,dry heat sterilized, reusable |
| Gold finger | Hangzhou Kangji Medical Instrument Co., Ltd | 101.237Φ10*350mm | Sterile,dry heat sterilized, reusable |
| Non-absorbable suture | Johnson & Johnson MEDICAL (CHINA) Ltd | 2-0/W2512 | Sterile, ethylene oxide sterilized, disposable |
| Non-traumatic forceps | Hangzhou Kangji Medical Instrument Co., Ltd | Φ10×260 | Sterile,dry heat sterilized, reusable |
| Soft rubber ureteric catheter | Yangzhou Jinhuan Medical Appliance factory | Type A 5.3mm(16Fr) | Sterile, ethylene oxide sterilized, disposable |
| Trocar | Zhejiang Geyi Medical Instrument Co.,Ltd | GYTR-I Φ5/Φ10/Φ12 | Sterile, ethylene oxide sterilized, disposable |
References
- Fujiyama, Y., et al. Latest findings on minimally invasive anatomical liver resection. Cancers (Basel). 15 (8), 2218 (2023).
- Reig, M., et al. Bclc strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol. 76 (3), 681-693 (2022).
- Morimoto, M., et al. Minimally invasive anatomic liver resection: Results of a survey of world experts. J Hepatobiliary Pancreat Sci. 29 (1), 33-40 (2022).
- Yang, J. D., Heimbach, J. K. New advances in the diagnosis and management of hepatocellular carcinoma. Bmj. 371, m3544 (2020).
- Cherqui, D. Laparoscopic liver resection: A new paradigm in the management of hepatocellular carcinoma. J Hepatol. 63 (3), 540-542 (2015).
- Morimoto, M., et al. Glissonean pedicle isolation focusing on the laennec's capsule for minimally invasive anatomical liver resection. J Pers Med. 13 (7), 1154 (2023).
- Sugioka, A., Kato, Y., Tanahashi, Y. Systematic extrahepatic glissonean pedicle isolation for anatomical liver resection based on laennec's capsule: Proposal of a novel comprehensive surgical anatomy of the liver. J Hepatobiliary Pancreat Sci. 24 (1), 17-23 (2017).
- Kato, Y., et al. Laparoscopic isolated liver segmentectomy 8 for malignant tumors: Techniques and comparison of surgical results with the open approach using a propensity score-matched study. Langenbecks Arch Surg. 407 (7), 2881-2892 (2022).
- Kim, J. H., Kim, H. Laparoscopic right hemihepatectomy using the glissonean approach: Detachment of the hilar plate (with video). Ann Surg Oncol. 28 (1), 459-464 (2021).
- Maeda, K., et al. Pure laparoscopic right hemihepatectomy using the caudodorsal side approach (with videos). J Hepatobiliary Pancreat Sci. 25 (7), 335-341 (2018).
- Wakabayashi, G., et al. Recommendations for laparoscopic liver resection: A report from the second international consensus conference held in morioka. Ann Surg. 261 (4), 619-629 (2015).
- Gotohda, N., et al. Expert consensus guidelines: How to safely perform minimally invasive anatomic liver resection. J Hepatobiliary Pancreat Sci. 29 (1), 16-32 (2022).
- Kato, Y., et al. Minimally invasive anatomic liver resection for hepatocellular carcinoma using the extrahepatic glissonian approach: Surgical techniques and comparison of outcomes with the open approach and between the laparoscopic and robotic approaches. Cancers (Basel). 15 (8), 2219 (2023).
- Xi, C., et al. A novel difficulty scoring system of laparoscopic liver resection for liver tumor. Front Oncol. 12, 1019763 (2022).
- Liu, J., et al. The difference in prolonged continuous and intermittent pringle maneuver during complex hepatectomy for hepatocellular carcinoma patients with chronic liver disease: A retrospective cohort study. Cancer Med. 10 (23), 8507-8517 (2021).
- Hu, Y., et al. Laennec's approach for laparoscopic anatomic hepatectomy based on Laennec's capsule. BMC Gastroenterol. 19 (1), 194 (2019).
- Zhang, Y., et al. Intermittent pringle maneuver versus continuous hemihepatic vascular inflow occlusion using extra-glissonian approach in laparoscopic liver resection. Surg Endosc. 30 (3), 961-970 (2016).
- Park, J. B., et al. Effect of intermittent hepatic inflow occlusion with the pringle maneuver during donor hepatectomy in adult living donor liver transplantation with right hemiliver grafts: A prospective, randomized controlled study. Liver Transpl. 18 (1), 129-137 (2012).
- Kawaguchi, Y., Fuks, D., Kokudo, N., Gayet, B. Difficulty of laparoscopic liver resection: Proposal for a new classification. Ann Surg. 267 (1), 13-17 (2018).
- Benson, A. B., et al. Hepatobiliary cancers, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 19 (5), 541-565 (2021).
- Cho, A., et al. Relationship between right portal and biliary systems based on reclassification of the liver. Am J Surg. 193 (1), 1-4 (2007).
- Cho, A., et al. Anterior fissure of the right liver--the third door of the liver. J Hepatobiliary Pancreat Surg. 11 (6), 390-396 (2004).
- Cho, A., et al. Relation between hepatic and portal veins in the right paramedian sector: Proposal for anatomical reclassification of the liver. World J Surg. 28 (1), 8-12 (2004).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved